Applied Mathematics
Vol.07 No.06(2016), Article ID:65164,14 pages
10.4236/am.2016.76050
Modeling Insecticide Resistance in Endemic Regions of Kenya
Josephine Wairimu, Marilyn Ronoh
School of Mathematics, University of Nairobi, Nairobi, Kenya

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 30 November 2015; accepted 27 March 2016; published 30 March 2016
ABSTRACT
In this study, we develop an SIS model for two types of mosquitoes, a traditional one and one that is resistant to IRS and ITNs. The resistant mosquito develops behavioral adaptation to control measures put in place to reduce their biting rate. They also bite early before dusk and later after dark when people are outside the houses and nets. We determine the effect of the two types of mosquitoes on malaria transmission in Kenya. The basic reproduction number 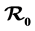 is established as a sharp threshold that determines whether the disease dies out or persists in the population. Precisely, if
is established as a sharp threshold that determines whether the disease dies out or persists in the population. Precisely, if 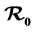 ≤ 1, the disease-free equilibrium is globally asymptotically stable and the disease always dies out and if
≤ 1, the disease-free equilibrium is globally asymptotically stable and the disease always dies out and if 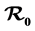 > 1, there exists a unique endemic equilibrium which is globally stable and the disease persists. The contribution of the two types of mosquitoes to the basic reproduction number and to the level of the endemic equilibrium is analyzed.
> 1, there exists a unique endemic equilibrium which is globally stable and the disease persists. The contribution of the two types of mosquitoes to the basic reproduction number and to the level of the endemic equilibrium is analyzed.
Keywords:
Malaria, Insecticide Resistance, ITNs, IRS, Reproduction Number

1. Introduction
Malaria is one of the leading causes of morbidity and mortality in Kenya and it kills an estimated 34,000 children under five every year. Economically, it is estimated that 170 million working days in Kenya are lost each year because of malaria illness.
http://kenya.usaid.gov/programs/health/72.
After 1990, pyrethroids were promoted as insecticides of choice especially for Insecticides Treated Nets (ITNs) and Indoor Residual Spray (IRS) [1] [2] , due to high efficacy, rapid rate of knockdown, strong mosquito excito-repellence and low mammalian toxicity [3] .
In Kenya, ITNs have mainly been distributed to pregnant women and children under 5 years old by the Kenya Ministry of Health and non-governmental organizations [4] [5] . Currently, ITN coverage for children under 5 years old has increased rapidly from 7% in 2004 to 67% in 2006; this increase has been associated with a 44% reduction in malaria deaths [6] . However there is an increasing case resistance of mosquitoes to pyrethroid. The likely zoonotic nature of P. falciparum and the behavioral changes of mosquitoes are many new features which indicate that malaria control is not yet achieved [7] - [9] . The gains made from ITNs and IRS therefore are threatened by the development of physiological or behavioral resistance in the malaria vectors, which is widely documented [10] . Anopheline mosquitoes exhibit two major mechanisms of pyrethroid resistance, they are:
1) Increased level of metabolic detoxification of the insecticide,
2) Reduced sensitivity in the target sites of the insecticide. The target site of the pyrethroids is the voltage- gated sodium channel.
The second type of resistance is caused when a point mutation in the region II of the para-type sodium channel genes causes a change in affinity between the insecticide and its binding site or the sodium channel, and it induces a phenotype termed knock-down resistance (KDR) in a range of insecticides [11] - [14] . Insensitivity at the sodium channel target site also leads to cross-resistance between different classes of insecticides [15] .
Reports from literature confirm that use of ITNs and IRS has led to a substantial reduction in mosquitoes, reduced malaria transmission and a 44% reduction in malaria deaths [16] - [18] . However, although there was a global reduction in overall malaria transmission, 57% of the population continued to live in areas where transmission remained moderate to intense in Africa [19] . The ITNs and IRS intervention can reduce malaria transmission by targeting mosquitoes when they feed upon sleeping humans and/or rest inside houses, livestock shelters or other man-made structures. Despite high coverage, malaria spreading mosquitoes can maintain robust transmission because they develop resistance hence limiting the achievable impact [20] [21] . High and patchy resistance to pyrethroid insecticide has been confirmed in the endemic region of western Kenya, leaving the government with limited option but to seek other control measures [7] .
In this study, we develop a mathematical model with two types of vectors, one which is sensitive to the insecticides and a resistant type which adapts easily and survives despite the two types of intervention. We assume that the An. fambiae and the An. fenestus mosquito species are either sensitive or resistant to insecticides.
In section 2 we develop the model and equations. In section 3 the basic properties of the model are shown for positive invariance and computation of the basic reproduction number is also done. Section 4, we show the local and global stability of the Disease Free Equilibrium and section 6 is the conclusion.
2. The Model Formulation and Equations
We shall subdivide the mosquito population in Western Kenya into the traditional (non resistant) group and the new resistant group. This new resistance type has been termed as a “super mosquito”, but for the sake of terminology, we shall refer to them generally as “resistant” mosquito vectors. We shall use the subscripts “n” to represent non resistant traditional vectors, while, “r” represents the resistant vectors.
In this model 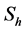 will represent the susceptible human hosts while
will represent the susceptible human hosts while 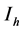 will represent the infectious human population. The variable
will represent the infectious human population. The variable 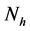 representing the total human population will be given by
representing the total human population will be given by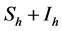 . The non resistant susceptible (infectious) vectors will be represented by
. The non resistant susceptible (infectious) vectors will be represented by 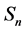 (
(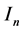 ), respectively, while the resistant vector population will be represented likewise as
), respectively, while the resistant vector population will be represented likewise as 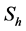 (
(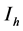 ), for the susceptible (infectious) population respectively. The total non resistant vector, therefore is given by
), for the susceptible (infectious) population respectively. The total non resistant vector, therefore is given by 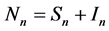 and the resistant vector population by
and the resistant vector population by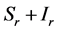 . We shall use
. We shall use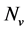 , reservedly for the total resistant and non resistant vector populations respectively, hence
, reservedly for the total resistant and non resistant vector populations respectively, hence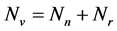 .
.
When there are no malaria deaths, the host population dynamics is given by
human and mosquito population size 

ulation size.
The non-resistant vector population defined by 


Model assupmtions:
the two types of vectors have different biting rates hence differentiated infectivity,
the two types coexist and no vector changes status during the entire life span, i.e. not resistance vector becomes non-resistant or vice versa,
The total vector and human populations are constant.
The following parameter symbols will be used in the equations:







The dynamics of our model will be governed by the following set of equations:

The term 
The transmission term 
The transmission term 
The infected hosts who recover 
The last terms 
In the susceptible vectors, 
The term 
Natural deaths affects all the groups as denoted by the parameter 

Both resistant and non resistant vectors, once infected, are assumed to remain infected till death as mosquitoes do not recover or develop immunity from the parasite [22] [23] .
All the parameters in the model are non negative and the model equations are well posed.
Equation (1) is defined in feasible region
where 



3. Well-Posedness of System
We use the relation 



3.1. A Compact Positively Invariant Set
In this section we prove that the following set
is a positively invariant compact set for system (2) by barrier theorems (e.g. [24] [25] ). Moreover 
Now we show that the vector field induced by the system is either tangent or entering 


when 






since 

since 

when 


when 


We denote the demographic equilibria by


The total human population is given by


The Integrating factor for this linear differential equation is given by
Thus
Integrating both sides we and applying the intial conditions (
The Equation for the Traditional non reistant mosquito 

The Integrating factor for this linear differential equation is given by
Thus
Integrating both sides we and applying the intial conditions (
Finally, the equation for the resistant mosquito given by
The Integrating factor for this linear differential equation is given by
Thus
Integrating both sides we and applying the intial conditions (
Thus the feasible set for the model system (1) is given by
3.2. Basic Reproduction Number

which can be simplified as

The expression 
preted from terms under the square root sign. The first term


man host. The square root sign represent the two generations that the disease has to undergo from a mosquito to a human being and to a mosquito again or vice versa for the infection to take place. It is a number that determines the threshold for disease spread, as well as a control tool that whose parameters can be targeted for control.
4. Stability of Disease-Free Equilibrium Solution
Jacobian evaluated at disease-free equilibrium solution:
Characteristic polynomial:

where
it is easy to show that
which is positive if
This means all the roots of the polynomial equations are negative, hence the system is locally asymptotically stable.
Global Stability of the DFE
The local dynamics of a general SIS and SI model is determined by the reproduction number






Theorem
If


Proof
We construct the following Lasalle-Lyapunov function 



We define
The system of ordinary differential equations given by Equation (2) can be written as

This can be written as 


If we define
then the derivative along the trajectories is given by 
We define the set
for which 











5. The Endemic Equilibrium, EE
5.1. Local Stability of the Endemic Equilibrium, EE
Theorem
The endemic equilibrium



Proof
The system of equations 5 can also be expressed as follows when we let

The Jacobian computed at the endemic equilibrium using the relations given by Equation (6) can be expressed as:
To determine the stability of the endemic equilibrium




The coefficient



The trace of J will be given as


To prove the Routh-Hurwitz stability criteria we compute 

The requirements of Routh-Hurwitz stability criteria are satisfied hence this proves that the endemic equilibrium is locally asymptotically stable
5.2. Global Stability of the EE
Theorem
The endemic equilibrium is globally asymptotically stable on 

Proof
We will prove the global stability of the Endemic Equilibrium by using the following Lyapunov function proposed by Cai and Li (2007). Thus we have:
Then the derivative of


Substituting the expressions of the model system 


where F represents the positive terms of the equation above and G represents the negative terms of the said equation. The expression of F and G are as follows:
Thus from equation 8 if 





We define the set
set 


NB: In an upcoming article, we include a human protection factor and the development of mosquito resistance during their life time. Wa also allow some resistant vectors to become sensitive to insecticides.
6. Conclusion
In this study, we formulated a malaria model representing the transmission of malaria by two types of vectors; the traditional mosquito which is sensitive to insecticides in ITNS and IRS, and a resistant type which is able to survive despite the control measures aimed at shortening their life span and limiting the biting rate. The basic reproduction number is determined as a contribution of the two types of vectors. The model is shown to be positively invariant, hence well posed. The Disease Free Equilibrium and the Endemic equilibrium are shown to be locally and globally asymptotically stable when 

Acknowledgements
We wish to thank Calistus Ngonghala for the contribution and advise he gave during the formulation of this model.
Cite this paper
Josephine Wairimu,Marilyn Ronoh, (2016) Modeling Insecticide Resistance in Endemic Regions of Kenya. Applied Mathematics,07,542-555. doi: 10.4236/am.2016.76050
References
- 1. Shanks, G.D., Hay, S.I., Omumbo, J.A. and Snow, R.W. (2005) Malaria in Kenya Western Highlands. Emerging Infectious Diseases, 11, 1425-1432.
http://dx.doi.org/10.3201/eid1109.041131 - 2. WHO (2011) Online Global Pathogen. Emerging Infectious Diseases, 7, 611.
- 3. Zaim, M. and Jambulingam, M. (2007) Global Insecticide Use for Vector-Borne Disease Control. Technical Report 3, World Health Organization.
- 4. Noor, A.M., Amin, A.A., Akhwale, W.S. and Snow, R.W. (2007) Increasing Coverage and Decreasing Inequity in Insecticide-Treated Bed Net Use among Rural Kenyan Children. PLoS Medicine, 4, e255.
http://dx.doi.org/10.1371/journal.pmed.0040255 - 5. Wacira, D., Hill, J., McCall, P.J. and Kroeger, A. (2007) Delivery of Insecticide Treated Net Services through Employer and Community Based Approaches in Kenya. Tropical Medicine & International Health, 12, 140-149.
- 6. Fegan, G.W., Noor, A.M., Akhwale, A.M., Cousens, S. and Snow, R.W. (2007) E-Ect of Expanded Insecticide-Treated Bed Net Coverage on Child Survival in Rural Kenya: A Longitudinal Study. The Lancet, 370, 1035-1039.
http://dx.doi.org/10.1016/S0140-6736(07)61477-9 - 7. Akogbeto, M.C., Djouaka, R.F. and Kinde-Gazard, R.F. (2006) Screening of Pesticide Residues in Soil and Water Samples from Agricultural Settings. Malaria Journal, 5, 22.
http://dx.doi.org/10.1186/1475-2875-5-22 - 8. Brouqui, P., Parola, P. and Raoult, D. (2012) Insecticide Resistance in Mosquitoes and Failure of Malaria Control. Expert Review of Anti-Infective Therapy, 10, 1379-1381.
- 9. Santolamazza, F., Calzetta, M., Etang, J., Barrese, E., Dia, I., et al. (2008) Distribution of Knockdown Resistance Mutations in Anopheles Gambiae Molecular Forms in West and West-Central Africa. Malaria Journal, 7, 74.
http://dx.doi.org/10.1186/1475-2875-7-74 - 10. Ramson, H., N’guessan, R., Lines, J., Moiroux, N., Nkuni, Z. and Corbel, V. (2011) Pyrethroid Resistance in African Anopheline Mosquitoes: What Are the Implications for Malaria Control. Trends Parasitol., 27, 91-98.
http://dx.doi.org/10.1016/j.pt.2010.08.004 - 11. Williamson, M.S., Martinez-Torres, D., Hick, C.A. and Devonshire., A.L. (1996) Identification of Mutations in the Houseflypara-Type Sodium Channel Gene Associated with Knockdown Resistance (kdr) to Pyrethroid Insecticides. Molecular Genetics and Genomics, 252, 51-60.
http://dx.doi.org/10.1007/BF02173204 - 12. Dong, K. (1997) A Single Amino Acid Change in the Para Sodium Channel Protein is Associated with Knockdown-Resistance (kdr) to Pyrethroid Insecticides in German Cock-Roach. Insect Biochemistry and Molecular Biology, 27, 93-100.
http://dx.doi.org/10.1016/S0965-1748(96)00082-3 - 13. Jamroz, R.C., Guerrero, F.D., Kammlah, D.M. and Kunz, S.E. (1998) Role of the kdr and Super-kdr Sodium Channel Mutations in Pyrethroid Resistance: Correlation of Allelic Frequency to Resistance Level in Wild and Laboratory Populations of Horn Flies (Haematobia irritans). Insect Biochemistry and Molecular Biology, 28, 1031-1037.
http://dx.doi.org/10.1016/S0965-1748(98)00094-0 - 14. Sina, B.J. and Aultman, K. (2001) Resisting Resistance. Trends Parasitol. Trends in Parasitology, 17, 305-306.
http://dx.doi.org/10.1016/S1471-4922(01)02007-4 - 15. Brengues, C., Hawkes, N.J., Chandre, F., Mccarroll, L., Duchon, S., Guillet, P., Manguin, S., Morgan, J.C. and Hemingway, J. (2003) Pyrethroid and ddt Cross-Resistance in Aedes Aegypti Is Correlated with Novel Mutations in the Voltage-Gated Sodium Channel Gene. Medical and Veterinary Entomology, 17, 87-94.
http://dx.doi.org/10.1046/j.1365-2915.2003.00412.x - 16. WHO (2013) World Malaria Report 2013 Geneva. WHO, Geneva.
- 17. WHO (2014) Global Strategic Framework for Integrated Vector Management. World Health Organization, Geneva.
- 18. Chouaïbou, M., Ngufor, C., Tchicaya, E., Loukou, B., Kesse, N., N’Guessan, R., et al. (2014) Combining Organophosphate-Treated Wall Linings and Long-Lasting Insecticidal Nets Fails to Provide Additional Control over Long-Lasting Insecticidal Nets alone against Multiple Insecticide-Resistant Anopheles Gambiae in Côte D’ivoire: An Experimental Hut Trial. Malaria Journal, 13, 396.
http://dx.doi.org/10.1186/1475-2875-13-396 - 19. Noor, A.M., Kinyoki, D.K., Mundia, C.W., Kabaria, C.W., Mutua, J.W., Alegana, V.A., et al. (2014) The Changing Risk of Plasmodium Falciparum Malaria Infection in Africa 2000-10, a Spatial and Temporal Analysis of Transmission Intensity. The Lancet, 383, 1739-1747.
http://dx.doi.org/10.1016/S0140-6736(13)62566-0 - 20. Service, M.W. (1997) Mosquito (Diptera: Culicidae) Dispersal the Long and Short of It. Journal of Medical Entomology, 34, 579-588.
http://dx.doi.org/10.1093/jmedent/34.6.579 - 21. Killeen, G.F. (2014) Characterizing, Controlling and Eliminating Residual Malaria Transmission. Malaria Journal, 13, 330.
http://dx.doi.org/10.1186/1475-2875-13-330 - 22. Bailey, N.J.T. (1975) The Mathematical Theory of Infectious Diseases and Its Application. 2nd Edition, Macmillan Publishers.
- 23. Hethcote, H.W. (1976) Qualitative Analysis of Communicable Disease Models. Mathematical Bio-Sciences, 28, 335-356.
- 24. Bony, J.-M. (1969) Principe du maximum, inégalite de Harnack et unicité du problème de Cauchy pour les opérateurs elliptiques dégénérés. Annales de l’institut Fourier (Grenoble), 19, 277-304.
http://dx.doi.org/10.5802/aif.319 - 25. Quincampoix, M. (1992) Di-Erential Inclusions and Target Problems. SIAM Journal on Control and Optimization, 30, 324-335.
http://dx.doi.org/10.1137/0330020









































