Journal of Modern Physics
Vol.4 No.5(2013), Article ID:31602,4 pages DOI:10.4236/jmp.2013.45088
Correlation between Diffusion Equation and Schrödinger Equation
Department of Applied Mathematics, Faculty of Engineering, Oita University, Oita, Japan
Email: okino@oita-u.ac.jp
Copyright © 2013 Takahisa Okino. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 28, 2013; revised March 20, 2013; accepted April 27, 2013
Keywords: Diffusion Coefficient; Diffusion Equation; Schrödinger Equation
ABSTRACT
The well-known Schrdöinger equation is reasonably derived from the well-known diffusion equation. In the present study, the imaginary time is incorporated into the diffusion equation for understanding of the collision problem between two micro particles. It is revealed that the diffusivity corresponds to the angular momentum operator in quantum theory. The universal diffusivity expression, which is valid in an arbitrary material, will be useful for understanding of diffusion problems.
1. Introduction
For micro particles such as atoms or molecules in the homogeneous time and space of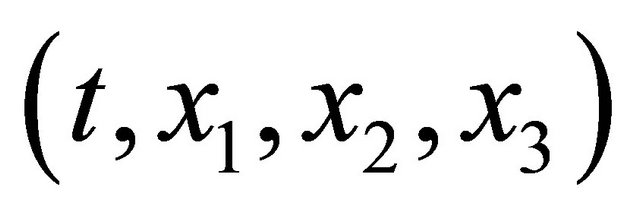 , the macro behavior of their collective motions is presented by the well-known diffusion equation of
, the macro behavior of their collective motions is presented by the well-known diffusion equation of
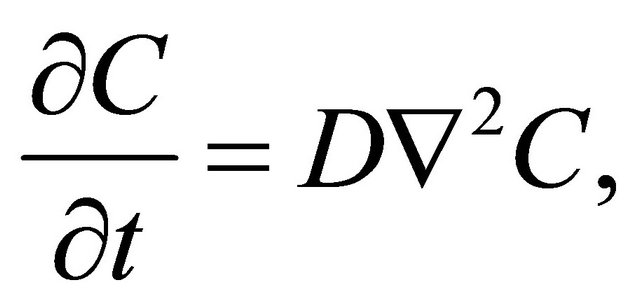 (1)
(1)
where 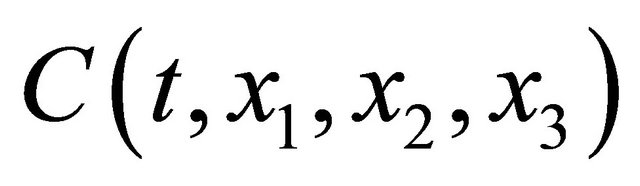 is the concentration of them and
is the concentration of them and
 the diffusivity when it does not depend on
the diffusivity when it does not depend on 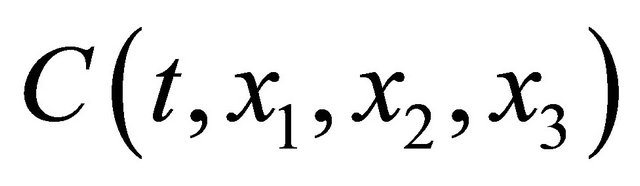 [1].
[1].
The motion of a micro particle is presented by quantum mechanics and its behavior is investigated by using the Schrödinger equation of
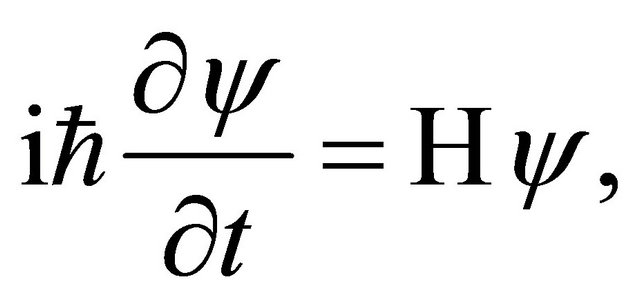 (2)
(2)
where  is
is 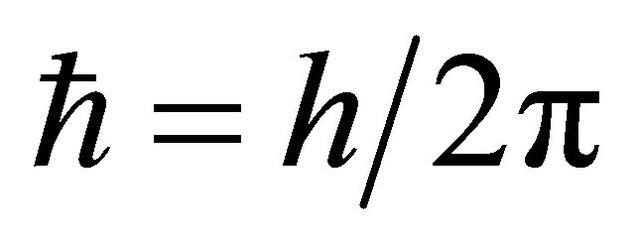 using the Plank constant
using the Plank constant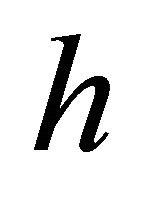 ,
, 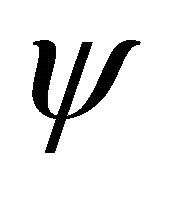 the state vector and
the state vector and 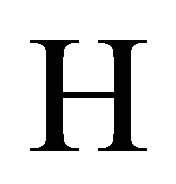 the Hamiltonian meaning the total energy in the given physical system [2]. In case of a free particle, it is given by
the Hamiltonian meaning the total energy in the given physical system [2]. In case of a free particle, it is given by
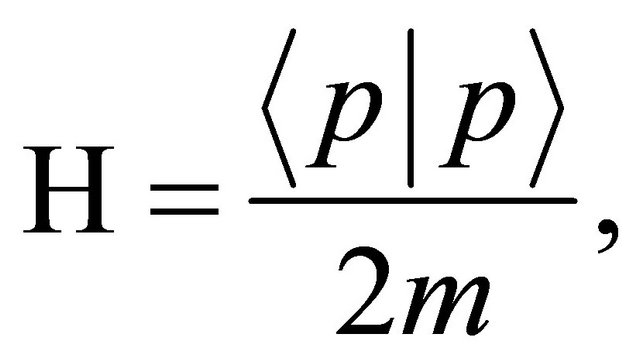 (3)
(3)
where 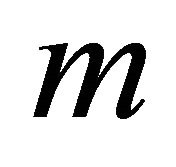 is the particle mass and
is the particle mass and 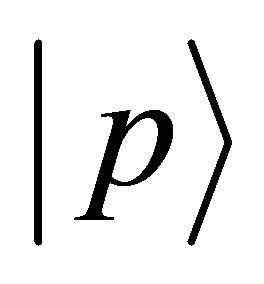 the momentum.
the momentum.
In the present study, the correlation between (1) and (2) was investigated. It was found that the Schrödinger equation (2) is reasonably derived from the diffusion equation (1) by means of using the imaginary time for (1). As a result, we revealed that the diffusivity 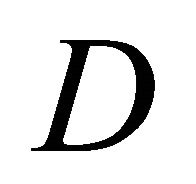 in (1) corresponds to the angular momentum operator
in (1) corresponds to the angular momentum operator 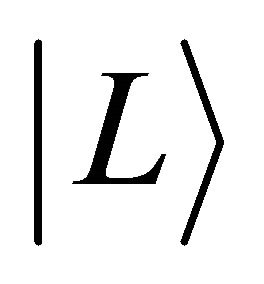 in quantum mechanics. The obtained new diffusivity will be useful for understanding of an elementary process of diffusion [3].
in quantum mechanics. The obtained new diffusivity will be useful for understanding of an elementary process of diffusion [3].
2. Necessity of Imaginary Time
The micro particle in a solid crystal jumps instantly to the nearest lattice site through an energy barrier when it obtains an activation energy caused by the thermal fluctuation. The micro particle in a fluid collides with another one via the movement of the averaged free path and the particle jumps to a neighbor site.
For a Brownian particle of mass m, the well-known Langevin equation is
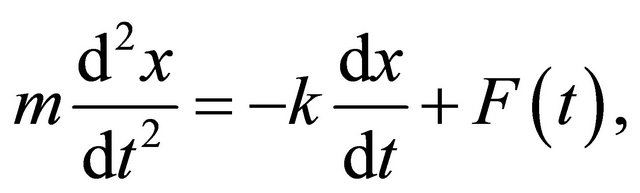 (4)
(4)
where the velocity 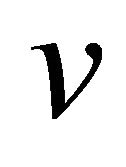 and the viscosity resistance f are
and the viscosity resistance f are
 and
and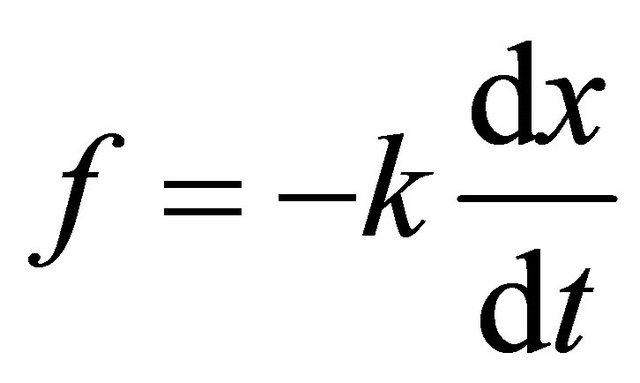 , respectively [4]. In (4), the time-averaged value of external force
, respectively [4]. In (4), the time-averaged value of external force 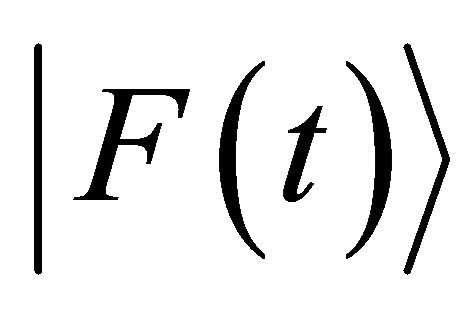 satisfies
satisfies
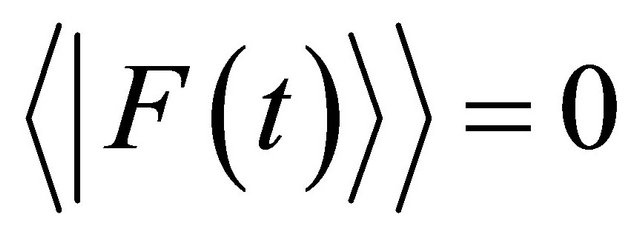 in a collision problem. Hereafter, we do not discuss
in a collision problem. Hereafter, we do not discuss 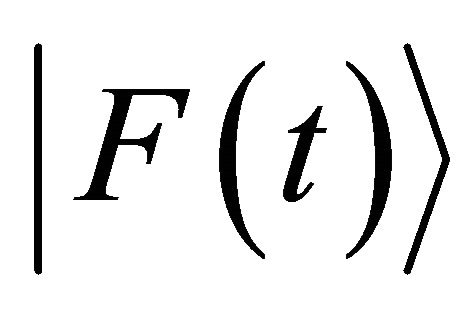 but the acceleration in a collision problem between two micro particles. In the three dimensional space
but the acceleration in a collision problem between two micro particles. In the three dimensional space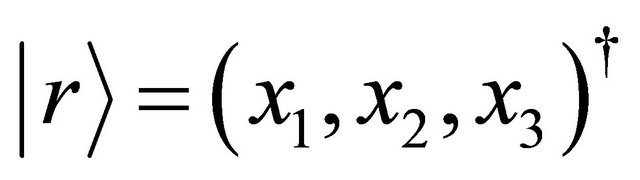 , the acceleration is expressed as:
, the acceleration is expressed as:
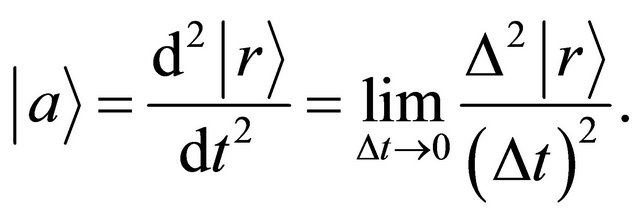 (5)
(5)
Since the physical essence is still kept even if we consider the simplest collision problem of one dimensional case, we thus investigate a perfect elastic collision problem between a micro particle A and a particle B of the same kind. When the particle A moves at a velocity 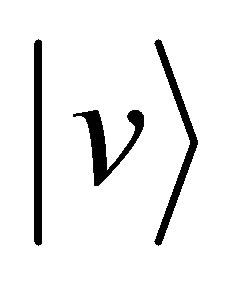 and collides at time
and collides at time 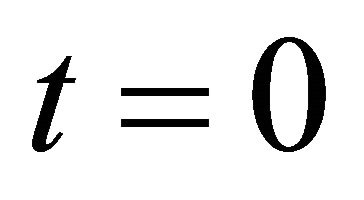 with the particle B in the standstill state, if we can clarify the distinction between A and B after the collision, the particle A decelerates from the velocity
with the particle B in the standstill state, if we can clarify the distinction between A and B after the collision, the particle A decelerates from the velocity 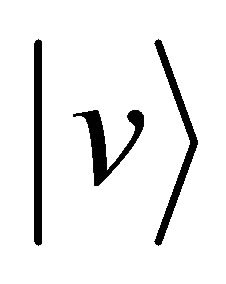 to the velocity zero and the particle B accelerates from the velocity zero to the velocity
to the velocity zero and the particle B accelerates from the velocity zero to the velocity 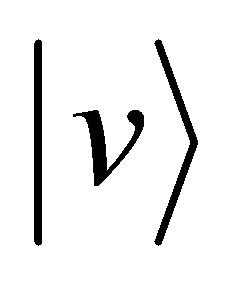 between
between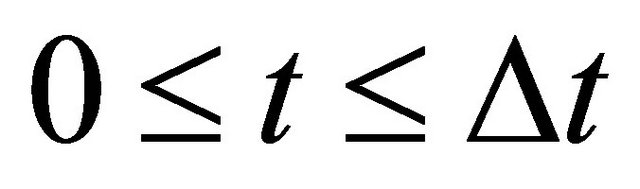 . On the other hand, if we cannot clarify the distinction between A and B after the collision, it seems that the particle A decelerates from the velocity
. On the other hand, if we cannot clarify the distinction between A and B after the collision, it seems that the particle A decelerates from the velocity 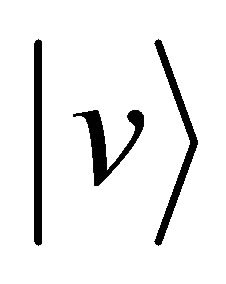 to the velocity zero between
to the velocity zero between 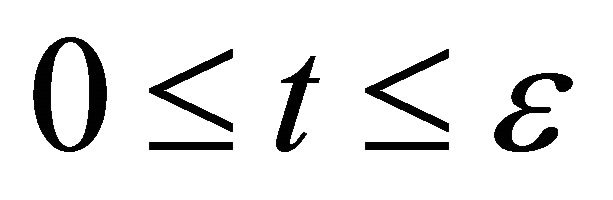 and subsequently accelerates again from the velocity zero to the velocity
and subsequently accelerates again from the velocity zero to the velocity 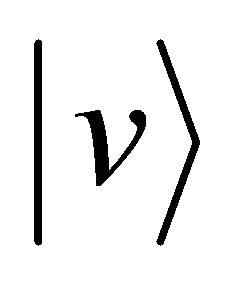 between
between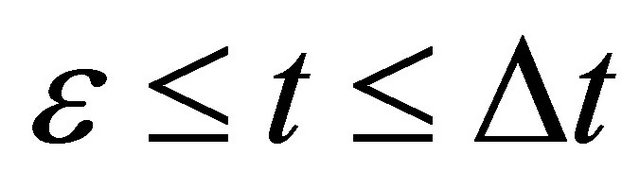 . In other words, the particle motion seems as if there is no collision process.
. In other words, the particle motion seems as if there is no collision process.
If we notice the acceleration of  in the above latter case, the relation of
in the above latter case, the relation of  between
between 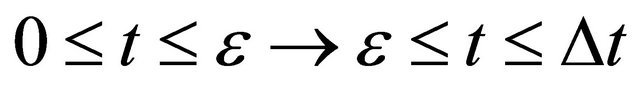 is valid in the three dimensional collision process, using a probabilistic parameter
is valid in the three dimensional collision process, using a probabilistic parameter 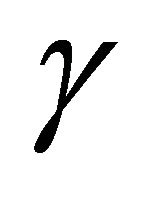 of
of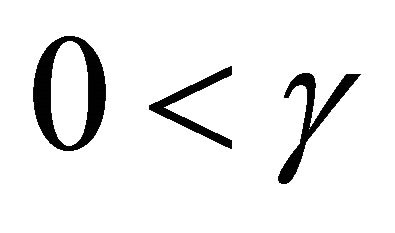 . Therefore, this indicates that the impossibility of discrimination between the particles A and B yields
. Therefore, this indicates that the impossibility of discrimination between the particles A and B yields 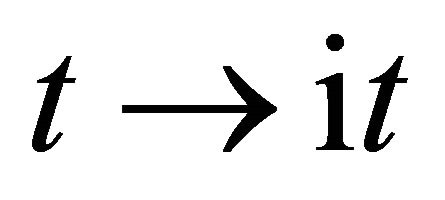 or
or 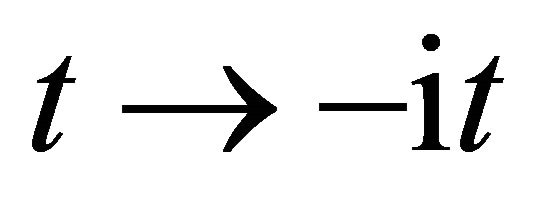 between
between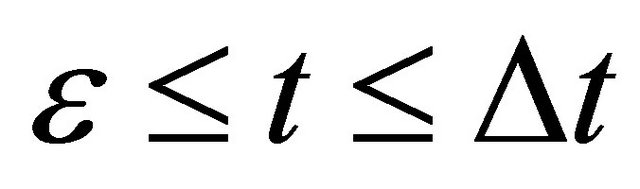 , as can be seen from the expression of (5).
, as can be seen from the expression of (5).
In the present study, we thus accept the imaginary time 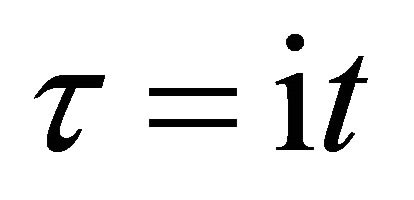 as an essential characteristic of a micro particle caused by the impossibility of discrimination between micro particles. In a collision problem, the acceleration is meaningless, although
as an essential characteristic of a micro particle caused by the impossibility of discrimination between micro particles. In a collision problem, the acceleration is meaningless, although 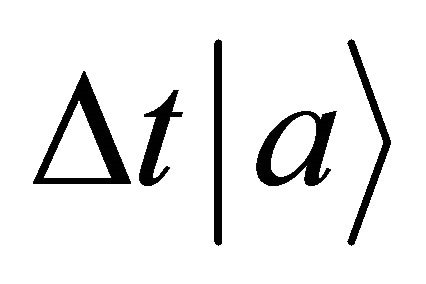 is finite at the limit of
is finite at the limit of 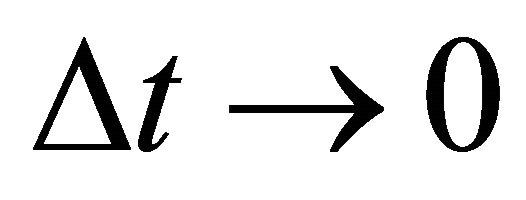 and
and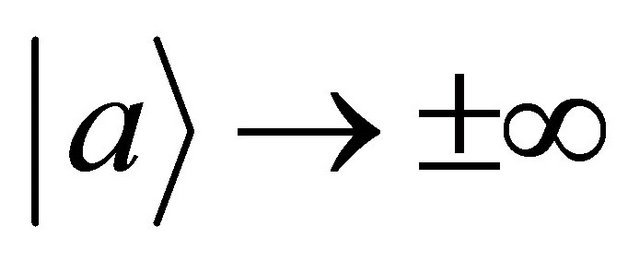 .
.
3. Diffusion Equation of Imaginary Time
Rewriting the concentration 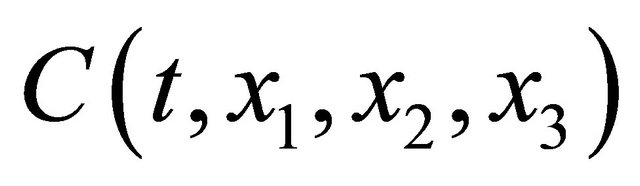 of diffusion particles into a quantity of state expressed by a complex function
of diffusion particles into a quantity of state expressed by a complex function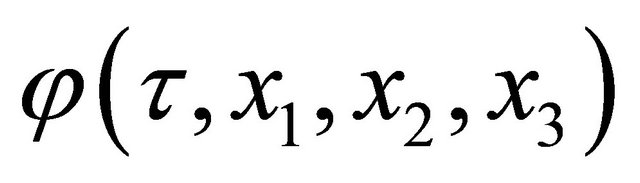 , (1) is presented as:
, (1) is presented as:
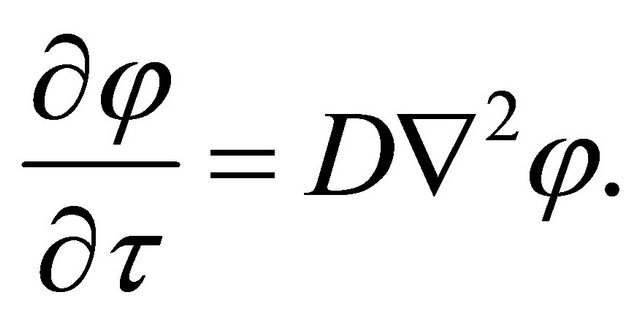 (6)
(6)
Assuming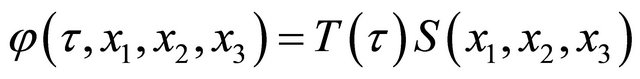 , (6) can be solved by the separation method of variables. Using complex numbers
, (6) can be solved by the separation method of variables. Using complex numbers 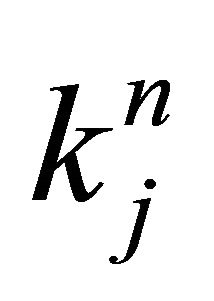 and
and 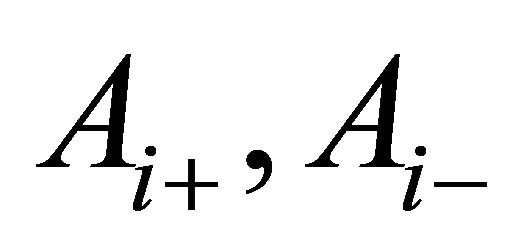 determined from the initial and boundary conditions, the general solution of (6) is obtained as;
determined from the initial and boundary conditions, the general solution of (6) is obtained as;
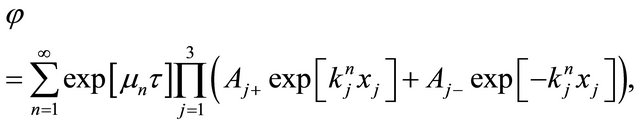 (7)
(7)
where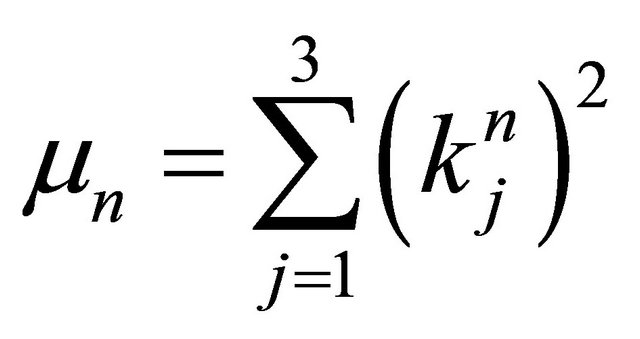 . Substituting
. Substituting 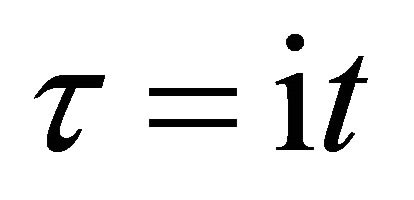 into (7), it becomes
into (7), it becomes
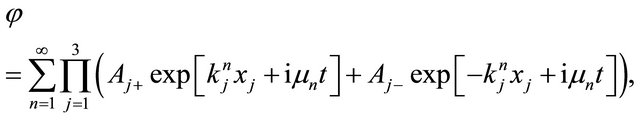
and using the real function 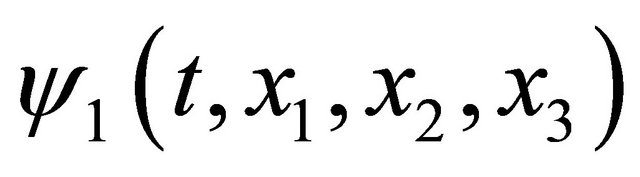 and
and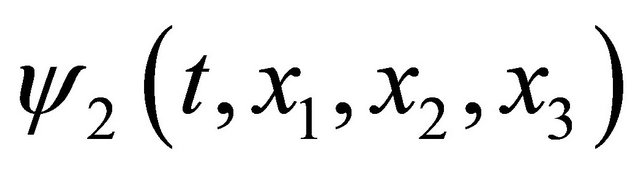 , we rewrite the complex function
, we rewrite the complex function  into the complex-value function yielding
into the complex-value function yielding
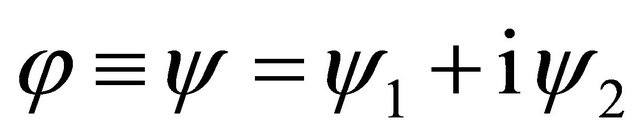 . (8)
. (8)
Further, substituting (8) and 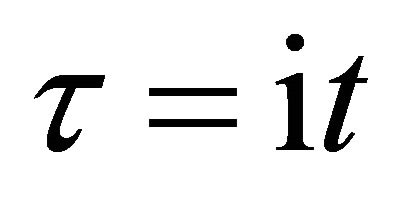 into (6) and multiplying the both-side of (6) by
into (6) and multiplying the both-side of (6) by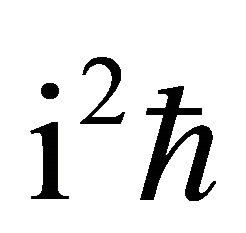 , (1) is rewritten as:
, (1) is rewritten as:
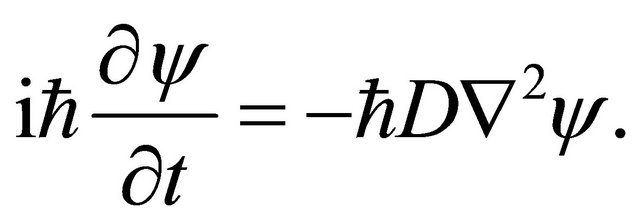 (9)
(9)
4. Diffusion Coefficient of Micro Particle
The function 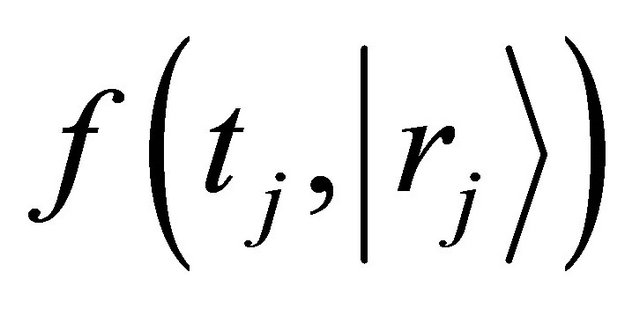 is defined as a probability density which a diffusion particle in the initial state of
is defined as a probability density which a diffusion particle in the initial state of 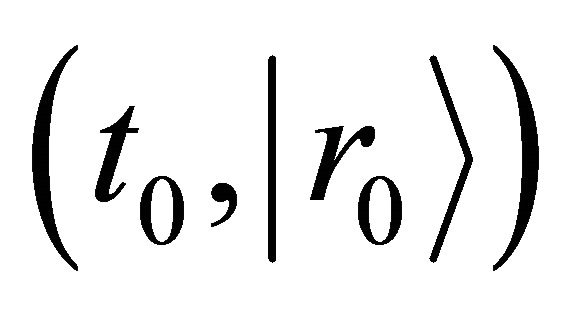 exists in the state of
exists in the state of 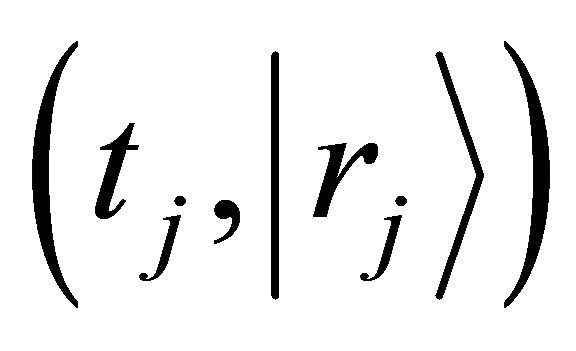 after j times jumps. A diffusion particle moves at random and it is, therefore, considered that the jump frequency
after j times jumps. A diffusion particle moves at random and it is, therefore, considered that the jump frequency 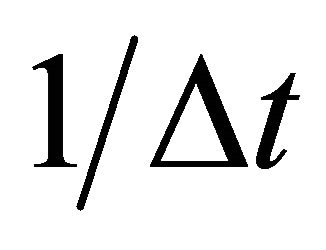 and jump displacement
and jump displacement 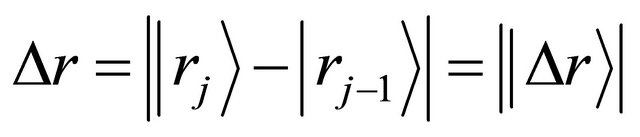 are equivalent in probability to their mean values of all diffusion particles in the collective system. Since it is also considered that the probability of diffusion-jump from the state of
are equivalent in probability to their mean values of all diffusion particles in the collective system. Since it is also considered that the probability of diffusion-jump from the state of 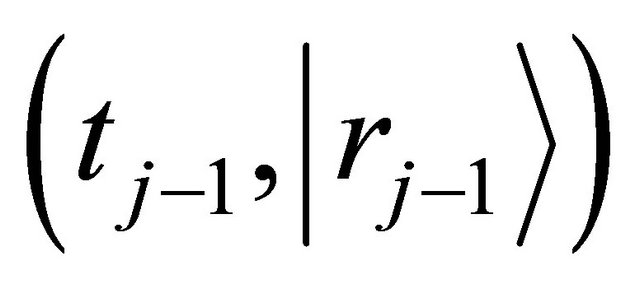 to
to 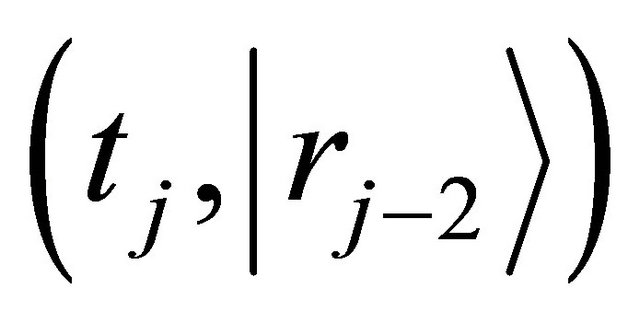 is equivalent to one from the same state to
is equivalent to one from the same state to , the relation of
, the relation of
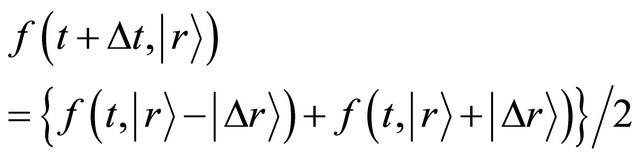 (10)
(10)
is thus valid.
The Taylor expansion of the left-hand side of (10) yields
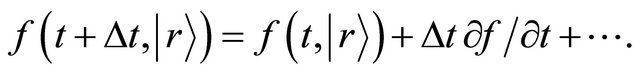 (11)
(11)
The Taylor expansion of the right-hand side of (10) also yields
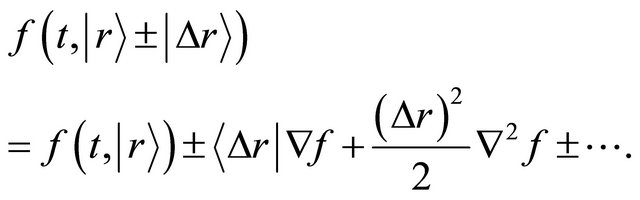 (12)
(12)
The substitution of (11) and (12) into (10) gives
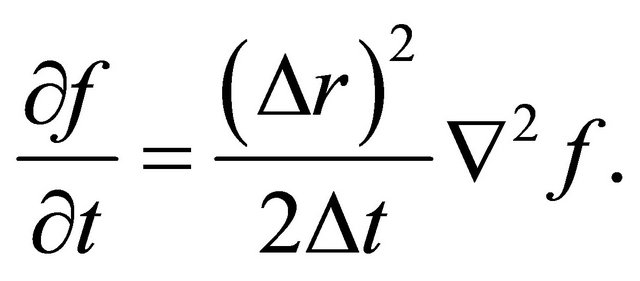 (13)
(13)
Since the probability density function f of a diffusion particle corresponds to the normalized concentration C, the comparison of (1) with (13) gives the diffusion coefficient yielding
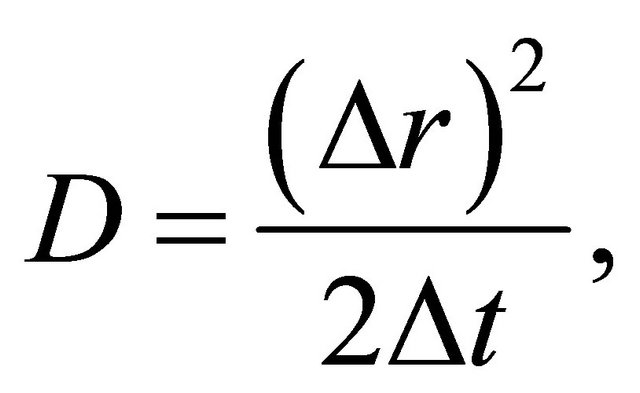 (14)
(14)
as a relation satisfying the well-known parabolic law [5].
5. Diffusion Coefficient and Angular Momentum
When a micro particle randomly jumps from a position to another one, the jump orientation becomes the spherical symmetry in probability. Using the equation of

relevant to the angular momentum 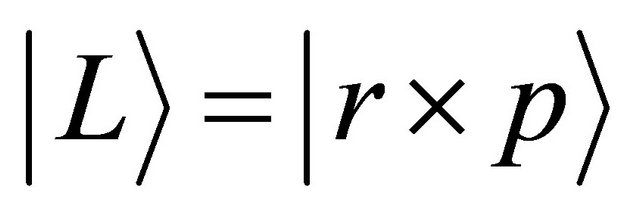 defined by a position vector
defined by a position vector  and a momentum
and a momentum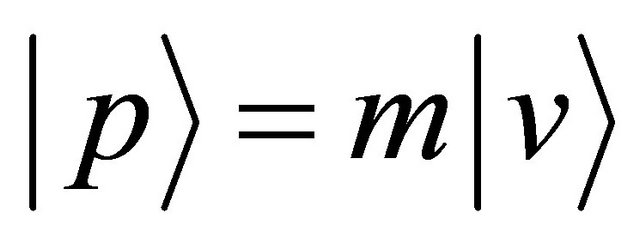 , the right-hand side of (14) is rewritten as:
, the right-hand side of (14) is rewritten as:
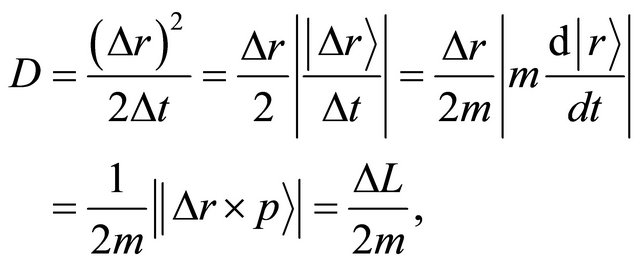
where 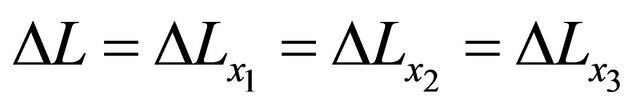 is valid in the spherical symmetry space. Considering the eigenvalue, the relation of (14) is thus rewritten as an operator relation of
is valid in the spherical symmetry space. Considering the eigenvalue, the relation of (14) is thus rewritten as an operator relation of
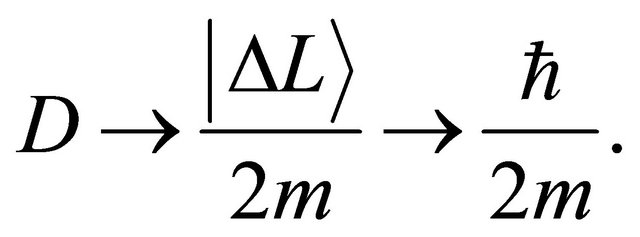 (15)
(15)
Substituting (15) into (9) gives
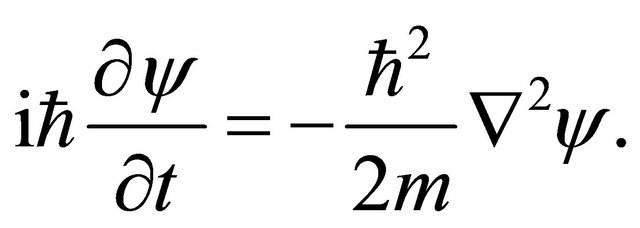 (16)
(16)
Here, if we define the relation given by
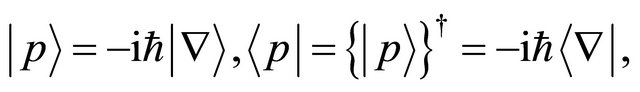 (17)
(17)
(16) becomes the equation of
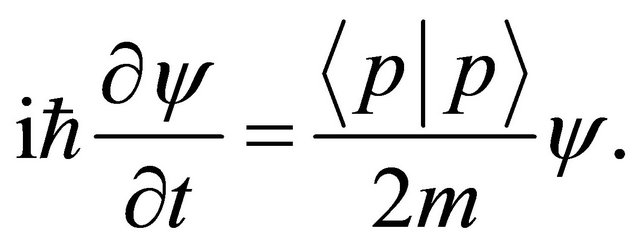 (18)
(18)
Further, the substitution of (3) into (18) yields the well-known Schrödinger equation (2).The defined equation (17) is one of the basic operators in quantum mechanics.
Hereinbefore, the Schrödinger equation was reasonably derived from the diffusion equation. It was also found that the diffusivity corresponds to the angular momentum operator in quantum mechanics. The relation of (15) is concretely investigated in the following section.
6. Discussion and Conclusion
In mathematics, it was clarified that we can transform the diffusion equation for the collective motion of micro particles into the Schrödinger equation for a micro particle. In physics, energy E, momentum  and angular momentum
and angular momentum  are expressed as operators yielding
are expressed as operators yielding
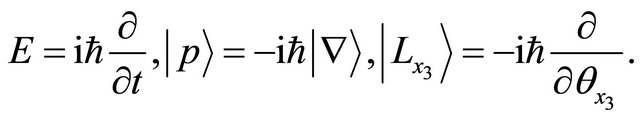
We cannot observe imaginary physical quantities. Therefore, the eigenvalues of their operators are meaningful in quantum mechanics.
As previously mentioned in a collision problem, the impossibility of identification between micro particles corresponds to introducing the imaginary time 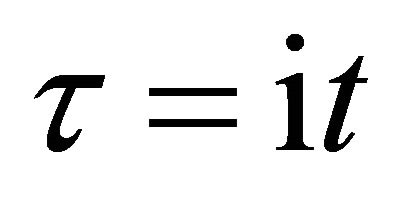 into those motions and also it corresponds to yielding the meaningless acceleration. It is considered that the physical concept obtained here is generally valid for the micro particle motions. Thus, the concept of acceleration disappears in quantum mechanics.
into those motions and also it corresponds to yielding the meaningless acceleration. It is considered that the physical concept obtained here is generally valid for the micro particle motions. Thus, the concept of acceleration disappears in quantum mechanics.
Except constant physical quantities, physical variables containing an imaginary number i should be accepted as physical operators in quantum mechanics. Here, note that the kinetic energy 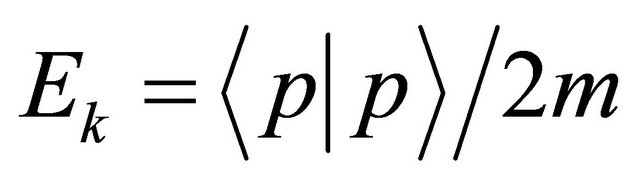 in Hamiltonian is acceptable as an operator
in Hamiltonian is acceptable as an operator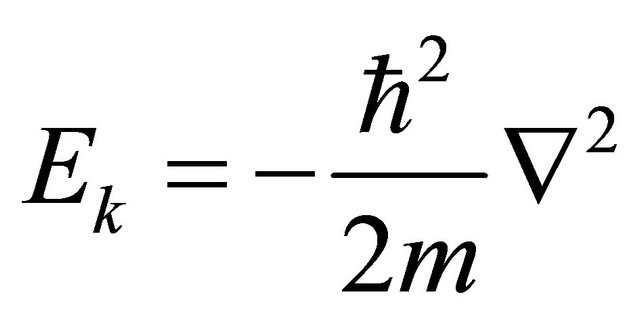 . On the other hand, the photon energy
. On the other hand, the photon energy 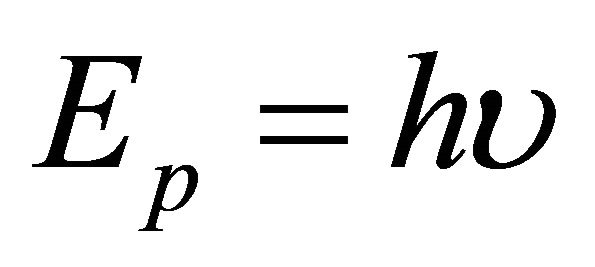 expressed by using a frequency
expressed by using a frequency 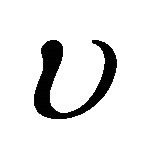 is acceptable as an operator
is acceptable as an operator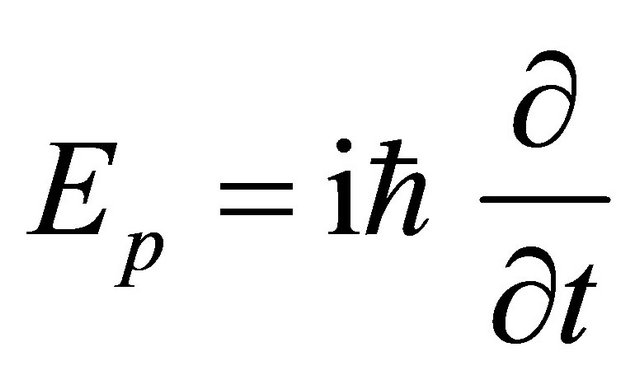 although
although 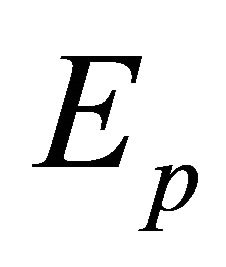 as well as
as well as 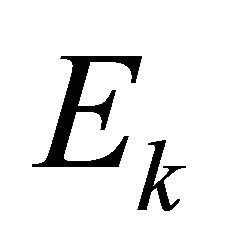 is also an energy representation.
is also an energy representation.
The existence probability of a micro particle in a collective system of heat quantity Q and absolute temperature T is given by the well-known Boltzmann factor of
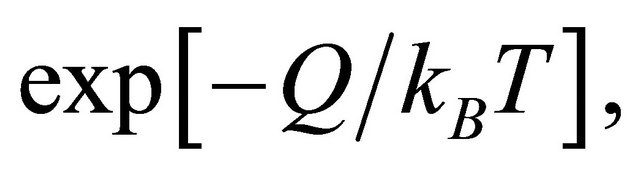 (19)
(19)
where 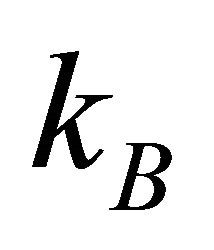 is the Boltzmann constant [6]. There is an energy barrier for a diffusion particle in order to jump from a site to another site. Therefore, it is necessary for a diffusion particle to obtain the activation energy Q from the thermal fluctuation. In a collective system composed of micro particles, the diffusion coefficient D is thus directly proportional to the probability factor of (19).
is the Boltzmann constant [6]. There is an energy barrier for a diffusion particle in order to jump from a site to another site. Therefore, it is necessary for a diffusion particle to obtain the activation energy Q from the thermal fluctuation. In a collective system composed of micro particles, the diffusion coefficient D is thus directly proportional to the probability factor of (19).
The jump of a diffusion particle in a solid crystal depends on a factor 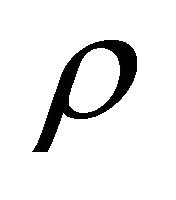 derived from the atomic configuration and on the entropy S derived from an elastic strain. In a solid crystal, therefore, (15) is rewritten as
derived from the atomic configuration and on the entropy S derived from an elastic strain. In a solid crystal, therefore, (15) is rewritten as
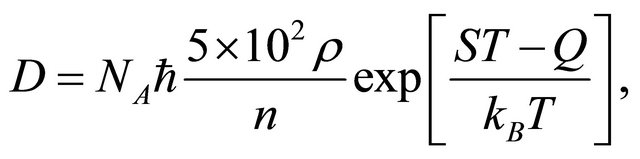 (20)
(20)
where 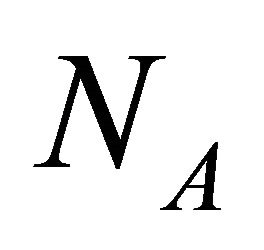 and
and 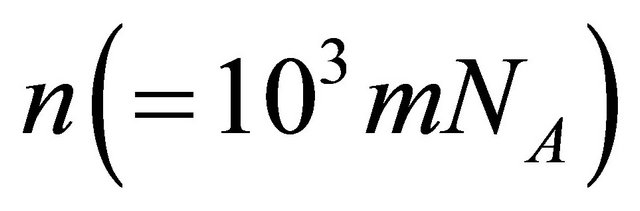 are the Avogadro constant and the molecular or the atomic weight. Here, (20) was obtained as a new representation of diffusion coefficient.
are the Avogadro constant and the molecular or the atomic weight. Here, (20) was obtained as a new representation of diffusion coefficient.
If we consider  in the given diffusion system of an arbitrary material, the universal diffusivity expression of
in the given diffusion system of an arbitrary material, the universal diffusivity expression of
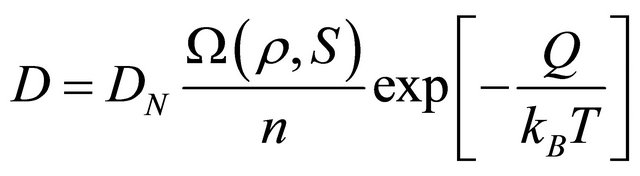 (21)
(21)
is thus obtained, where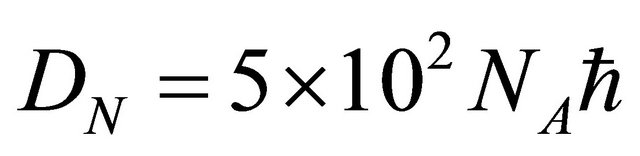 .
.
The correlation between the diffusion equation and Schrödinger equation was clarified. We revealed that the diffusion coefficient D in classical mechanics corresponds to the angular moment 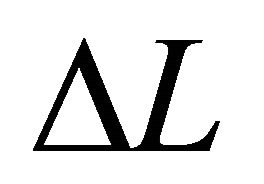 in quantum mechanics. The physical constant of
in quantum mechanics. The physical constant of
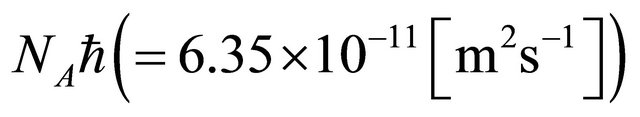 in (20) is an essential quantity in the diffusion problems.
in (20) is an essential quantity in the diffusion problems.
REFERENCES
- A. Fick, Philosophical Magazine Journal of Science, Vol. 10, 1855, pp. 31-39.
- E. Schrödinger, Annalen der Physik, Vol. 79, 1926, pp. 361-376. doi:10.1002/andp.19263840404
- T. Okino, Journal of Modern Physics, Vol. 3, 2012, pp. 1388-1393. doi:10.4236/jmp.2012.310175
- P. Langevin, Comptes Rendus de l’Academie des Sciences (Paris), Vol. 146, 1908, pp. 530-533.
- A. Einstein, Annalen der Physik, Vol. 18, 1905, pp. 549- 560. doi:10.1002/andp.19053220806
- L. Boltzmann, Wiener Berichte, Vol. 66, 1872, pp. 275- 370.

