Journal of Modern Physics
Vol.05 No.14(2014), Article ID:49252,19 pages
10.4236/jmp.2014.514131
Motion Characteristics of Single Electrons of Atoms of Atomic Gas of Hydrogen and Single Electrons of Hydrogen-Like Ions in Form Gas or Vapour during Decays of Such Atoms and Ions. Emission Line Spectra
Ivan Antonovych Strilets
Kyiv, Ukraine
Email: anteo@global-hit.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 March 2014; revised 12 April 2014; accepted 8 May 2014
ABSTRACT
For the first time the vector differential equation of central motion of single electron in electric field of an atomic nucleus as in external central electric field is set up and solved. Here the following findings are reported. Each of single electrons of a part of atoms of atomic gas of hydrogen and a part of hydrogen-like ions in the form of a gas or a vapour revolves around corresponding atomic nucleus in a flat spiral which has an interior maximum of turns density. The distance between each of these single electrons and corresponding atomic nucleus increases while a speed of single electron decreases. Such motion of single electrons takes place with no expenditures of external energy and points to decays of foregoing parts of atoms and ions. The electric field strength of the atomic nuclei of atoms of atomic gas of hydrogen and hydrogen-like ions in the form of a gas or a vapour is inversely proportional to the distance between the atomic nucleus and the corresponding single electron by greater than the power of 3. Calculated cyclic frequency (rough value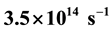 ) of revolution of the electron around the nucleus of atom of atomic gas of hydrogen (in interior maximum of turns density of the flat spiral), which moves at speed
) of revolution of the electron around the nucleus of atom of atomic gas of hydrogen (in interior maximum of turns density of the flat spiral), which moves at speed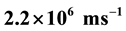 , and central cyclic frequency of
, and central cyclic frequency of
 -line of Balmer series
-line of Balmer series
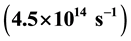 have the same order of magnitude. This fact and line structure of experimental emission line spectra confirm the formation of all lines of these spectra by continuous slight emission of light front by single electrons. The formation of series of lines of emission line spectra is linked to repeated creations of atoms of atomic gas of hydrogen and hydrogen-like ions in the form of a gas or a vapour.
have the same order of magnitude. This fact and line structure of experimental emission line spectra confirm the formation of all lines of these spectra by continuous slight emission of light front by single electrons. The formation of series of lines of emission line spectra is linked to repeated creations of atoms of atomic gas of hydrogen and hydrogen-like ions in the form of a gas or a vapour.
Keywords:
Atomic Gas of Hydrogen, Hydrogen-Like Ions, Slight Emission of Light Front by Single Electron, Emission Line Spectra

1. Introduction
Atomic gas of hydrogen (atomic hydrogen) and hydrogen-like ions in the form of a gas or a vapour have been used for experimental study of emission line spectra (see e.g. [1] ). Theory of these spectra was proposed by N. Bohr (1913) and developed in quantum mechanics and quantum electrodynamics.
However phenomena of atomic physics and optics have been described not only by quantum theory. Generally known example [1] is Cerenkov radiation (1934), which was interpreted on the basis of classical electrodynamics. This effect applies to slight emission of light front in the form of a conical surface by charged particles (for example, by electrons), passing through a transparent medium at a speed greater than the speed of light in that medium. According to [1] the effect is similar to that of a sonic boom when an object moves faster than the speed of sound.
In our time at the end of XX century the effect of substantial reduction of light speed in atomic gas of Na with the high atomic density was discovered [2] .
The discoveries of Cerenkov radiation and afore-named effect of substantial reduction of speed of light give ground for a new research of emission line spectra with such supposition. I have supposed that a speed of single electrons of atoms of atomic gas of hydrogen and a speed of single electrons of hydrogen-like ions in the form of a gas or a vapour are greater than the speed of light in electric field of corresponding atomic nuclei. Consequently, these single electrons, moving around atomic nuclei, simultaneously emit slight light front in the form of a conical surface (like Cerenkov radiation), i.e., form a line of emission line spectrum.
In order to establish fact of formation of a line and identify the way of formation of series of lines with slight emission of light front by single electrons a necessity appeared to find out the motion characteristics of single electron (especially the characteristics of its velocity) in electric field of an atomic nucleus. As far as I know these characteristics with a strict physical and mathematical argumentations have never been ascertained.
In the beginning of my work I had found out that motion of single electron around an atomic nucleus of a part of atoms of atomic gas of hydrogen and a part of hydrogen-like ions in form a gas or a vapour may be considered as plane. To define these motion characteristics I have set up the vector differential equation of central motion using the formulas for plane motion of a moving point (see e.g. [3] - [5] ). After transformations of this equation I have found equivalent to it final system of two scalar differential equations, which describe the changes of magnitude and direction of the velocity of single electron. On the basis of solution of final system the characteristics of plane motion of single electron were ascertained by me. In particular, I have found out that single electrons of foregoing parts of atoms of atomic gas of hydrogen and hydrogen-like ions in form a gas or a vapour move away from corresponding atomic nuclei, i.e., such parts of atoms and ions inevitably decay.
The ascertained characteristics, the slight emission of light front by single electrons which move around corresponding atomic nuclei, and also creation according to [4] of new atoms of atomic gas of hydrogen and new hydrogen-like ions in form a gas or a vapour define the formation of emission line spectra.
All novel results of the paper are formulated further in Section 12, Results.
2. Methods
This investigation is theoretical. The object of the investigation is plane motion of single electrons of a part of atoms of atomic gas of hydrogen and a part of hydrogen-like ions in form a gas or a vapour. A single electron is considered as a point, which has electric charge, mass and an initial speed, and moves on definable distance from an atomic nucleus.
The criterion of choice of mentioned parts of atoms and ions is the distance between single electron and an atomic nucleus. I have chosen this distance as enough big when it is possible to study the system: an atomic nucleus―single electron as two-body problem, while the motion of single electron may be considered as plane motion. Reducing two-body problem to one-body problem [4] , I passed to the question about definition of motion of single electron in electric field of an atomic nucleus as in external central electric field. According to such model I have set up the vector differential equation of central motion of single electron. This equation was transformed by me to equivalent system of two scalar differential equations which I have solved as extreme- value problem and obtained the answers to such questions:
-how does single electron move relative to atomic nucleus;
-does a speed of single electron change and what changes may be;
-what is a curve along which single electron moves;
-what is instantaneous angular rate of revolution of this electron around atomic nucleus;
-what is an electric field strength of an atomic nucleus as a function of the distance between atomic nucleus and single electron.
Additionally I have considered the process of formation of emission line spectra. This process is defined by motion of single electrons not only in separately taken atoms of hydrogen or hydrogen-like ions (during decays of such atoms and ions), but also in a gas or a vapour, which consist of such atoms or ions. I have compared results of this consideration with the results [6] of experimental study of emission line spectra.
3. Electric Field Strength of Atomic Nuclei
Let us elucidate, where in electric field single electrons of atoms of atomic gas of hydrogen and hydrogen-like ions in form a gas or a vapour may be found. Accordingly quantum mechanics an electron is located in an atom or molecule in a region of space, which is called orbital [1] . Taking into account this formulation of quantum mechanics one may affirm that distance
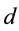 between single electron and corresponding atomic nucleus of the part of foregoing atoms and the part of foregoing ions satisfies condition
between single electron and corresponding atomic nucleus of the part of foregoing atoms and the part of foregoing ions satisfies condition
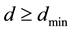 (1)
(1)
where
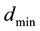 is the distance to atomic nucleus which approximately equals to atom sizes (of the order of
is the distance to atomic nucleus which approximately equals to atom sizes (of the order of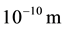 ) and is much more than nucleus sizes (of the order of
) and is much more than nucleus sizes (of the order of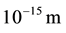 ) [1] . Consequently, on the distance
) [1] . Consequently, on the distance
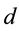 we can consider an atomic nucleus as a point and its electric charge as a point charge.
we can consider an atomic nucleus as a point and its electric charge as a point charge.
Then vector
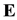 of the electric field strength of an atomic nucleus may be written down in a simple form
of the electric field strength of an atomic nucleus may be written down in a simple form
 (2)
(2)
where
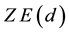 is the positive magnitude of the electric field strength of the nucleus (
is the positive magnitude of the electric field strength of the nucleus (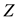 is number of protons of the nucleus: if
is number of protons of the nucleus: if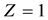 , the question is the electric field strength of atomic nuclei of atoms of atomic gas of hydrogen; if
, the question is the electric field strength of atomic nuclei of atoms of atomic gas of hydrogen; if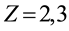 , etc., the question is the electric field strength of atomic nuclei of corresponding hydrogen-like ions in form a gas or a vapour;
, etc., the question is the electric field strength of atomic nuclei of corresponding hydrogen-like ions in form a gas or a vapour;





4. Coordinate Systems and Frame of Reference
Let mutually perpendicular axes







where


I have studied the motion of single electron around an atomic nucleus (distanced from other the same nuclei) in inertial frame of reference. Taking into account condition (1) I have considered the motion of single electron in the scope of two-body problem: an atomic nucleus and single electron. According to [4] the center-of-mass system in two-body problem is inertial frame of reference. I have chosen just such frame of reference on the condition, that its center of mass was motionless. For that I had matched the point

Also I had directed axis

Thus, single electron moves at some initial speed along the plane curve, whose coordinates satisfy the system of parametric equations

where











continuous first derivatives with respect to





unit vector of tangent to the curve (4);








because




5. Acceleration of Plane Motion of Single Electron
Let us consider the acceleration of motion of single electron. I write down this acceleration in inertial center-of- mass system. Accordingly the second Newton’s law single electron accelerates in electric field of an atomic nucleus

where the acceleration vector












6. Vector Differential Equation of Central Motion of Single Electron
According to [3] the indefinite integral

оf a suitable vector function


which may be replaced by a set of differential equations for the components of

In Equations (7) and (8) I have changed







The Equation (9) may be solved after its replacement accordingly [3] by the set of two differential equations for physical components of







7. Transformations of Vector Differential Equation (9)
According to [3] - [5] I have decomposed derivative



normal


where



letter






Then I have transformed Equation (10)

In Equation (11) replacement of angular rate

is expedient. According to [3]



where




fined the angle








Decomposition of derivative


ration is illustrated by Figure, that gives the graphic idea about the plane motion of single electron in electric field of an atomic nucleus.
On this Figure 1is shown, in particular, that angle


Figure 1.Decomposition of derivative





Further, according to [4] I have decomposed





Here the directional derivative





Then I have substituted in Equation (11)






Let us rewrite the vector differential Equation (14) in that way

Now I calculate the vector products




Let us substitute results of Equations (16) and (17) into Equation (15):

Obtained Equation (18) is equivalent to Equation (9).
8. Finding of Final System of Two Scalar Differential Equations
I have replaced the vector differential equation (18) by the set of two differential equations for physical components of


The set of Equations (19) may be written down in scalar form

After elementary transformations of the set (20) I have found the derivatives


In the system (21)


In Equations (21)-(23) the quantities





atives




The values of the function


where


The final system (21) is equivalent to Equation (9) and describes the revolution of single electron around instantaneous position of the center of mass of foregoing parts of atoms and ions. Instantaneous angular rate






9. Solution of Final System (21) As Extreme-Value Problem [3]
To solve the system (21) as extreme-value problem it is necessary to find the conditions which maximize the angle



and also derivative
In the beginning according to [3] let us write down the conditions for the existence of interior maximum and interior minimum of angle
a) if






Here the first derivative


b) if








(Finding of second derivative

tem (21) is also used, see in App. D.)
According to condition (25) and the second differential equation of system (21) I have obtained the equality

which relates a value

Then according to App. D I have determined the value of second derivative



Taking into account the equality (28) in Equation (29) I have obtained

Accordingly condition (27) I have investigated the function


because in the paper
Consequently, according to Equation (31) condition (27) takes place in two cases:
1) if

and

2) and vice versa, if

and

Both these cases have no physical sense. In the case 1) values of derivative

may be neither zero nor positive, because the function




Further I have investigated the function


because
Consequently, accordingly Equation (36) the condition (26) takes place in such two cases:
1) if

and

2) and vice versa, if

and

Investigating interior maximum of the function
physical sense, because values of derivative

(the function



Let us consider the case 1). From condition (37) one can see the values of angle

with the values of angle

ly. That corresponds to definition of the function




10. Motion Characteristics of Single Electron as Results of Maximization of Angle
Maximization of angle


From the range of values of angle


It means, that for an suitable function






For the range of values of angle




From condition (42) and the first differential equation of system (21) I have concluded, that

i.e., speed of single electron only decreases during its revolution around an atomic nucleus. The inequality (43)
means also, that tangential component




lustrated by arrow on the curve (4) (see Figure), is confirmed by solution of system (21).
There are two reasons of decrease of speed of single electron: slight emission of light front (see 1. Introduction) and self-ionization of atom or ion, which is contained single electron. If both these reasons lead to a small
decrease of speed of single electron, then one may assign

Now one may explain, how interior maximum of turns density of flat spiral is formed. Such maximum is connected with maximum of angle


-increase of turns density of flat spiral;
-approach of turns form of this spiral to circle form.
So long as for



Let us consider the characteristics of revolution of single electron around an atomic nucleus in the interior maximum of turns density of flat spiral. According to Equation (23) value of instantaneous angular rate

because value
Now let us consider equality, that I have obtained from the relation (28):

The right side of Equation (45) approximately equals to value of angular rate



In maximum of turns density of spiral the form of these turns approaches to the form of a circle, consequently the motion of single electron may be characterized by cyclic frequency. Dividing (44) and (46) by



In Equations (47) and (48)



At last let us consider the result of maximization of angle




Let us pass from the function






11. Formation of Separate Line and Series of Lines of Emission Line Spectra
A. Formation of separate line
In my supposition (see Section 1. Introduction), a separate line is formed by single electron when it moves around an atomic nucleus and emits a slight light front in the form of a conical surface simultaneously. However such line is formed not by one single electron, but by many single electrons of the part of atoms of atomic gas of hydrogen (and the part of hydrogen-like ions in form a gas or a vapour). During the formation of separate line each single electron revolves around corresponding atomic nucleus in interior maximum of turns density of the spiral. Approximate value

From each of these single electrons the light front comes to spectral device one time per a turn of single electron around an atomic nucleus. Therefore central cyclic frequency



The formation of separate line is connected with the interior maximum of turns density of flat spiral and therefore this line has the form of a top which is confirmed in experiment [6] . Such form of the line indicates, that decrease of speed of single electrons is caused, in particular, by slight emission of light front.
B. Formation of series of lines
Formation of series of lines takes place, when the same single electrons move consecutively through electric fields of several atomic nuclei and simultaneously emit slight light front. Such motion of single electrons is possible because of the decays of the part of atoms of atomic gas of hydrogen (or the part of hydrogen-like ions in form a gas or a vapour) and also because of small decrease of a speed of single electrons.
Let us consider formation of series of lines using as example Balmer series. After creation of primary atoms of atomic gas of hydrogen (for example, from a vapour of water [6] ) each electron of the part of these atoms distances from corresponding atomic nucleus in flat spiral and forms the first line, i.e.,



where inferior index





After formation of






where inferior index






By analogy with considered formation of



From studying emission line spectra it is known [1] [6] , that for series of lines central cyclic frequency of emission of every next line is greater than corresponding frequency of previous line. I explain this fact, using also Balmer series as example. So long as all lines of a series are formed by the same single electrons, speed of which decreases during formation of lines, then
during creation of secondary atoms (in the way of capture [4] ) the more is value


(51). But intensities of lines decrease as their ordinal number increases. That may be explained by decrease of probability of capture of the electrons with atomic nuclei because of decrease of quantity of these electrons and nuclei (in particular, because of scattering). From this explanation it is clear, that way of formation of series of lines is linked to repeated creations of atoms of atomic gas of hydrogen (and hydrogen-like ions in form a gas or a vapour) after decays of such atoms and ions.
Formation of other series of lines both atoms of atomic gas of hydrogen and any hydrogen-like ions in form a gas or a vapour takes place like formation of Balmer series. But, in my opinion, the first line of each such series (except the first lines of main series of hydrogen-like ions) is formed with the help of electrons of gas discharge, if a speed of these electrons may be assigned in experiment. Probably, last lines of exceptionally long Balmer series are formed also by the electrons of gas discharge.
Let us illustrate, how just separate line is formed by the electrons at assigned speed.
C. Formation of separate line by electrons at assigned speed
Firstly I have considered the following. If atomic gas of hydrogen is bombarding by the electrons with assigned energy 13.6 eV, these electrons will move through electric fields of free moving atomic nuclei (which are in atomic gas of hydrogen) at assigned speed







Then I have made such calculation. Using Equation (47) and the value




numerical value



The calculation by Equation (47) gives
tude the value








12. Results
In the paper the model of motion of single electron in electric field of an atomic nucleus as in external central electric field is developed. During development of this model I have found out, that after creation of atoms of atomic gas of hydrogen and hydrogen-like ions in form a gas or a vapour the motion of single electrons of the parts of mentioned atoms and ions one may consider as plane motion. To define the characteristics of such motion of single electron the vector differential equation of central motion was set up. As a result of solution of the final system of differential equations, which is equivalent to aforementioned vector differential equation, I have found the following.
Not a single electron of foregoing parts of atoms and ions “falls” on corresponding atomic nucleus but only moves away from it, revolving around the nucleus along flat spiral and continuously emitting a slight light front in the form of a conical surface. The spiral has the interior maximum of turns density. Moving away from the nucleus, each single electron decelerates with its electric field and the speed of single electrons decreases slightly. As a result single electrons move away from electric fields of atomic nuclei with no expenditures of external energy. I have proved, that the electric field strength of an atomic nuclei of atoms of atomic gas of hydrogen and hydrogen-like ions in form a gas or a vapour for a point of single electron location (accordingly condition (1)) is inversely proportional to the distance between the corresponding atomic nucleus and single electron by greater than the power of 3. I have also found relations (47) and (48) for calculation of cyclic frequency of revolution of single electron around an atomic nucleus in maximum of turns density of flat spiral.
One can see in the paper, that calculated cyclic frequency (rough value



13. Discussion
The results of the paper may be conditionally classified into two groups. The first group of results has a bearing on motion characteristics of single electron, the second one applies to the formation of emission line spectra.
Using the second Newton’s law, I have ascertained the motion characteristics of single electrons of the part of atoms of atomic gas of hydrogen and the part of hydrogen-like ions in form a gas or a vapour. Ascertained characteristics mean these parts of atoms and ions inevitably decay, that was unknown before. During their decays emission of light front and self-ionization take place, which need a small expenditures of internal energy, i.e., expenditures of external energy are not necessary (according to currently accepted opinion, expenditures of definite external energy are necessary, that is incorrectly).
Also I have proved, that the electric field strength of an atomic nucleus (in considered region of electric field) is inversely proportional to the distance from nucleus by the power

Note that both mentioned results: decays and condition



In a future research it is necessary to elucidate why the atoms of hydrogen which are connected in chemical compounds (for example, in molecules of water) are stable, whereas the atoms of atomic gas of hydrogen decay, and their electrons emit light front in this time. To approach the answers to these and, perhaps, other questions, I have made the following. From Equation (50) when


where all quantities are known:





For atomic nucleus with


Let us discuss now the second group of results of the paper?the formation of emission line spectra. I have shown, that all lines of these spectra are formed with slight emission of light front by single electrons. The hypothesis of slight emission of light front is confirmed by line structure of these spectra, which was discovered in experiments.
In future research it is necessary to ascertain, in particular, the values of frequency of revolution of single electron around atomic nucleus of atoms of atomic gas of hydrogen and hydrogen-like ions in form a gas or a vapour (in maxima of turns density of spiral) as function of values of speed of this electron in mentioned maxima.
14. Conclusions
In conclusion such statements may be added.
The second Newton’s law may be applied to define the motion characteristics of single electron in electric field of an atomic nucleus.
Coulomb’s law does not describe the interaction between electric charges of single electron and an atomic nucleus.
Emission line spectra are formed with slight emission of light front by single electrons during their revolution around corresponding atomic nuclei.
Energy of decays of the part of atoms of atomic gas of hydrogen and the part of hydrogen-like ions in form a gas or a vapour may be used in practice.
Cite this paper
Ivan AntonovychStrilets, (2014) Motion Characteristics of Single Electrons of Atoms of Atomic Gas of Hydrogen and Single Electrons of Hydrogen-Like Ions in Form Gas or Vapour during Decays of Such Atoms and Ions. Emission Line Spectra. Journal of Modern Physics,05,1302-1320. doi: 10.4236/jmp.2014.514131
References
- 1. Daintith, J. (2009) A Dictionary of Physics (Oxford Paperback Reference). Sixth Edition, Oxford University Press, Oxford.
- 2. Hau, L.V., Harris, S.E., Dutton, Z. and Behroozi, C.H. (1999) Light Speed Reduction to 17 Metres Per Second in an Ultracold Atomic Gas. Nature, 397, 594-598.
http://dx.doi.org/10.1038/17561 - 3. Korn, G.A. and Korn, T.M. (2000) Mathematical Handbook for Scientists and Engineers. Definitions, Theorems, and Formulas for Reference and Review. Dover Publications, Inc., New York.
- 4. Synge, J.L. (1960) Classical Dynamics. Encyclopedia of Physics (Handbuch der Physik). Vol. III/1, Springer-Verlag, Berlin, Gottingen, Heidelberg.
- 5. Pohl, R.W. (1964) Mechanik, Akustik und Warmelehre. Springer-Verlag, Berlin, Gottingen, Heidelberg, New York.
http://dx.doi.org/10.1007/978-3-662-25338-0 - 6. Humphreys, C.J. (1953) The Sixth Series in the Spectrum of Atomic Hydrogen. Journal of Research of the National Bureau of Standards, 50, 2380.
Appendixes
Appendix A: Finding of Directional Derivative
If the direction of motion of single electron is assigned by the unit vector


where



where





According to the rules for operations with the operator nabla I have obtained

Let us substitute the result (A3) into Equation (A1)

where





The sign of directional derivative






Appendix B: Finding of Instantaneous Angular Rate
Accordingly [3] in terms of polar coordinates



Let us divide Equation (В1) by



Taking into account in Equation (В2), that


From Equation (В3) I have obtained instantaneous angular rate




In this paper


Appendix C: Finding of Derivatives

I have divided the first differential equation of the set (20) by


Then I have added the first differential equation of the set (С1) to the second one

and multiplied differential equation (С2) by

The left side of differential equation (С3) is equal to

Equation (В3) in Equation (С3) I have found


Then I have multiplied the first differential equation of the set (20) by



I have subtracted the first differential equation of the set (С5) from the second one

I have multiplied differential equation (С6) by

After elementary transformations of Equation (С7) I have found derivative

Let us consider the ranges of values of some quantities that contains Equations (C4) and (C8). The positive
angle














Equations (С4) and (С8) have the same conditions:

If we take into consideration









Appendix D: Finding of Second Derivative

Let us substitute the derivative


Let us transform Equation (D2)

Appendix E: Finding of Function
At the beginning I have solved the equation, which is connected with inequality (38). To make that, in inequality (38) I have replaced the sign “<” by sign “=” the value





Separating variables in differential Equation (Е1), I have integrated it with definite conditions


i.e.,

or

The function (Е4) is the solution of differential Equation (Е1), that I have obtained from inequality (38). If we
substitute the function


we will obtain




and is the function, that must be found.












