Materials Sciences and Applications
Vol. 3 No. 7 (2012) , Article ID: 21160 , 14 pages DOI:10.4236/msa.2012.37070
The Use of Exoskeletons of Shrimp (Litopenaeus vanammei) and Crab (Ucides cordatus) for the Extraction of Chitosan and Production of Nanomembrane
![]()
Post-Graduate Program in Mechanical Engineering, Federal University of Rio Grande do Norte, Natal, Brazil.
Email: mestrsan@yahoo.com.br/rasiah@ufrnet.br
Received March 14th, 2012; revised April 15th, 2012; accepted May 13th, 2012
Keywords: Chitin; Chitosan; Nanomembranes; Crystallinity; Shrimp; Crab
ABSTRACT
Chitin is widely distributed in nature, being the main structural component of the exoskeleton of crustaceans and is non-toxic, biodegradable and biocompatible. These exoskeletons once discarded become an industrial waste creating environmental pollutant. In order to find an alternative use, the present work exploits the extraction of the chitosan from chitin that is present in the exoskeletons of shrimps Litopenaeus vannamei and crabs Ucides cordatus and transforms it into high valued products, which can help solving the environmental problem as well to provide extra income to the fishermen. One example is the manufacture of nanomembranes from chitosan for the application in medical textiles. Nanomembranes using electrospinning of chitosan solutions (7% and 5 wt%) with 100:0 v/v (TFA/DCM) and 70:30 v/v (TFA/DCM) were produced. Morphological properties of chitin and chitosan were studied using SEM, DRX, and thermal properties through TG/DTG and molecular structure by FTIR analysis. TG/DTG showed thermal decomposition of chitosan samples. X-ray diffraction analysis indicated the semi-crystalline structure of chitosan, and highly crystalline structure for chitin. Morphologies of the nanomembranes were also observed from scanning electron micrographs. Results showed that the nanomembranes with 5% chitosan solutions with 70:30 v/v (TFA/DCM) showed facilitation in the formation of the nanomembranes. The nanomembranes of shrimp and crab with 5% 70:30 v/v (TFA/DCM) had higher breaking tension and breaking extension. With positive results obtained, the present work will help the authorities to organize the fishermen to have consciousness in the collection of exoskeleton waste as well as helping to have a better environment.
1. Introduction
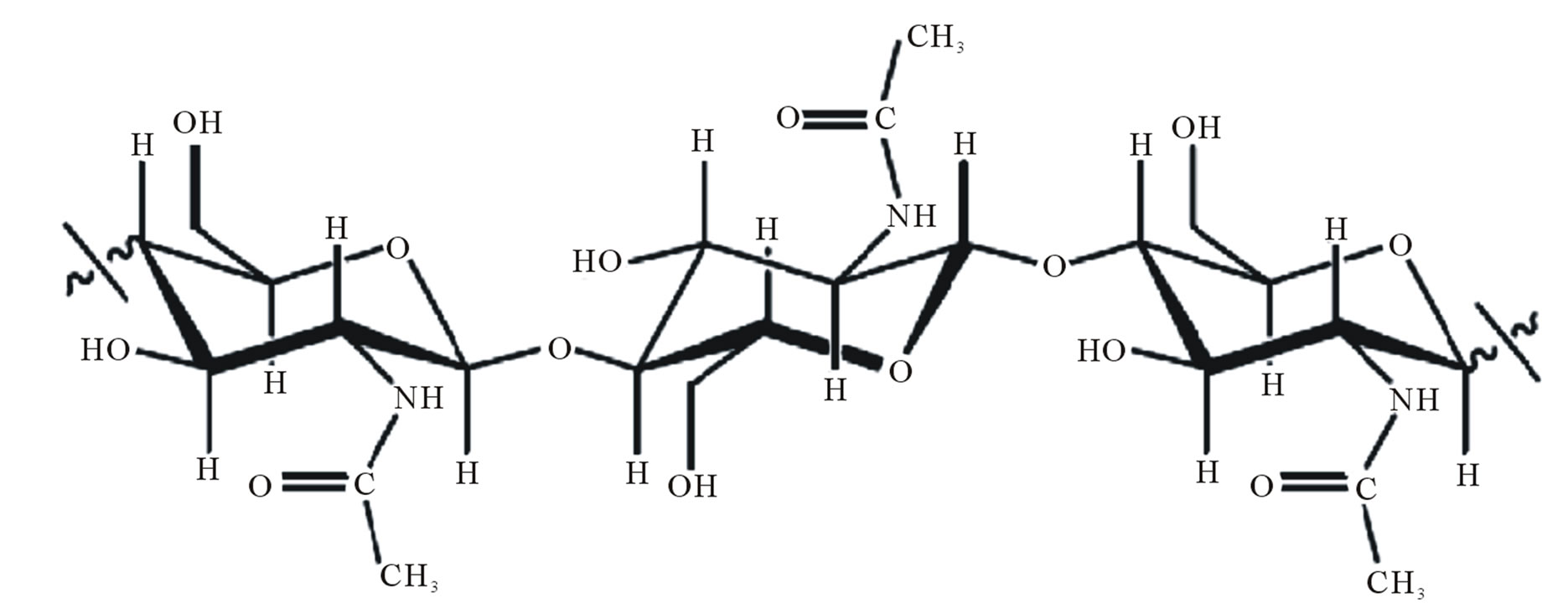
Chitin and chitosan, Figure 1 are polysaccharides polymeric materials, non-toxic, biodegradable, biocompatible and produced by the renewable natural source, whose properties have been exploited in industrial applications and technology for almost seventy years [1]. They are widely used in many areas such as, agriculture, food industry, textiles, pharmaceuticals, cosmetics and development of biomaterials, for instance gels, films, nanomembranes and polymer nanofibers [2].
Chitin is widely distributed in nature, being the main structural component of the exoskeleton of crustaceans (shrimp, crab, squid and lobster). It is also found in the insect cuticle and cell wall of fungi and yeast. The chitosan is obtained from deacetylation of chitin in alkaline solution. During this reaction, the acetamide groups (-NHCOCH3) of chitin are converted into amino groups (-NH2) leading to chitosan [4]. Depending on the source from where it is extracted, chitin can be found in three polymorphic conformations (α, β and γ) as in the Figure 2. This is according to the orientation of the polymer chains, which depend on their crystal structures. The polymorphism of chitin leads to low crystallinity [5].
(-NHCOCH3) of chitin are converted into amino groups (-NH2) leading to chitosan [4]. Depending on the source from where it is extracted, chitin can be found in three polymorphic conformations (α, β and γ) as in the Figure 2. This is according to the orientation of the polymer chains, which depend on their crystal structures. The polymorphism of chitin leads to low crystallinity [5].
The α conformation is found in the shells of crustaceans, insects and the cell walls of fungi, with features such as a structure where there is an alternate arrangement of parallel and anti-parallel chains. In the β form, predominant in microscopic squids and seaweeds, the
 (a)
(a)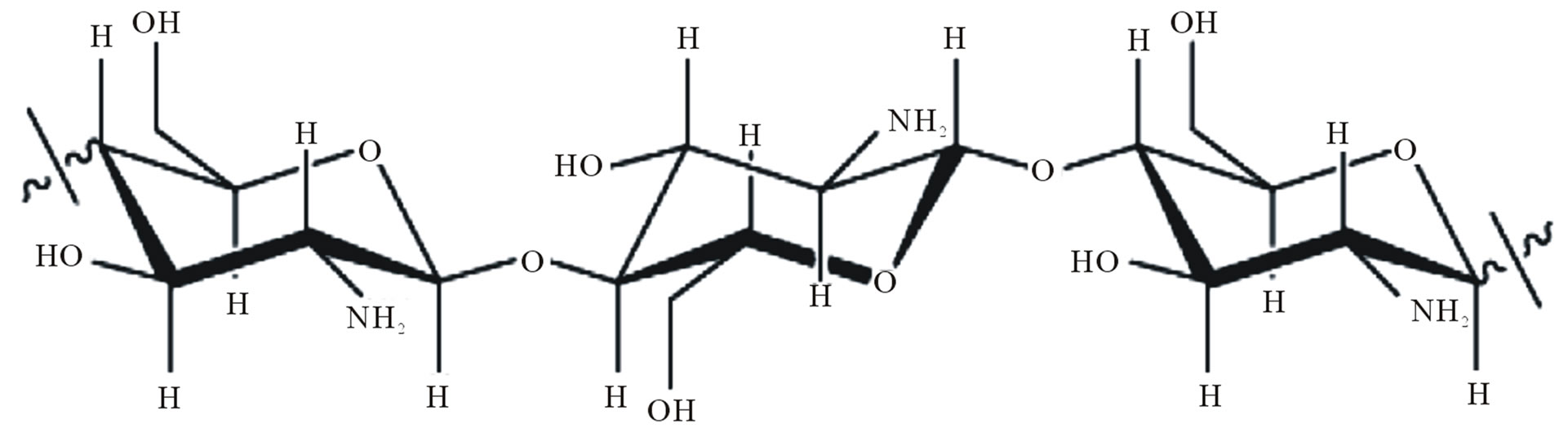 (b)
(b)
Figure 1. Chemical structure of the polysaccharide (a) chitin; (b) chitosan [3].
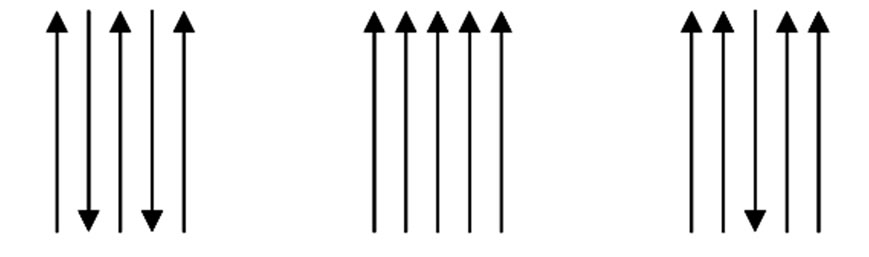
Figure 2. Schematic representation of the polymer chains in different forms of chitin [6].
arrangement is of parallel chains. α and β chitins are mostly known, αis being the most common and consequently the most studied one. The γ chitin is rarely found in nature thus its characteristics and possible uses are not well defined [7].
The production of chitin is currently from the shells of shrimp and crab wasted by canneries in various parts of the world including the USA (Oregan, Washington, Virginia), Japan and several fishing fleets in Antarctica [8]. The polymer is also produced on a smaller scale in India, Norway, Canada, Italy, Poland, Chile and Brazil [9].
Shrimp and crab are food with rich in protein, calcium, vitamins, and several components have been extracted and used as one of the most popular and important ingredients for food preparations in various countries. The northeast coast is considered ideal environment for shrimp farming because it has extensive coastal waters with warm temperatures throughout the year.
The kind of gray shrimp Litopenaeus vannamei in the Pacific Ocean was introduced in Brazil in the 80’s and spread throughout the northeast after their excellent adaptation to local climatic conditions, immediately contributing to a better performance of the creations [10]. Statistical data from the Brazilian Shrimp Farmers Association [11] show that the Brazilian production of these species grew between 1998 and 2005, and their production rose from 7.000 to 65.000 tons per year. The increased production of shrimp has generated a large amount of solid waste considering that the animal’s head and shell account for approximately 40% of its total weight, culminating in a strong environmental impact.
The crab Ucides cordatus is popularly known in Brazil as Uçá-crab, crustacean of greater importance in the mangroves and cited in reports at the beginning of the fourteenth century by the Jesuits and Portuguese travelers who lived in Brazil [12,13] It is among the most sought species of crustaceans in the coastal region of the country and their meat is enjoyed in cooking.
In the Brazilian mangroves, the activity of catching crabs Ucides cordatus is one of the most important sources of livelihood for the population living in these areas. The main occurrence and production are concentrated in the North and Northeast of Brazil. The capture of the Uçá-crab is probably one of the oldest activities in the extractive mangroves occurring on a large scale in the States of Pará, Maranhão, Piauí, Rio Grande do Norte, Sergipe and Bahia, and also in smaller volumes, in the States of Espirito Santo, Rio de Janeiro, São Paulo, Parana and Santa Catarina [14].
The annual production of waste from the shellfish industry is approximately 39.000 tons. Even though these are biodegradable, they do cause environmental problem. However, they create social disorder as they produce unpleasant smell and attract insects, which may cause damage to human health [15,16].
The residue from the production of shrimp contains about 15% to 20% chitin, 25% to 40% protein and 40% to 55% calcium carbonate. The residues of crabs possess high levels of nutrients such as N, P, Ca and Mg. The waste is usually buried underground or dumped into the sea or river, causing environmental problems.
The present study emphasizes the current problems and also aims at proposing an alternative model to reduce the organic waste of Ucides cordatus crab and Litopenaeus vannamei shrimp generated by beach huts which are located on the south coast in the Ponta Negra beach in Natal-RN and also in commercial establishments (local restaurants) by using them for alternative applications that are mentioned earlier.
The objective of this paper is to investigate the potential usage of shrimp and crab shells that are being discarded as waste, for the extraction of chitosan to produce high value products such as nanomembranes that has technical, economical and environmental, using a simple and effective technology. Characterization of the chitosan as well as the membrane produced will be carried out to elucidate their properties.
2. Materials and Methods
The raw materials used in this work were exoskeletons of crustaceans (Litopenaeus vannamei shrimp and Ucides cordatus crab) as shown in Figures 3(a) and (b) which were collected from the beach huts located on the south coast in the Ponta Negra beach and local restaurants in the city of Natal/RN-Brazil.
 (a)
(a) (b)
(b)
Figure 3. (a) Litopenaeus vannamei shrimp; (b) Ucides cordatus crab.
All reagents used in this stage of this work were of analytical grade, Table 1.
The process of extraction of chitin and chitosan from shells of crustaceans involve pre-treatment, demineralization, deproteinization, deodorizing and drying [17] were carried out in Labtex/UFRN, Brazil. Analysis of extracted chitin and chitosan were carried out at CTPETROINFRA e FINEP/LIEM, CSIR, Port Elizabeth-South Africa.
Initially the crab and shrimp shells were washed and cleaned thoroughly to remove any foreign materials, followed by grinding to get particle sizes in the order of 0.297 mm, Figure 4.
In the demineralization process for shrimp and crabs, 2.5% v/v and 7.0% v/v, hydrochloride acid was used. Both samples were washed repeatedly until the neutral pH was attained. This process has been carried out to reduce the ash content as well as the elimination of carbonates and phosphates from the samples.
Deproteinization step involved the use of sodium hydroxide (NaOH) (5% w/v) to reduce the nitrogen content of protein. The treated samples were subsequently washed to remove any traces of sodium hydroxide.
In the deodorization process, the deproteinized ma-
Table 1. List of reagents.

Figure 4.Granulation of samples of shrimps and crabs.
terial was mixed with a solution of sodium hypochlorite (NaOCl) (0.36% v/v) under stirring to reduce the odour of the material and also to remove any pigments. The samples were filtered, washed repeatedly to remove any traces of left over chemicals and soluble impurities. The filtered samples were then dried in an oven at 80˚C for 4 hours.
The dried deproteinized and deodorized samples of chitin were treated with NaOH (42.3%) and the solution was heated for 2 hours with constant stirring. At the end of the reaction time the samples were filtered and then washed until the neutral pH was obtained. 1% of acetic acid was added to dissolve the Chitosan at pH 6.0. The solution was then precipitated in an alkaline solution of NaOH followed by neutralized with acetic acid until pH 7.0. The purified Chitosan was dried in an oven at a constant temperature.
For the formation of chitosan nanomembranes worked up with a chitosan solution on 7% chitosan in 100:0 v/v (TFA/DCM) and 5 wt% chitosan in 70:30 v/v (TFA/ DCM). The solutions, Figure 5, were prepared and stirred continuously for 12 h at room temperature and later poured into a syringe of 5 ml, with distance of needle to collector was 5 and 10 cm. Both the syringe and the needle were tilted 45˚ from a horizontal baseline. The chitosan membrane was collected in aluminum sheet and the electrical potential was between 20 and 30 kV. The same were dried at 50ºC.
3. Results
3.1 Yield Percent
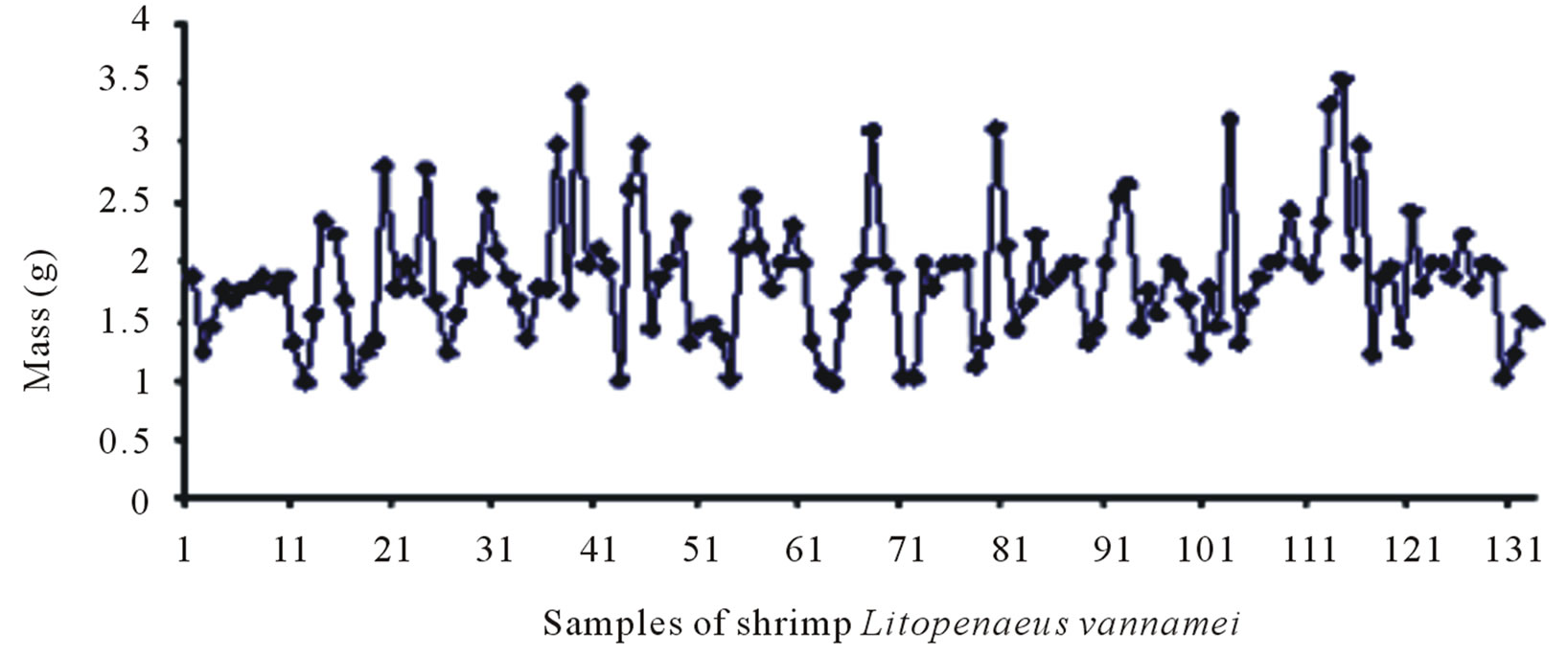
The shrimp and crab shells were weighed after cleaning and drying. 132 samples of shrimp shells were selected (amount to 1 Kg). Samples of 100g lots where in the individual weight of the shrimp shells varied from 0.98 g to 3.55 g were prepared. The selected lots were then dried in an oven at 50˚C for one hour and transferred to a desiccator. The final average weight of the lots obtained was 84.00 g, which indicates a weight loss of 16.00% due to


Figure 5. Solutions for chitosan membrane preparation 100:00 and 70:30.
drying.
Figures 6(a) and (b) show the samples of shrimp before and after drying respectively. Similarly the samples of crab before and after drying are depicted in Figures 6(c) and (d).
51 crab shells were selected and weighed for a total weight of 229.56 g, where the individual shell weights varied from 3.72 g to 6.30 g. After drying the total weight of the samples was about 189.39 g, having suffered a mass loss of 17.50% compared to the total weight of the samples before drying.
In the Tables 2 and 3, the total amount of raw material for both shrimp and crab shell samples after cleaning and drying at 50˚C to be used for the extraction of chitin and chitosan.
The yield of chitin presented was of 41% (shrimp) and 56% (crab) regarding the concentration the initial mass of chitin in the shrimp and crab exoskeleton. As the reaction of chitin deacetylation is more aggressive due to high alkalinity and temperature employed, the result of this step equaled 22% (shrimp) and 40% (crab) regarding the concentration of the initial mass used. The values obtained were in concordance with the results obtained by Moura [18].
3.2. Scanning Electron Microscopy
The topography of chitin and chitosan samples was inspected under scanning electron microscope (SEM) and the results showed that the samples possess similar structures with loosely attached geometrically irregular fine particles as seen in Figures 7(a)-(c). It is also seen that their surfaces are heterogeneous.
The micrographs substantiate that chitosan obtained from shrimp possesses smooth surface and grains of different size sand shapes that are randomly distributed than that of chitosan from crab in which it is seen that there are greater regularity in the average grain size and more porous. The micrographs of the samples also showed highly rough and fibrous surfaces as observed in Figures 7(d)-(f).
3.3. X-Ray Diffraction
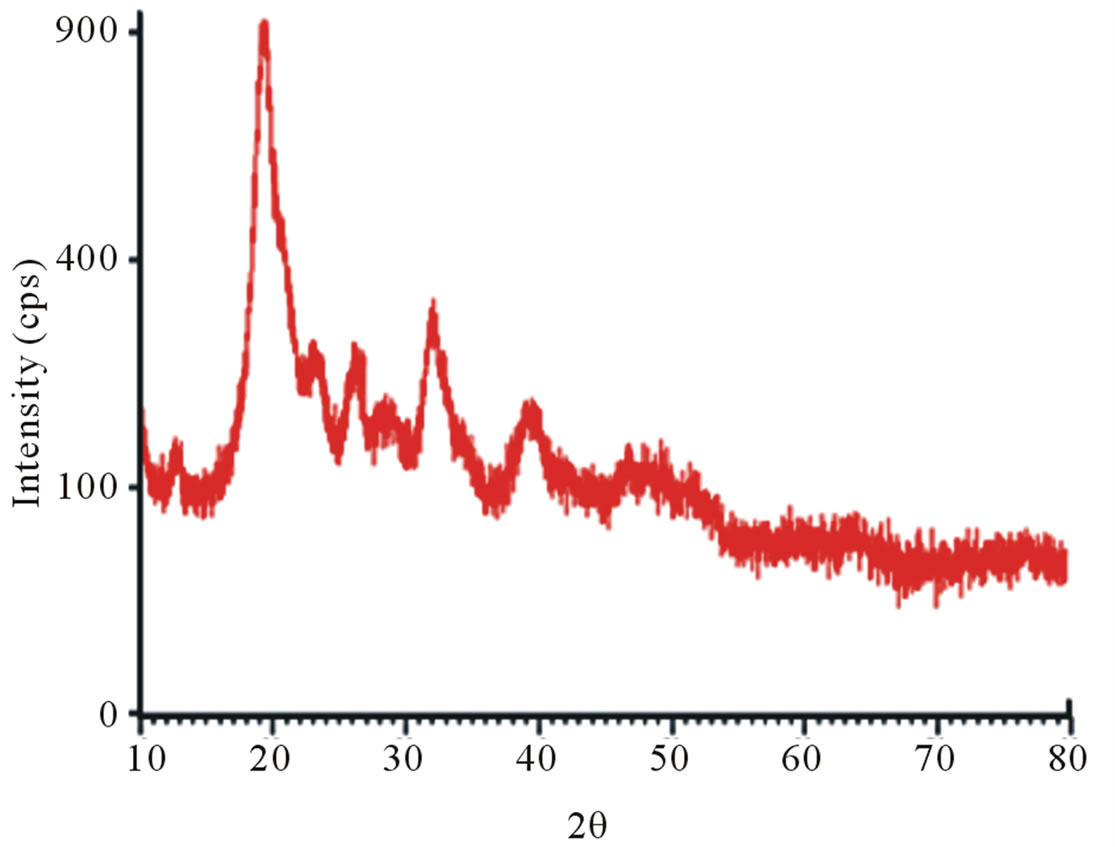
X-ray diffraction study was carried out using a Universal X-ray diffractometer; model Shimadzu XRD-6000 with Cu radiation, with 30 kV and 30 mA. The samples of chitin and chitosan were scanned from 10 to 80º.
X-ray diffraction clearly distinguishes the structure of raw chitin and its derivative, deacetylated chitosan. In fact, the diffractograms of shrimp chitin showed better resolved peaks and in greater numbers, Figure 8(a), than that observed in the diffractograms of chitosan from shrimp, Figure 8(b), which could be attributed to the
 (a)
(a)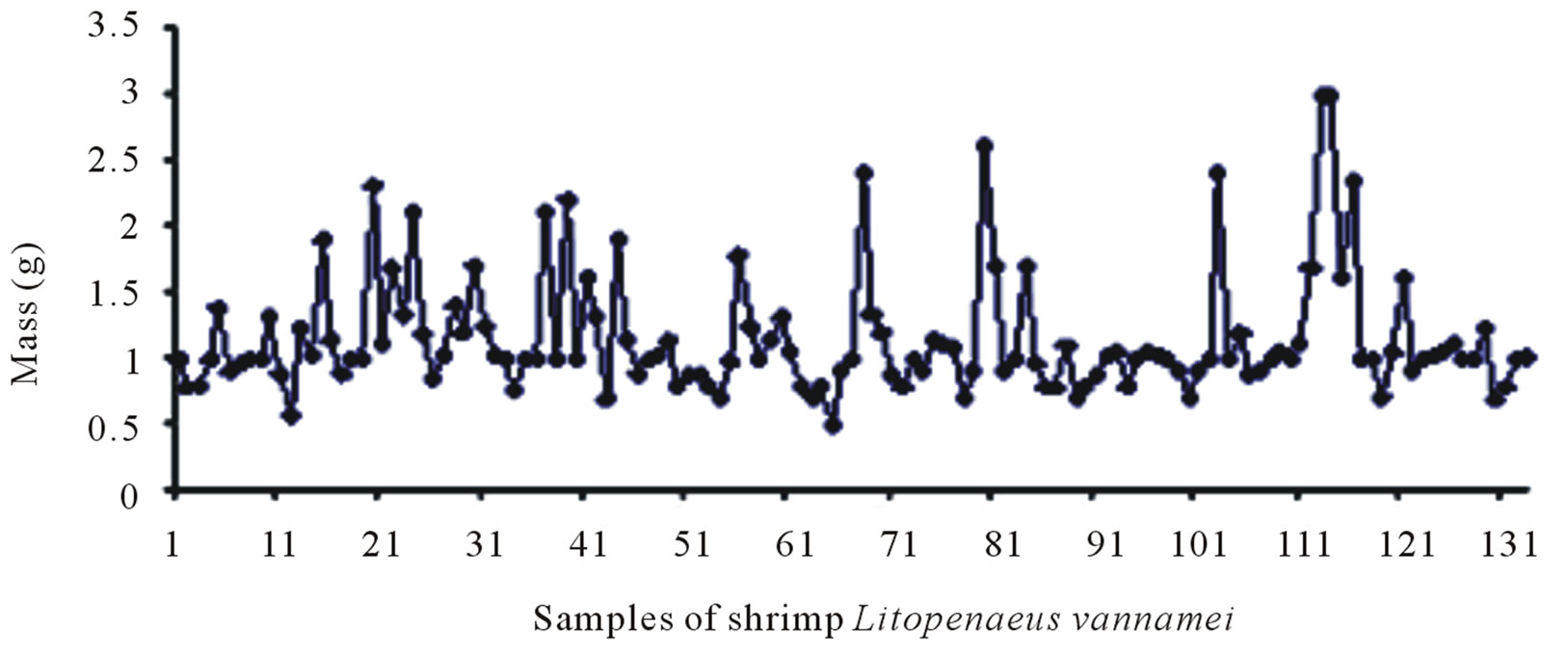 (b)
(b)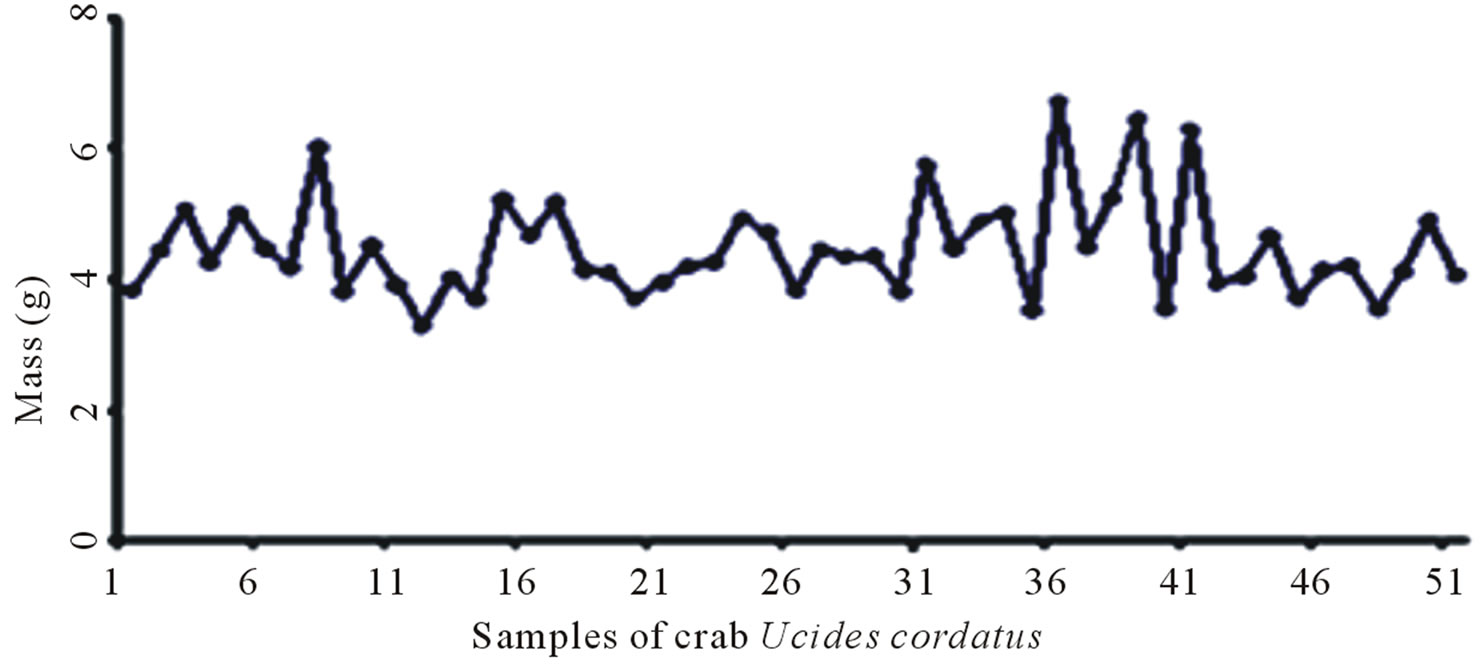 (c)
(c)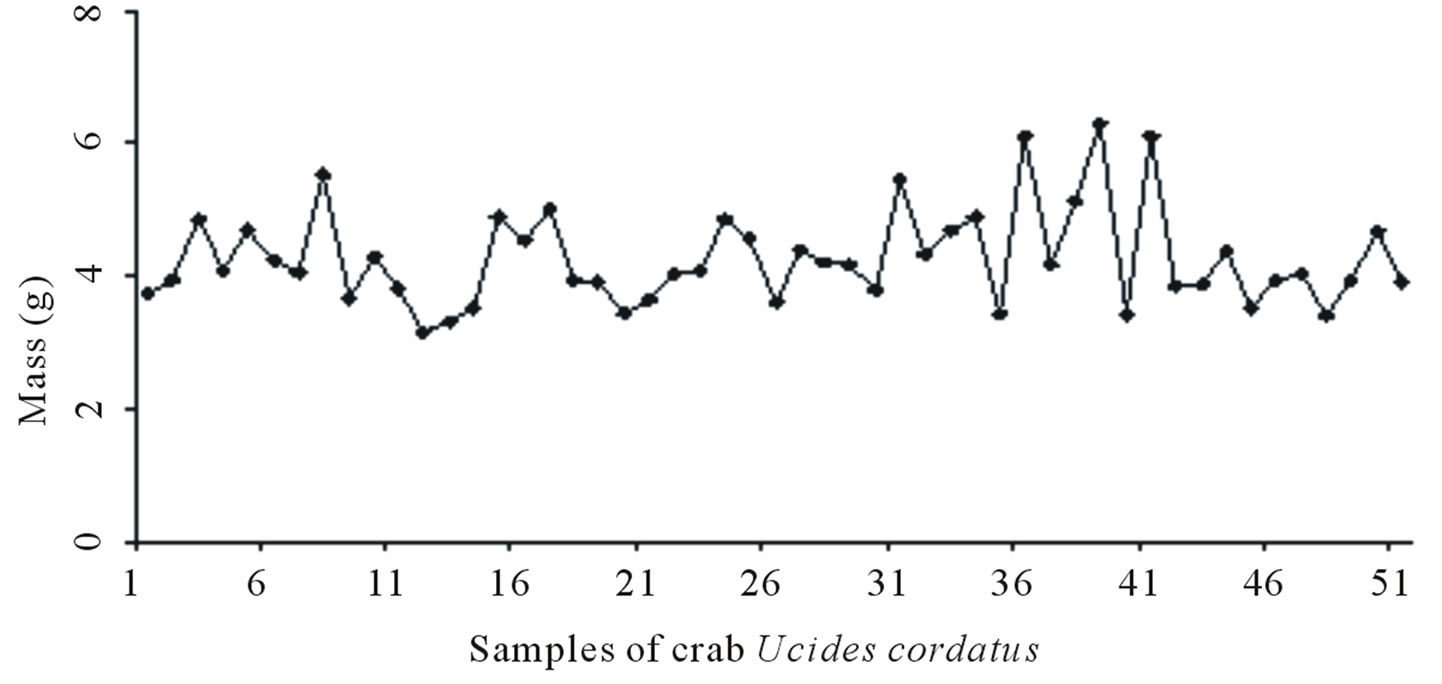 (d)
(d)
Figure 6. Sample population of shrimp before (a) and after (b) drying at 50˚C; sample population of crab before (c) and after (d) drying at 50˚C.
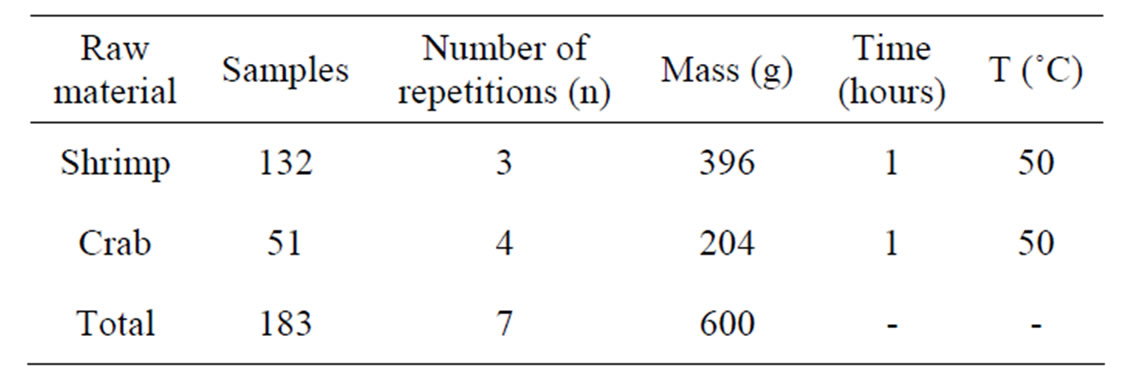
Table 2. Quantity of raw materials used for the extraction of chitin and chitosan.
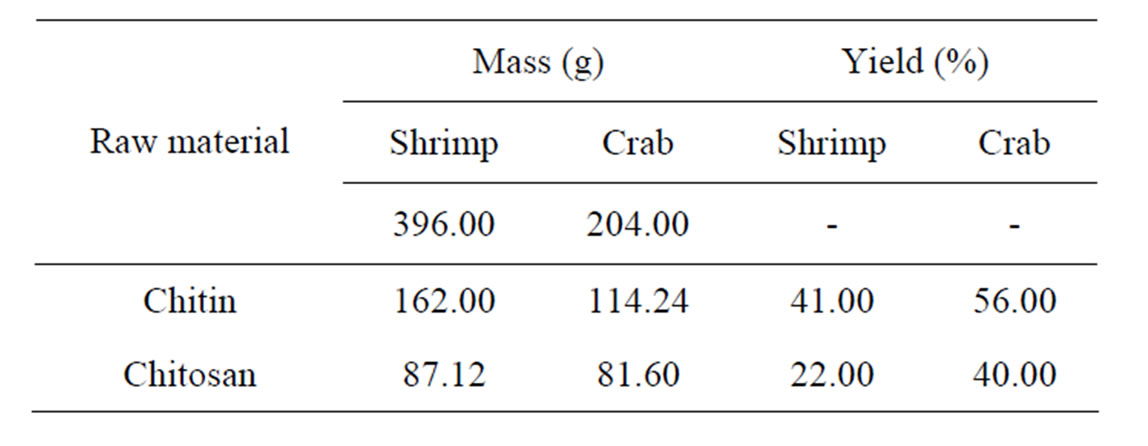
Table 3. Percent yield of chitin and chitosan.
 (a)
(a)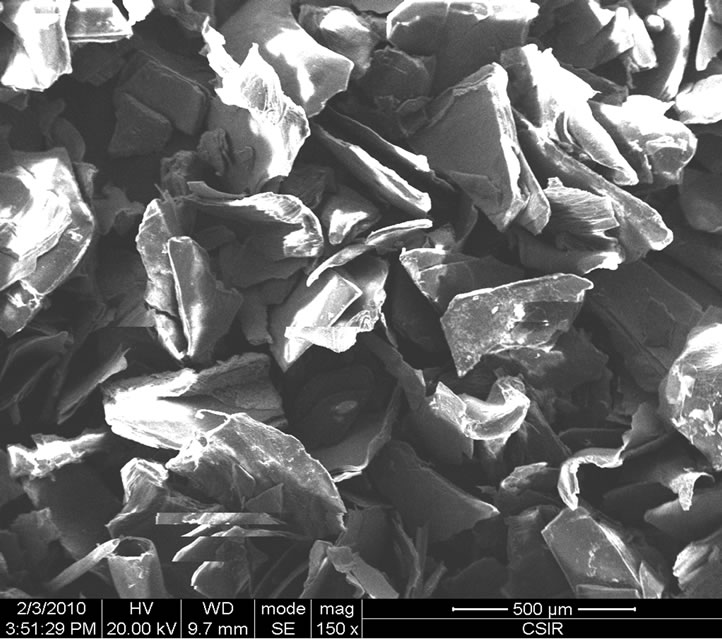 (b)
(b) (c)
(c)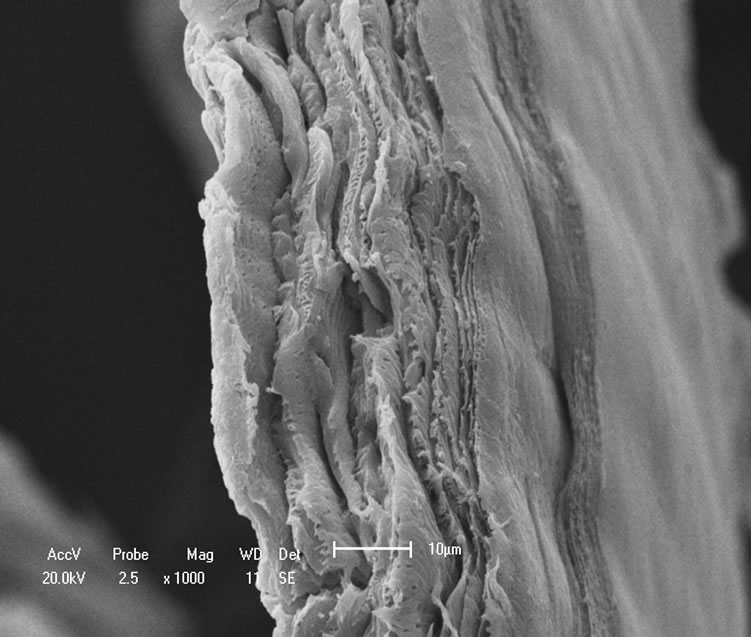 (d)
(d) (e)
(e)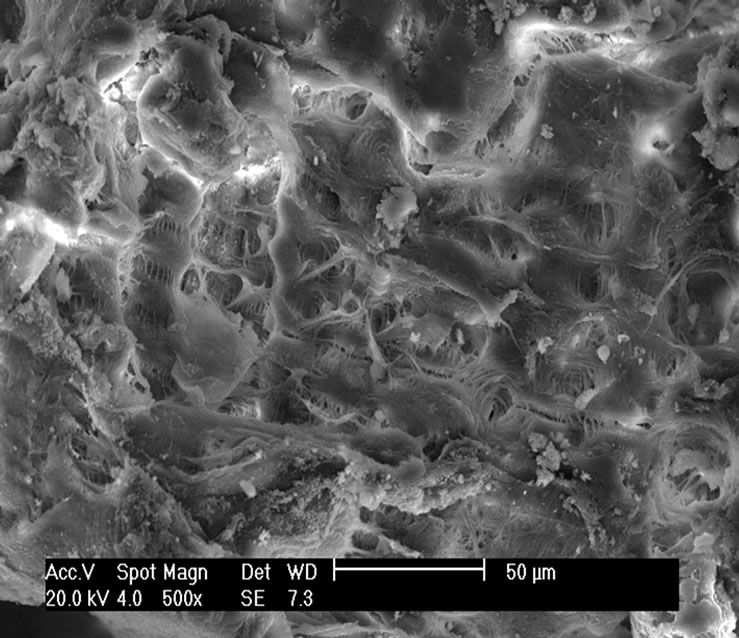 (f)
(f)
Figure 7. Micrographs: (a) Shrimp chitin 50×; (b) Shrimp chitosan 50×; (c) Crab chitosan 50×; (d) Shrimp chitin 1000×; (e) Shrimp chitosan 1000×; (f) Crab chitosan 500×.
presence of more crystalline area in chitin than that in chitosan [19].
The diffractograms of crab chitin showed better-resolved peaks and in greater numbers with a higher degree of crystallinity, Figure 8(c), than observed in the diffractograms of crab chitosan, Figure 8(d).
It should also be noted that the crystallinity of the samples depends on several factors, such as the nature of the organism from which the chitin was extracted and the conditions employed in the extraction of the polymer [20]. Besides, polymorphism of chitin leads to low crystallinity of chitosan, as well as the steps of chemical treatments carried out to extract chitosan from chitin [5].
3.4. Thermogravimetric Analysis (TG/DTG)
The thermogravimetric curves were obtained at a heating rate of 10˚C·min–1 under a dynamic atmosphere of air in the temperature range of 25˚C - 700˚C. The profiles of the thermal decomposition of chitin and chitosan are shown in Figures 9(a)-(d).
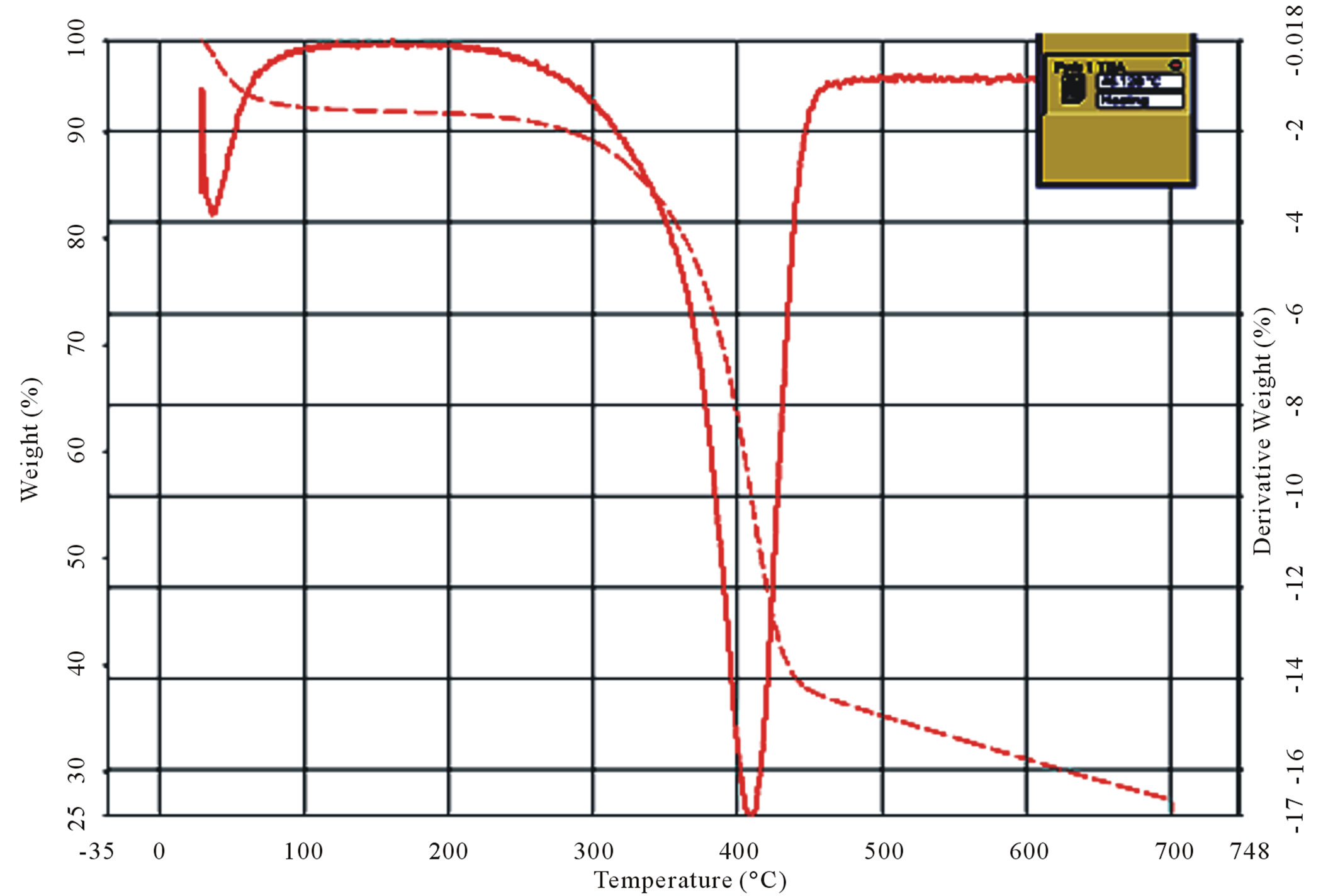
It is observed from the thermograms of both the shrimp and crab chitin Figures 9(a) and (b) that the decomposition takes place at two stages. The degradation starts in the range of 50˚C - 100˚C and is attributed to evaporation of water molecules followed by second stage of decomposition at 400˚C - 500˚C resulting the degradation of saccharide structure of the molecules that include the dehydration of saccharide rings and polymerization and also decomposition of the acetylated and deacetylated units of chitin.
The thermogram of shrimp chitosan, Figure 9(c) also shows the two stage decomposition, as found in shrimp
 (a)
(a)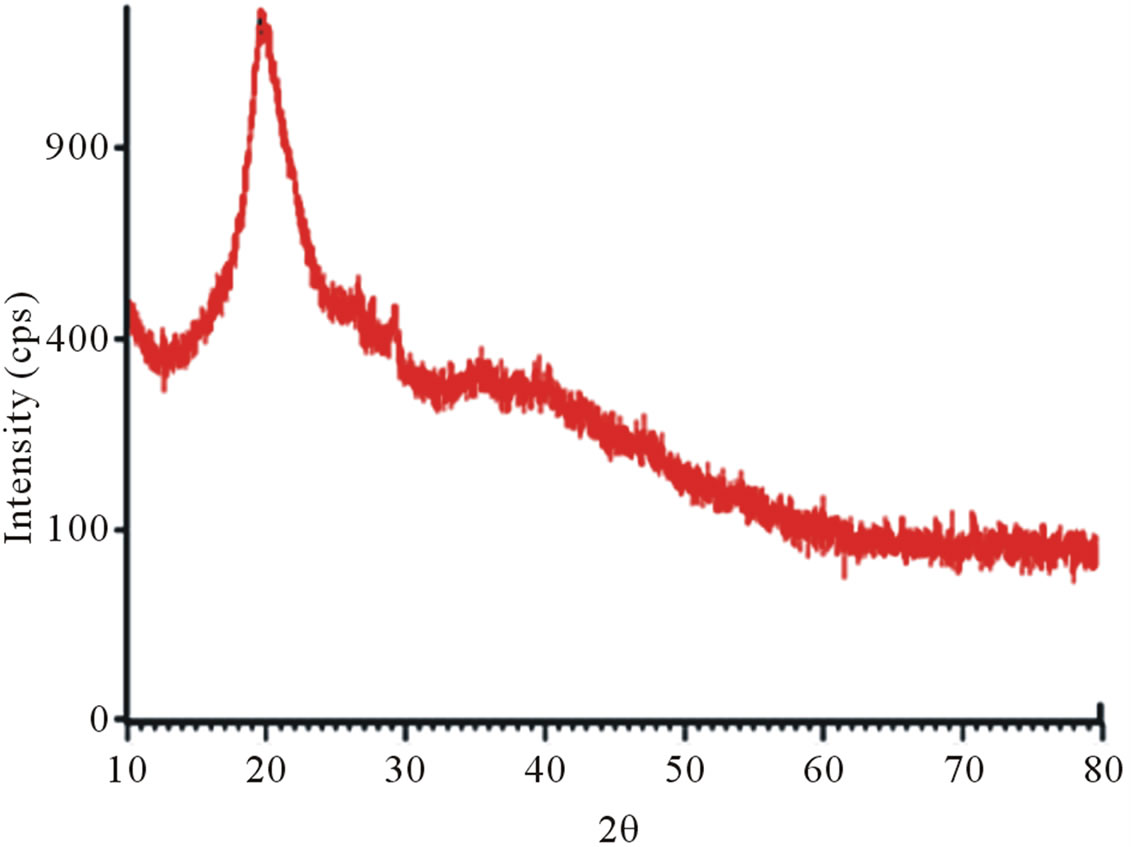 (b)
(b)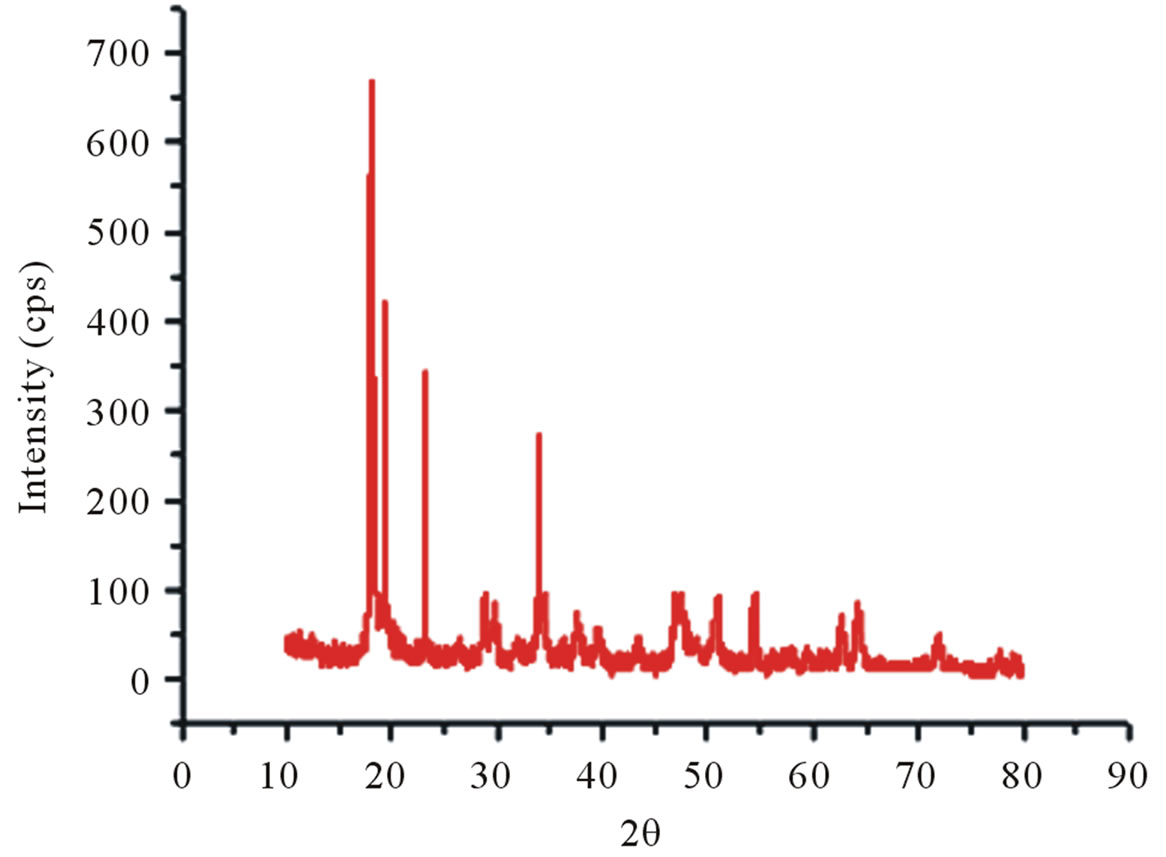 (c)
(c)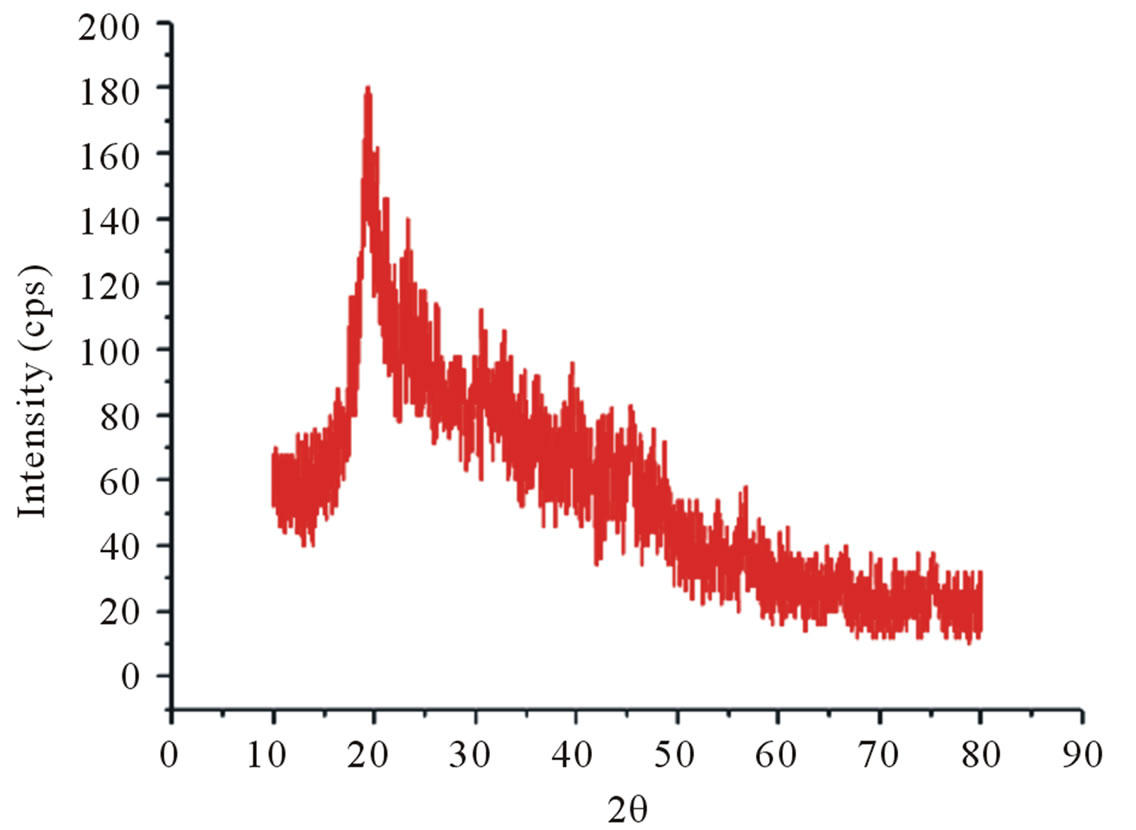 (d)
(d)
Figure 8. X-ray diffractograms, from 10˚ to 80˚ (2θ). (a) Shrimp chitin; (b) shrimp chitosan; (c) Crab chitin; (d) Crab chitosan.
and crab chitin, which occurs respectively in the ranges of 40˚C - 100˚C and 400˚C - 500˚C. However in the case of crab chitosan, Figure 9(d), there are four stages of decomposition occurred respectively in the ranges of 40˚C - 100˚C, 300˚C - 400˚C, 400˚C - 500˚C and 600˚C - 700˚C.
It was observed from the TG/DTG curves, Figure 9(d) that the decomposition stage of chitosan occurred, between temperatures of 300˚C - 400˚C, where for the chitin the decomposition occurred between 400˚C - 500˚C, which suggests that chitosan had a lower thermal stability.
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
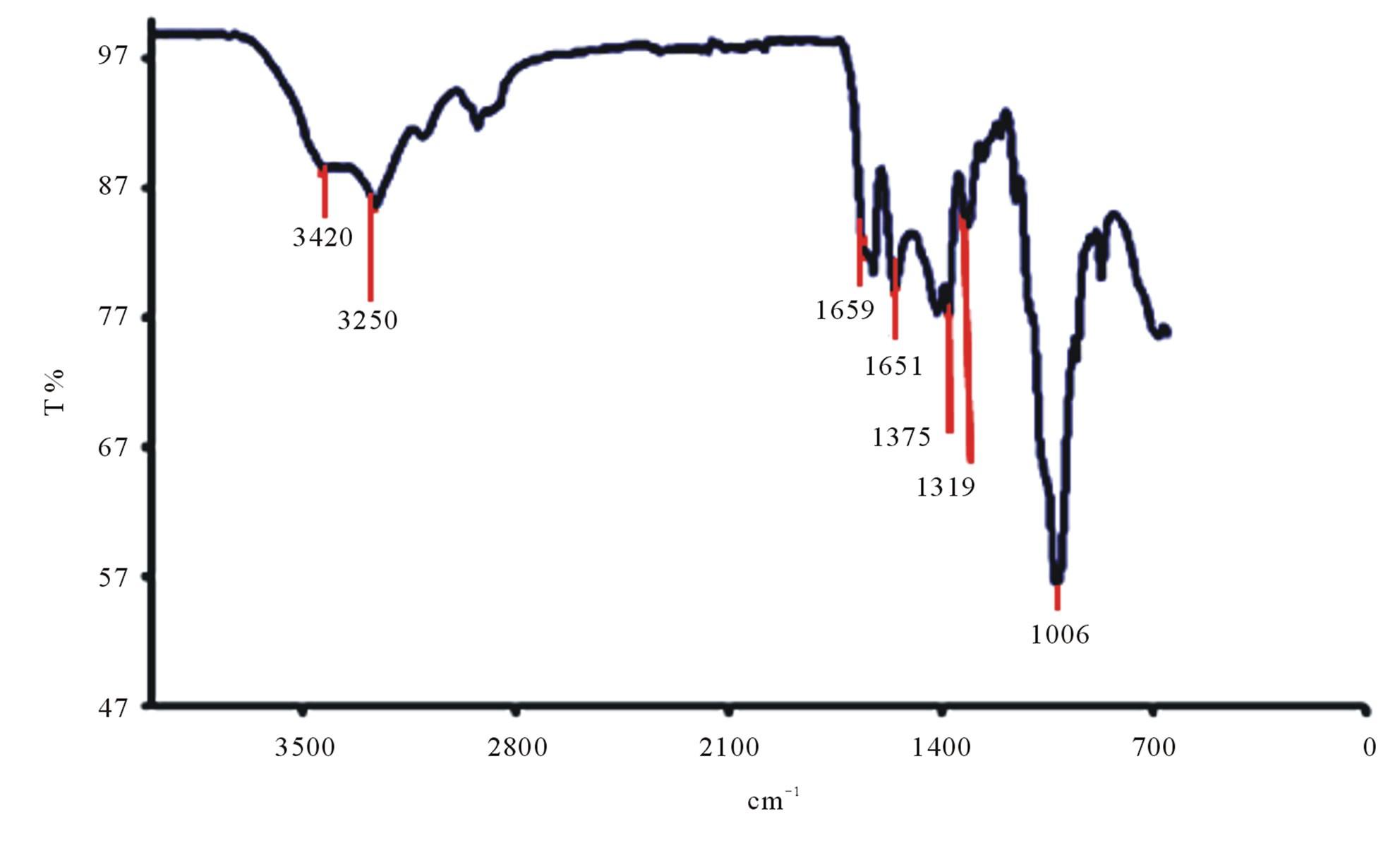
The characteristic bands of Litopenaeus vannamei shrimp chitin can be seen in Figure 10(a). It is observed that that the band at 1659 cm–1 has high intensity and is attributed to carbonyl band C=O, known as amide I band. The band at 1561 cm–1 correspond to -NH deformation and stretching C-H called of amide II band and 1319 cm–1 attributed to the -CO-NH deformation and the -CH2 group. The latter band has been named as a band of amide III. The acute band of 1375 cm–1 is attributed to the -CH3 symmetric deformation. The band at 3250 cm–1 is attributed to -NH stretching vibration and the band at 3420 cm–1 corresponds to -OH stretch.
The Figure 10(b) shows the characteristic bands of Litopenaeus vannamei shrimp chitosan. It can be observed that the band at 3425 cm–1 corresponds to angular deformation of OH present in structure of chitosan, over lapping band connecting NH, which is also present in the same region, at 1659 cm–1 is the band of -C=O, amide I. A decrease in that band at 1659 cm–1 showed that it happened during deacetylation of the chitin. The region at 1420 cm–1 corresponds to the axial deformation of the C-N of amide. The peak at 2920 cm–1 confirms the -CH
 (a)
(a)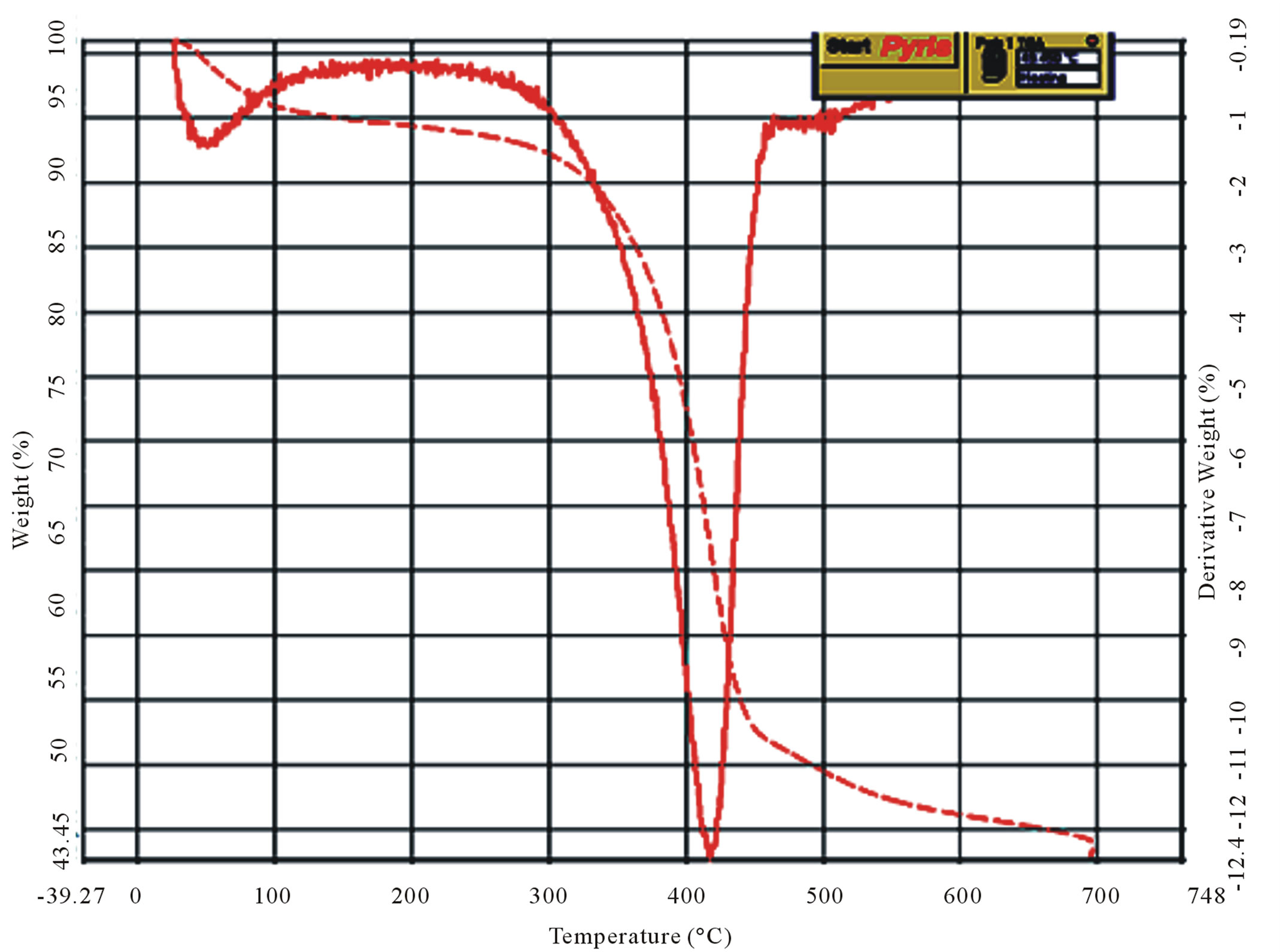 (b)
(b)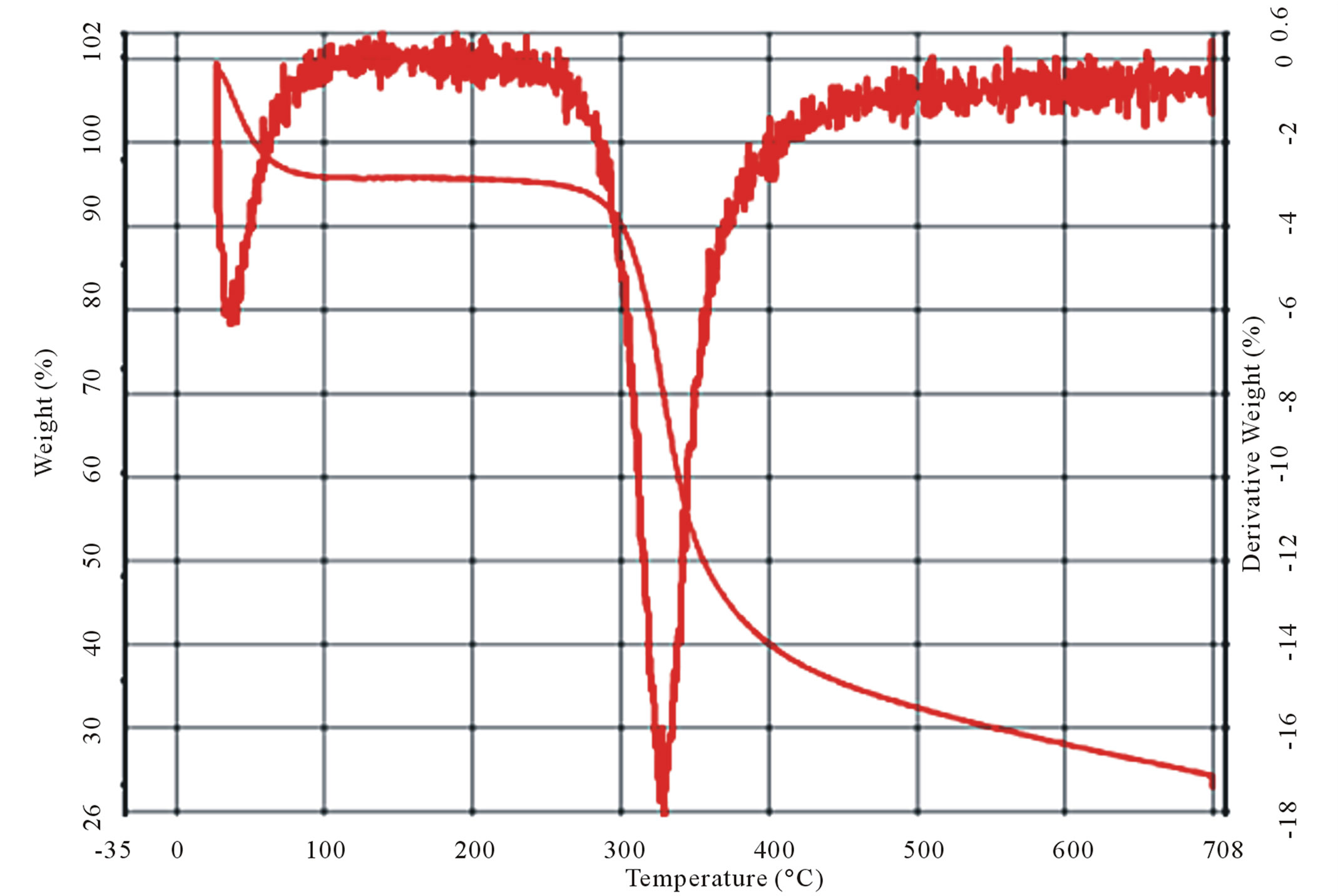 (c)
(c)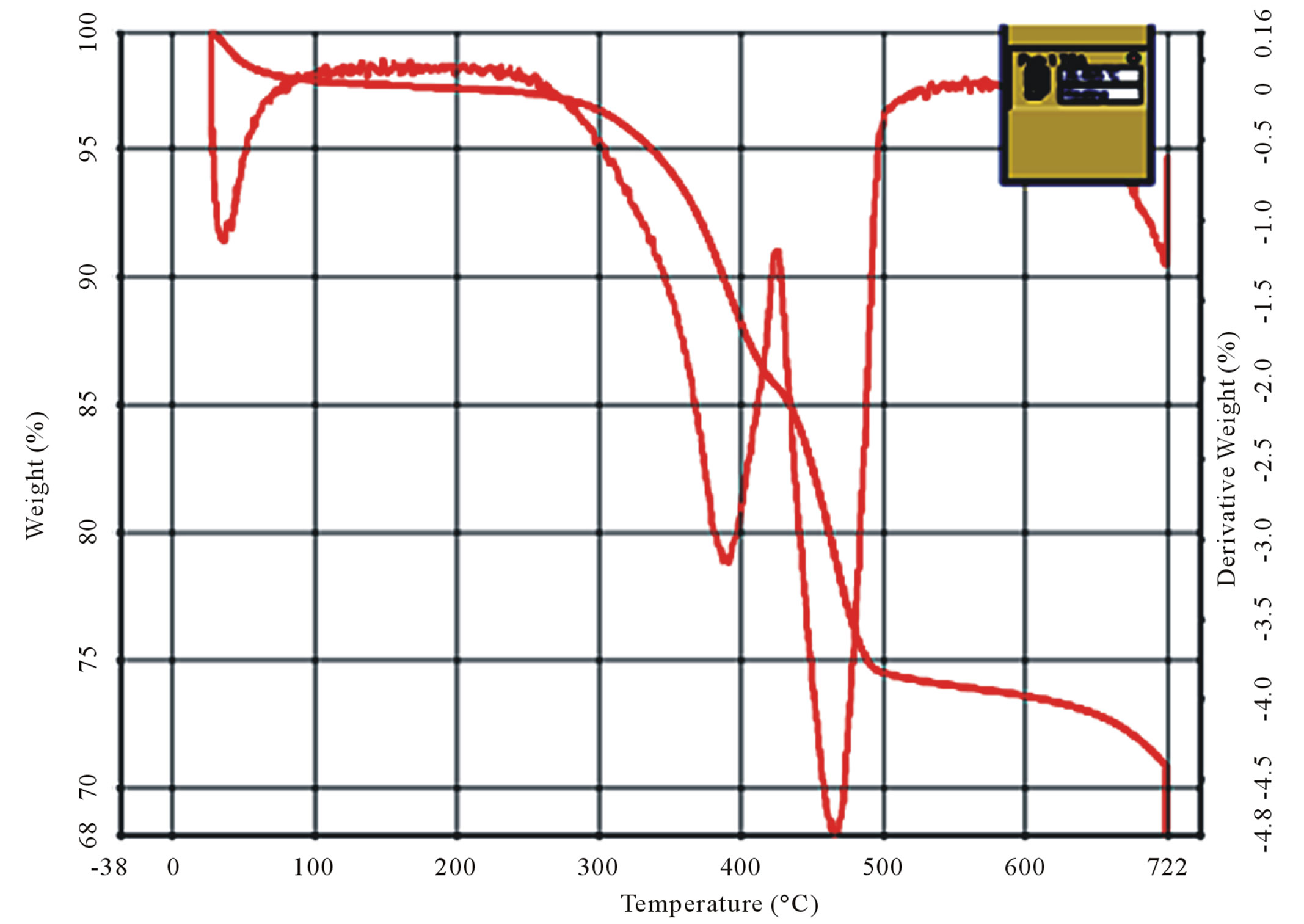 (d)
(d)
Figure 9. Curves of TG/DTG (a) and (b) shrimp and crab chitin; (c) and (d) shrimp and crab chitosan.
stretching and the region near 1150 cm–1 corresponds to -COC (polysaccharide). The peaks at 1319 cm–1, 1360 cm–1, 1379 cm–1 correspond to the strong vibrations of bending of the -NH primary, secondary and tertiary bonds respectively.
The spectrographs of Ucides cordatus crab chitin are shown in Figure 10(c). On analyzing the spectra of chitin, it is seen that there are two bands located about 3250 and 3420 cm–1 assigned respectively to the amide -NH group and OH of groups’ acetylated. The presence of the absorption band 870 cm–1 indicates the polysaccharides structure. It is observed that the band at 1620 cm–1 has greater intensity which corresponds to -C=O carbonyl bond.
The spectrographs of Ucides cordatus crab chitosan are shown in Figure 10(d). It is evident from the spectra
 (a)
(a)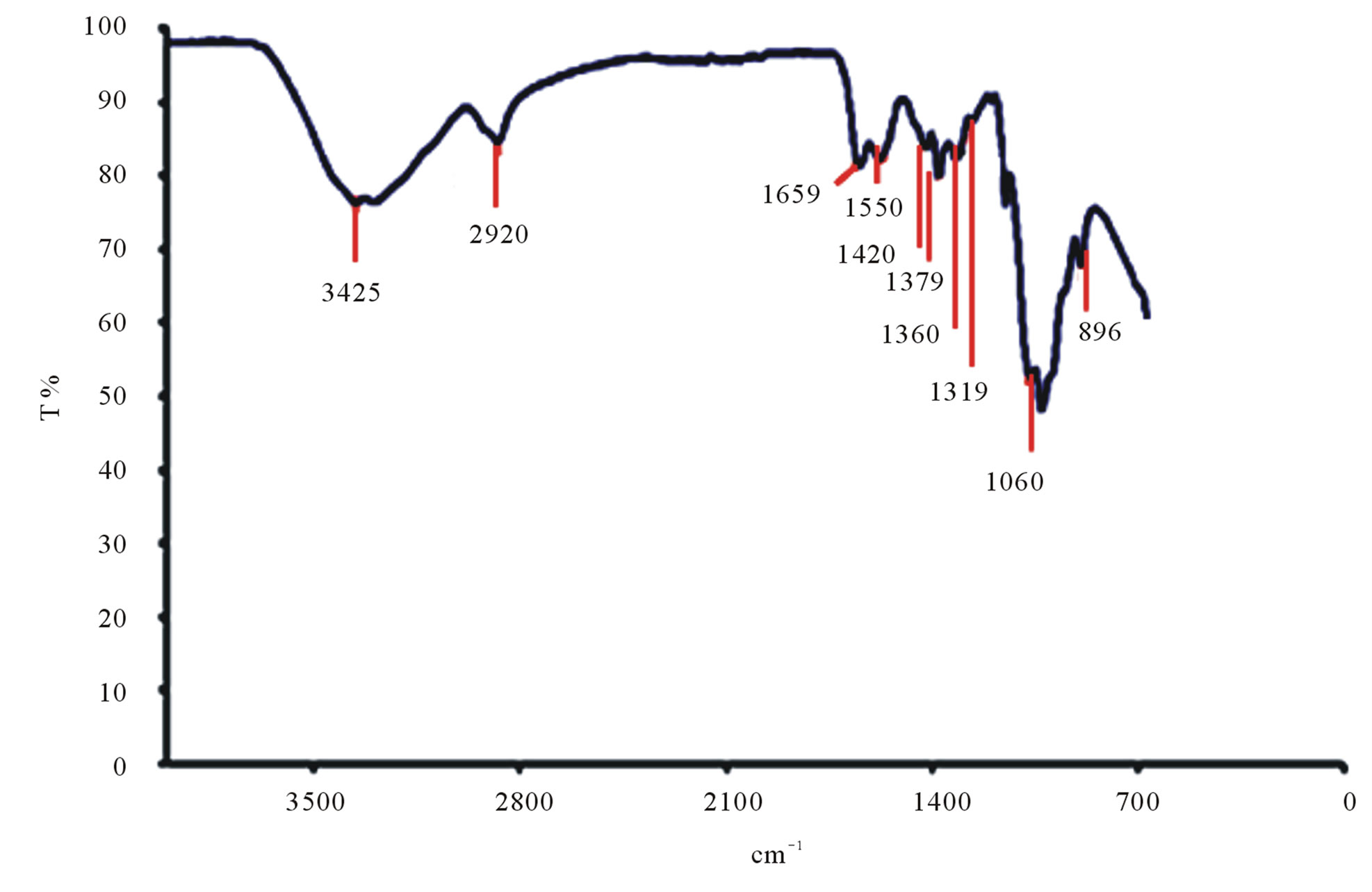 (b)
(b)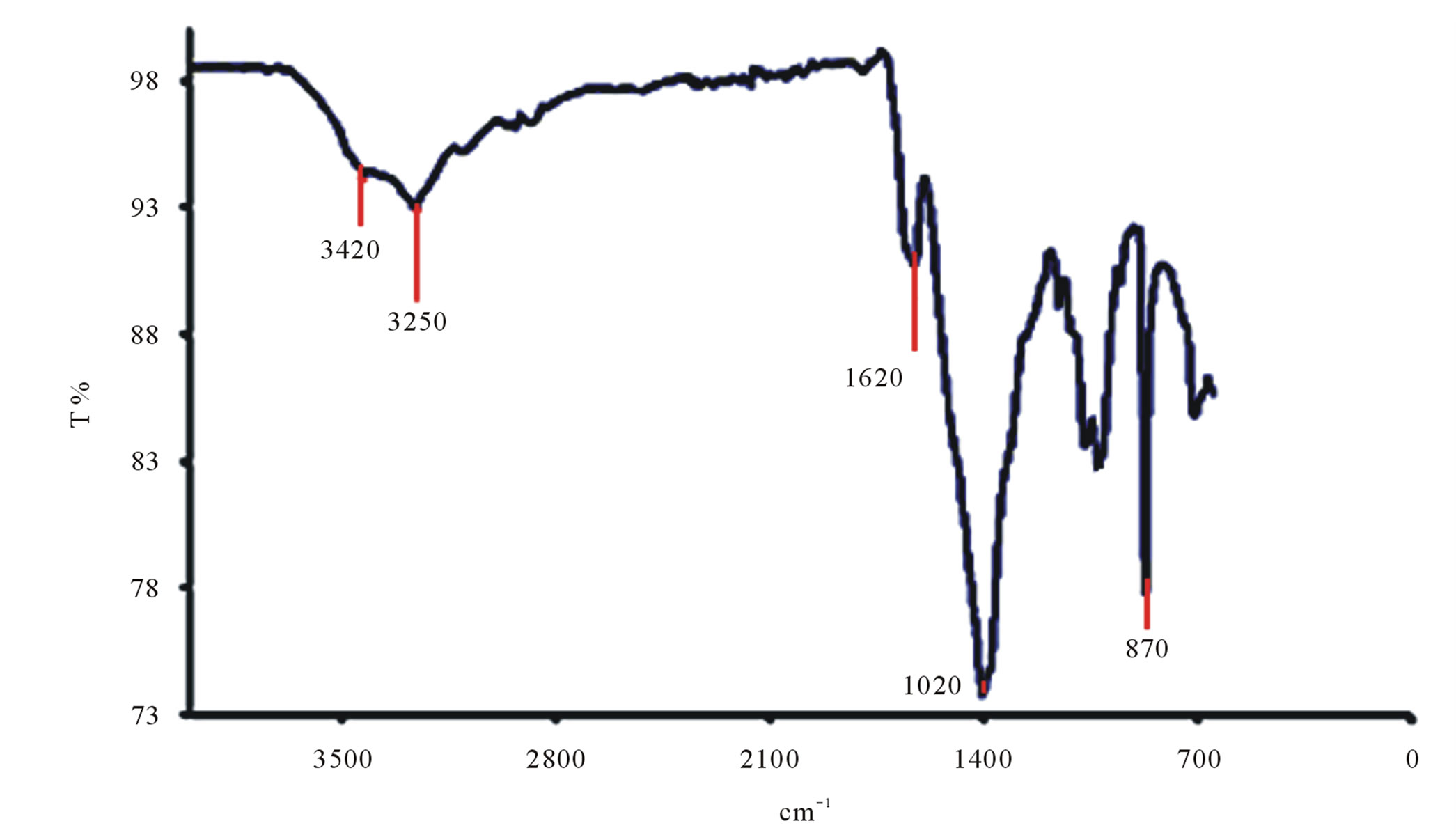 (c)
(c)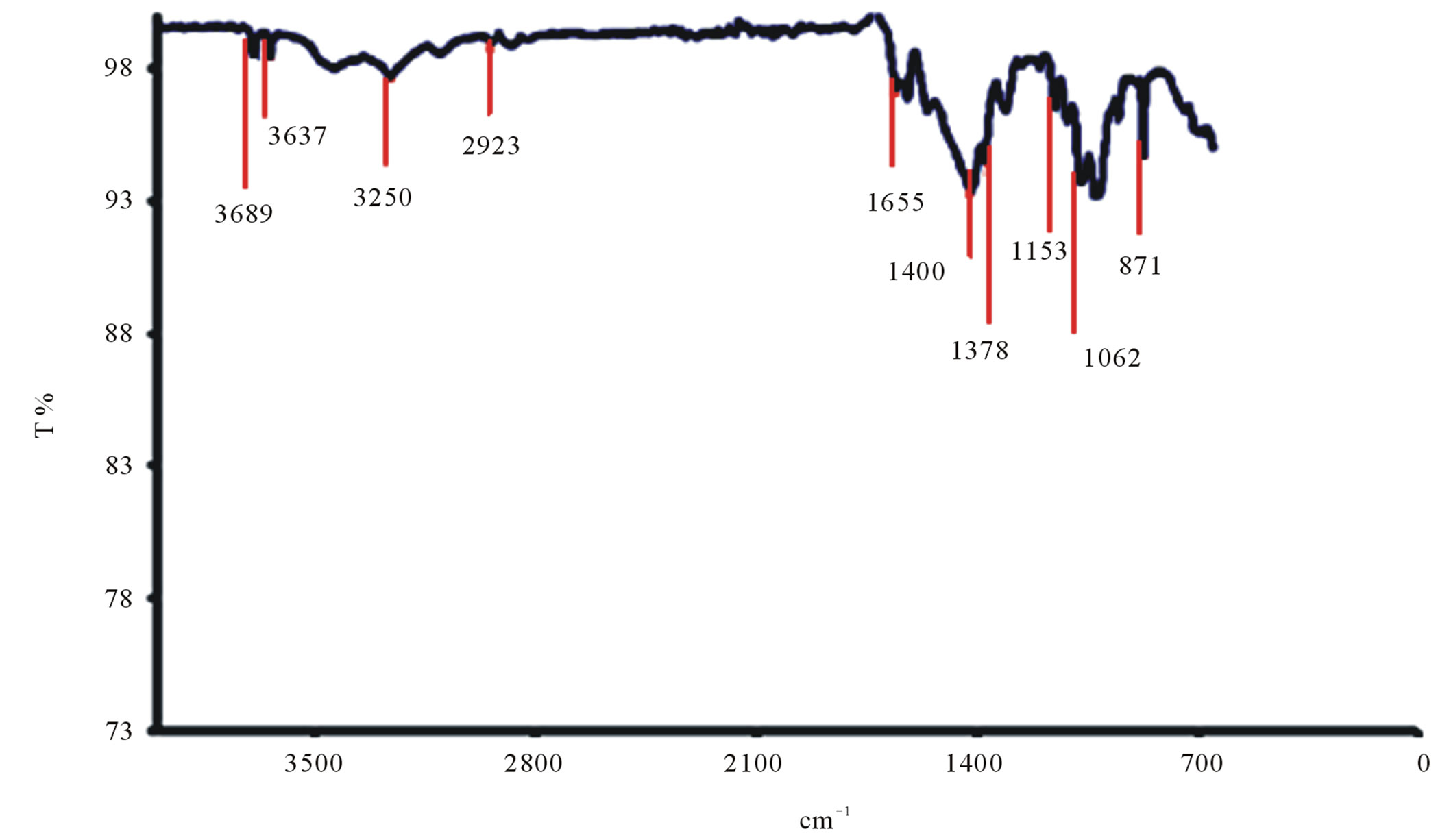 (d)
(d)
Figure 10. (a) FTIR spectrogram of Litopenaeus vannamei shrimp chitin; (b) FTIR spectrogram of Litopenaeus vannamei shrimp chitosan; (c) FTIR spectrogram of Ucides cordatus crab chitin; (d) FTIR spectrogram of Ucides cordatus crab chitosan.
that there are significant changes in the band at 1400 cm–1 where a reduction in the peak is observed. The intensity of the band at 1655 cm–1 associated with carbonyl -(C=O) decreases as the degree of deacetylization of the chitosan increases. The region from 3000 cm–1 to 3700 cm–1 indicates the bands corresponding to the stretching of -OH and -NH groups.
The peaks at 870 cm–1 to 1153 cm–1 refer to the bands of polysaccharide structures and the peak at 1378 cm–1 refers to the angular deformation and symmetry of -CH3 group. The region between the bands 1323 and 1378 cm–1 refer to axial deformation of -CN of amino groups. The band 2923 cm–1 is attributed to the -C-H stretching links.
All bands observed are similar to those described in second the literature [21].
3.6. Scanning Electron Microscope in Nanomembranes
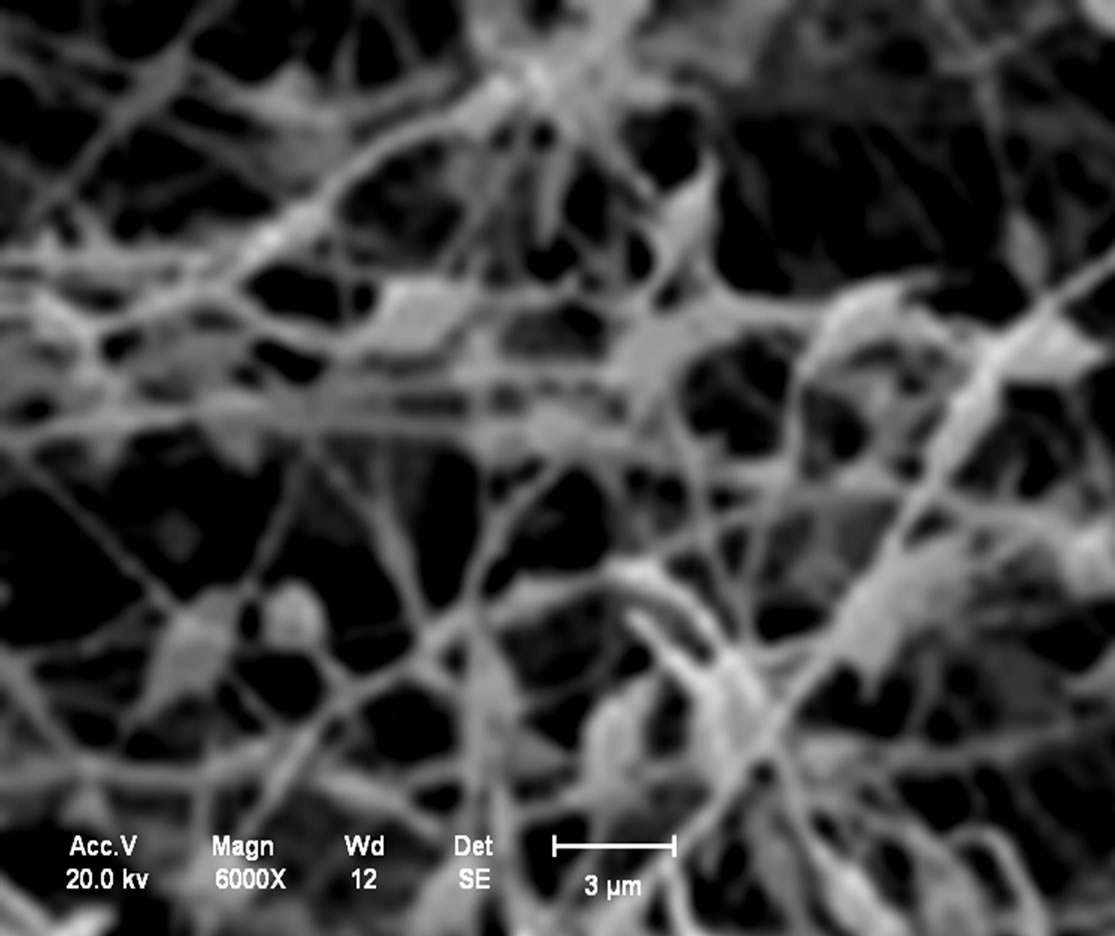
Analysis in the chitosan nanomembranes was performed in the scanning electron microscope (SEM), which are showed the in Figures 11(a)-(d). The nanomembranes in Figures 11(a) and (b) were produced at a 20 kV, on 7 wt.% chitosan solution was dissolved in 100:0 v/v (TFA/ DCM). The formation of beads in the nanomembranes occurred when the distance between the needle and the collector was 5 cm.
In the Figures 11(c) and (d), the chitosan nanomembranes were produced at a voltage of 25 kV, using a 5 wt.% chitosan in 70:30 v/v (TFA/DCM). No bead formation was observed with the distance of the needle from the collector increased to 10 cm.
3.7. Mechanical Analysis of the Nanomembrane
The chitosan nanomembranes were subjected to tension/extension tests. For Litopenaeus vannamei shrimp, with 5% in 70:30 (TFA/DCM) the result obtained was 12.44 MPa and the breaking extension was 82%. While
 (a)
(a)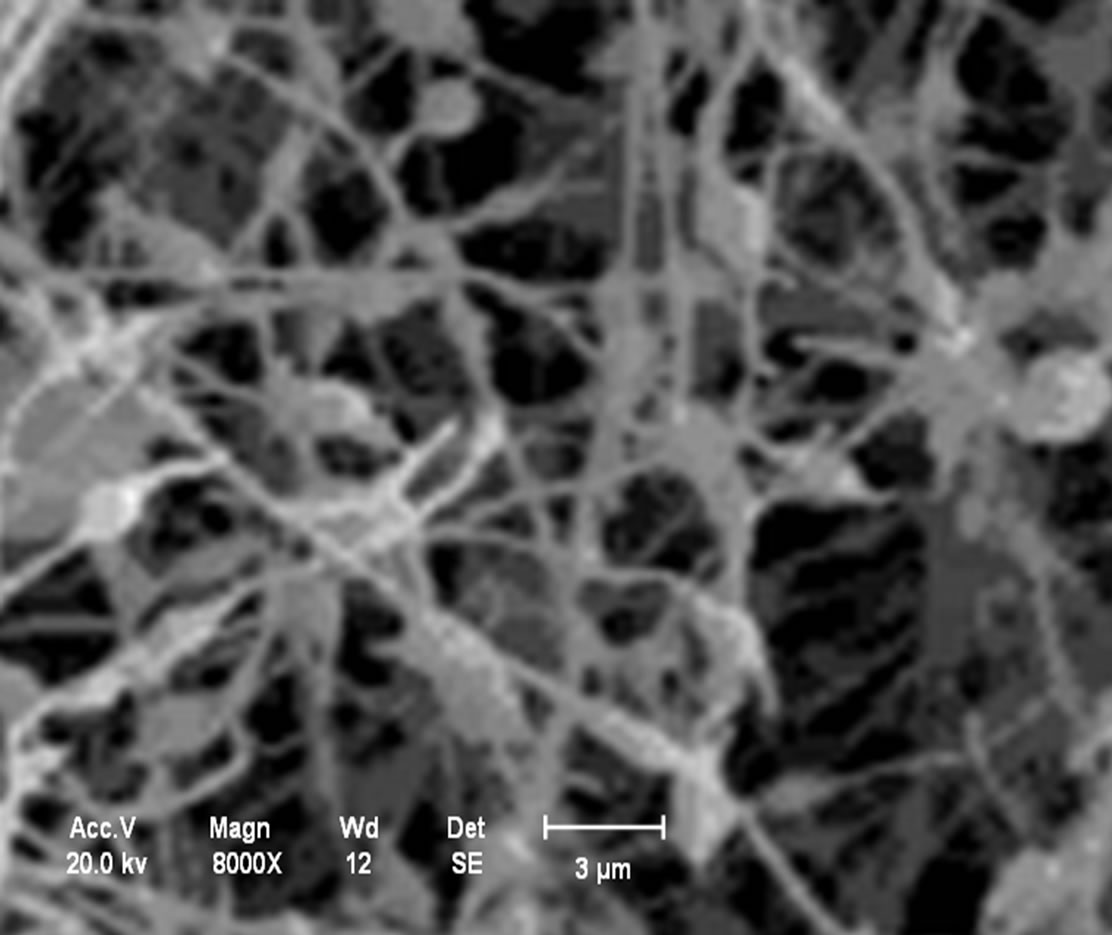 (b)
(b)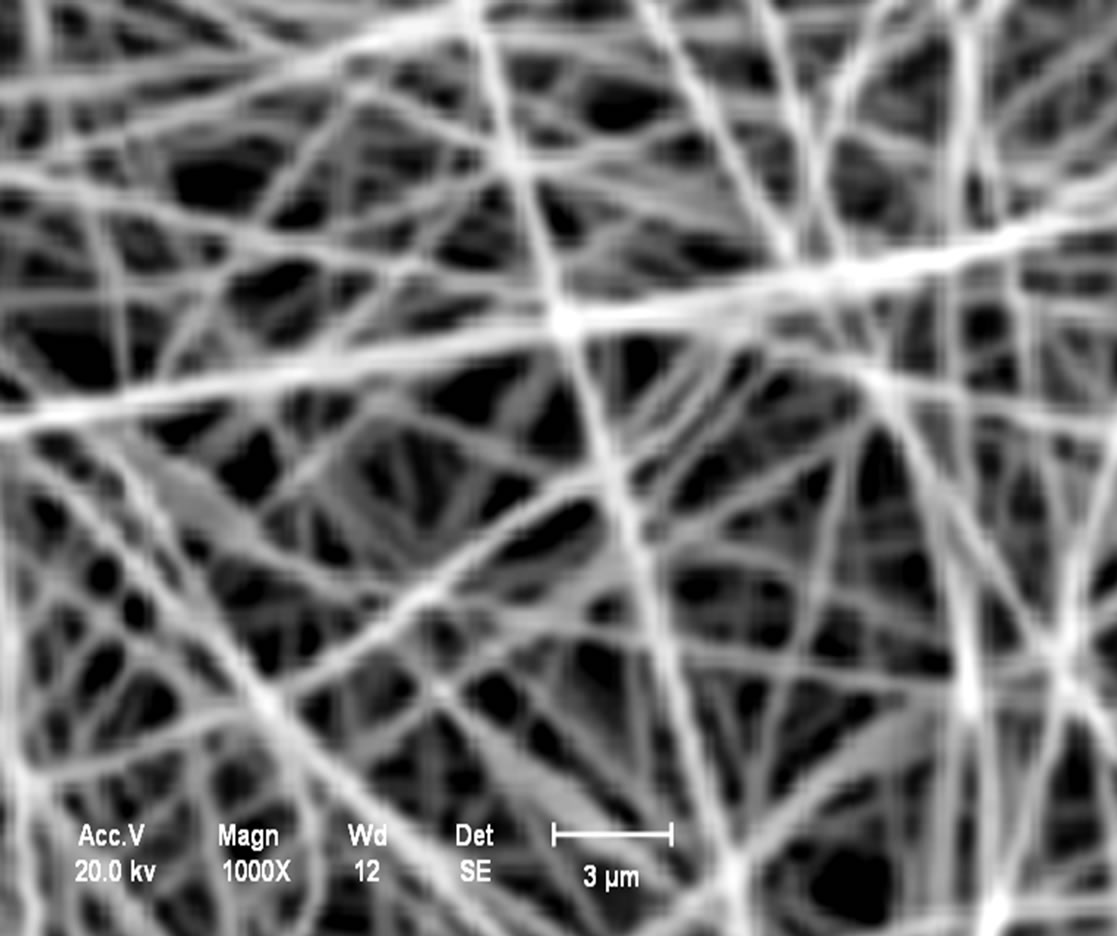 (c)
(c)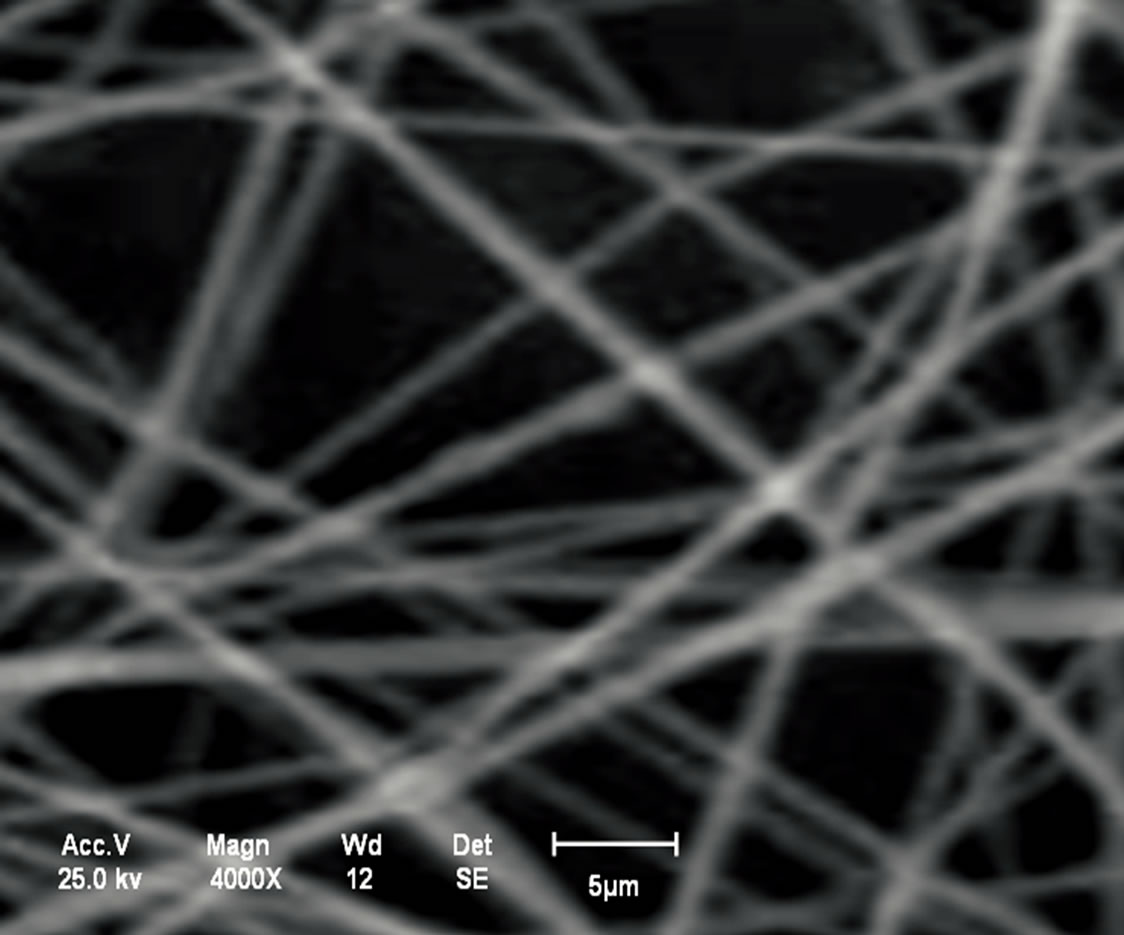 (d)
(d)
Figure 11. (a) Chitosan nanomembranes—Litopenaeus vannamei with 7% in 100:0 (TFA/DCM)—6000×; (b) Chitosan nanomembrane—Ucides cordatus with 7% in 100:0 (TFA/DCM)—8000×; (c) Chitosan nanomembranes—Litopenaeus vannamei with 5% in 70:30 v/v (TFA/DCM)—1000×; (d) Chitosan nanomembrane—Ucides cordatus with 5% in 70:30 v/v (TFA/DCM)—4000×.
for 7% in 100:0 (TFA/DCM), the breaking tension was 10.32 Mpa and the breaking extension was 66%, Table 4.
The chitosan nanomembranes for crab Ucides cordatus with 5% in 70:30 (TFA/DCM) the result obtained was 13.75 MPa and the breaking extension was 68%. While for the 7% in 100:0 (TFA/DCM), the breaking tension was 11.78 MPa and the breaking extension was 36%, Table 5.
It could be seen from the results, that by using 5% chitosan in 70:30 v/v (TFA/DCM) combination in the manufacture of nanomembranes from shrimp as well as crab chitosan showed better breaking tension with higher breaking extension in comparison with the 7% 100:0 (TFA/DCM) composition. As TFA is a nonaquous solvent and being non-volatile than DCM, when used in combination of the two solvents, showed better results.
4. Conclusion
This paper describes the extraction of chitin and chitosan obtained from waste Litopenaeus vannamei shrimp and Ucides cordatus crab and the manufacture of nanomembranes. Analysis by scanning electron microscopy was important to observe the structure of chitin and chitosan, thus verifying the formation of the particles. It was observed from the X-ray diffraction analysis that the chitosan has a semi-crystalline structure compared to chitin which is more crystalline, due to the polymorphism of chitin, which leads to low crystallinity of chitosan as well as the extraction steps performed in demineralization, deproteinization and deodorization. The time and temperature control were also important to prevent polymer degradation. The thermogravimetric analysis and X-ray diffraction revealed that the deacetylation reduces the
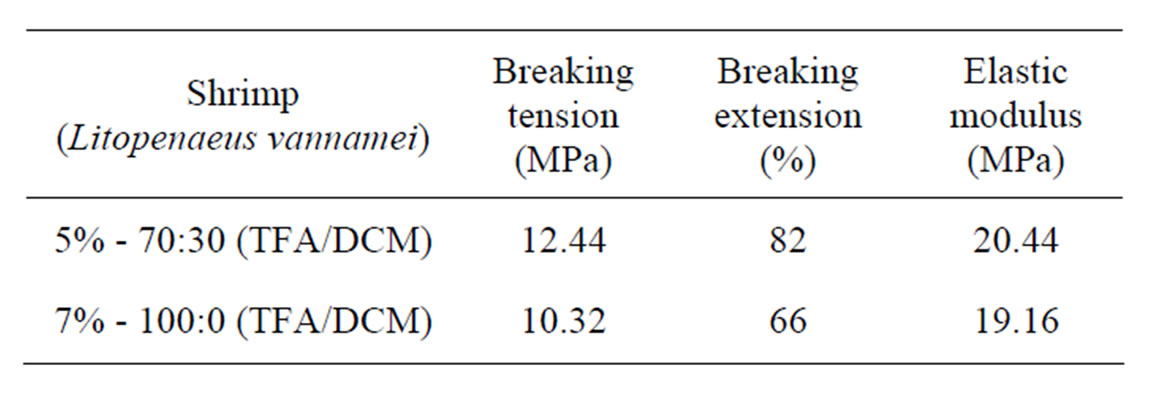
Table 4. Mechanical analysis of shrimp chitosan nanomembranes.
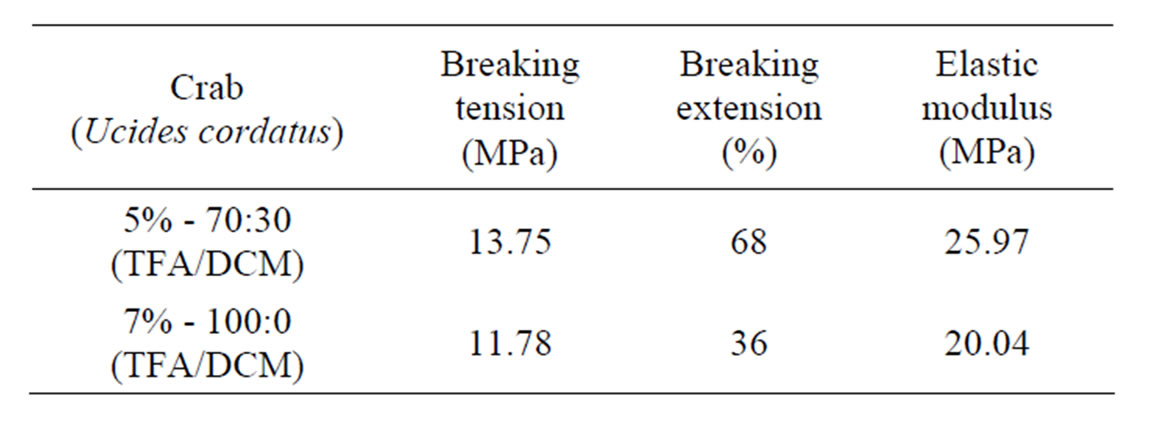
Table 5. Mechanical analysis of crab chitosan nanomembranes.
thermal stability and crystallinity of the samples. Infrared spectrographs showed differentiation between chitin and chitosan. The peaks in the infrared region showed characteristics through the bands that present to be chitin and chitosan according to BRUGNEROTTO et al., 2001. In the present study, electrospinning was used for the production of chitosan nanomembranes. It was observed that the quality of chitosan and nanomembranes produced depend mainly on the extraction conditions, of the origin of raw material, of chemicals used and the immersion time in the sequence of treatments. The chitosan nanomembranes produced with the distance of 10 cm from the needle to the collector no showed beads formation. The incomplete evaporation of the solvent and distance of needle to the collector of 5 cm might have caused the formation of the agglomerates. Because, TFA is a solvent less volatile than DCM, and the presence of DCM in the solution of 70:30 v/v (TFA/DCM) promoted the increase in the volatility of the system, contributing to decrease the number of agglomerates and facilitate in the formation of the electrospun nanomembranes. This could be the reason that the nanomembranes manufactured with the combination of the two solvents showed better mechanical results as seen in Tables 4 and 5.
5. Acknowledgements
The authors wish to thank God, CAPES for the research grant, as well as to PPGEM, The CTPETRO-INFRA, FINEP/LIEM e CSIR.
REFERENCES
- A. Gildberg and E. Stenberg, “A New Process for Advanced Utilization of Shrimp Waste,” Process Biochemistry, Vol. 36, No. 8-9, 2001, pp. 809-812. doi:10.1016/S0032-9592(00)00278-8
- E. Tonhi and A. M. G. Plepis, “Obtenção e caracterização de blendas colágeno-quitosana,” Química Nova, vol. 25, No. 6, 2002, pp. 943-948.
- k. Okuyama, k. Noguchi, m. Kanenari, t. Egawa, k. Osawa and k. Ogawa, “Structural diversity of chitosan and Its Complexes,” Osaka prefecture university, Sakai, 1999.
- I. Y. Kimura, J. R. Gonçalves, J. Stolberg, M. C. M. Laranjeira and V. T. Favere, “Efeito do pH e do tempo de contato na adsorção de corantes reativos por microesferas de quitosana,” Polímeros, Vol. 9, No. 3, 1999, p. 51.
- Y. Noishiki, Y. Nishiyama, M. Wada, S. Okada and S. Kuga, “Inclusion complex of Beta-Chitin and Aliphatic Amines,” Biomacromolecules, Vol. 4, No. 4, 2003, pp. 944-949. doi:10.1021/bm034024k
- F. S. P. Campana, D. Britto, E. Curti, F. R. Abreu, M. B. Cardoso, M. V. Battisti, P. C. Sim, R. C. Goy, R. Signini, R. L. Lavall, “Extração, estruturas e propriedades de ae b-quitina,” Química Nova, vol. 30, no. 3, 2007.
- F. C. Wu, R. L. Tseng and R. S. Juang, “Enhanced abilities of Highly Swollen Chitosan Beads for Colour Removal and Tyrosinase Immobilization,” journal of hazardous materials, vol. 81, no. 1-2, 2001, pp. 167-177. doi:10.1016/S0304-3894(00)00340-X
- M. Rinaudo, “Chitin and Chitosan Properties and applications,” Progress in Polymer Science, vol. 31, No. 7, 2006, pp. 603-632. doi:10.1016/j.progpolymsci.2006.06.001
- P. Abram, “Chitin e Chitosan: Obtención caracterización y aplicaciones,” Pontificia Universidad Católica-Perú, 2004.
- R. M. Madri, “Camarões-Como está o agro negócio camarão marinho,” Revista Brasileira de Agropecuária, vol. 11, 2001, p. 66.
- ABCC-Associação Brasileira de Criadores de Camarão. http://www.abccam.com.br/abcc
- G. A. S. Melo, “Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro,” Editora Plêiade, São Paulo, 1996.
- G. G. Castilho, “Aspectos reprodutivos do caranguejouçá,Ucides cordatus (Crustacea,Brachyura, Ocypodidae), Baía de Antonina, Paraná, Brasil. Curitiba.” Dissertação (Mestrado em Ciências Veterinária)—Setor de Ciências Agrárias, Universidade Federal do Paraná, 2006.
- M. A. A. Pinheiro and A. G. Fiscarelli, “Manual de apoio à fiscalização do caranguejo-uçá (Ucides cordatus),” Santa Catarina, Centro de Pesquisa e Gestão de Recursos Pesqueiros do Litoral Sudeste e Su—CEPSUL, Itajaí, 2001.
- A. A. Craveiro, A. C. Craveiro and D. C. Queiroz, “Quitosana: A fibra do futuro,” Editora Universitária, Pernambuco, 1998.
- I. P. Rocha, J. Rodrigues and L. A. Amorim, “carcinicultura brasileira em 2003,” Revista da Associação Brasileira de Criadores de Camarão-30, 2004.
- N. M. Soares, C. M. Moura, J. Rizzi, S. R. Vasconcelos, V. O. B. Santos and L. A. A. Pinto, “Study of the chitosan production starting from Shrimp Residues in Pilot Scale,” XVI CRICTE_Regional Congress of Scientific and Technological Initiation in Engineering, Ijuí, 2003.
- J. M. Moura, F. C. Ferreira, F. M. M. Silva, J. Rizzi and L. A. A. Pinto, “Obtenção de quitina a partir de carapaças de siri—Uso de um Planejamento Experimental na Etapa de Desmineralização,” vol. 15, 2005, pp. 7-17.
- G. A. F. Roberts, “Chitin Chemistry,” The Macmillan Press Ltd., Hong Kong, 1992.
- N. A. R. Gow, G. W. Goodway, J. D. Russell and M. J. Wilson, “Infrared and X-Ray Diffraction Data on chitins of Variable Structure,” Carbohydrate Research, Vol. 165, 1987, pp. 105-110.
- J. Brugnerotto, J. Lizardi, F. M. Goycoolea, W. Arguellas-Monal, J. Desbrières and M. Rinaudo, “An Infrared Investigation in relation with chitin and Chitosan Characterization”, Polymer, Vol. 42, No. 8, 2001, pp. 35-69. doi:10.1016/S0032-3861(00)00713-8

