Advances in Bioscience and Biotechnology
Vol.1 No.3(2010), Article ID:2368,4 pages DOI:10.4236/abb.2010.13029
Properties of crude laccase from Trametes versicolor produced by solid-substrate fermentation
![]()
1Department of Biotechnology, University of Food Technologies, Plovdiv, Bulgaria;
2Department of Automatics, Information and Control Systems, University of Food Technologies, Plovdiv, Bulgaria.
Email: abt-kr@rocketmail.com
Received 7 April 2010; revised 22 April 2010; accepted 25 April 2010.
Keywords: Laccase; Solid-Substrate Fermentation; Trametes versicolor; Dye Decolorization
ABSTRACT
A low-cost process for the production of laccases is necessary for a sustainable enzymatic wastewater treatment. Therefore, it is necessary to establish an easy and low-cost procedure for the production of laccase. In the present study the properties of crude laccase from Trametes versicolor produced by solidsubstrate fermentation is investigated. The application of the enzyme for dye decolorization is also studied. Crude laccase from the studied culture established maximal activity at 45ºC. The enzyme retained over 90% of its activity in the temperature range 40- 47ºC and pH 4.5. The kinetic constants of the crude enzyme was also determined. In the presence of KCl, NaCl, CaCl2, MnSO4 and MgSO4, laccase demonstrated high stability—over 50% of its initial activity was still retained after 4-month incubation. Complete loss of enzymatic activity was observed in the presence of CuCl2, FeCl2, FeCl3 and NaN3 after 30 min of incubation. 100% decolorization by investigated crude laccase was completed in the case of Indigo Carmine for 4 h, Remazol Brilliant Blue R—for 6 h, Orange II— for 48 h and Congo Red—for 13 d.
1. INTRODUCTION
Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) are extracellular, multicopper enzymes that use molecular oxygen to oxidize various aromatic and nonaromatic compounds by a radical-catalysed reaction mechanism [1]. Laccases can convert o-and p-diphenols, aminophenols, methoxy-substituted phenols, benzenethiols, polyphenols, polyamines, hydroxyindols, some aryl diamines and a considerable range of other compounds but do not oxidize tyrosine (whereas the tyrosina ses do).
Laccases can be very strongly inhibited by various reagents. Small anions such as azide, halides, cyanide, thiocyanide, fluoride and hydroxide bind to the type 2 and type 3 Cu, resulting in the interruption of internal electron transfer and inhibition of activity. Other inhibitors include metal ions (e.g. Hg2+), fatty acids, sulfhydryl reagents, hydroxyglycine, kojic acid, desferal and cationic quaternary ammonium detergents the reactions with which may involve amino acid residue modifications, conformational changes or Cu chelation [2,3]. Regarding conformational changes, it is known that these are highly dependent on the state of oxidation of the copper atoms. Laccases generally are more stable at alkaline pH than at acidic pH, probably due to the OH− inhibition of autooxidation.
Laccases are increasingly being used in a wide variety of industrial oxidative processes such as delignification, dye or stain bleaching, bioremediation, plantfibre modification, ethanol production, biosensors, biofuel cells, etc. Industrial uses require overproduction of the enzyme, generally in a heterologous host, as an indispensable prerequisite. Indeed, most commercial laccases are produced in Aspergillus hosts. The functional expression of the Myceliophthora thermophila laccase in S. cerevisiae by directed molecular evolution has been reported, which enables this system to be tuned up for new and challenging applications [4]. Many white-rot fungi produce multiple laccase isoforms under the appropriate inductive conditions [5,6]. Most fungal laccases studied are extracellular proteins, but intracellular laccases have been detected in some fungi [7].
The genus Trametes, which belongs to the white-rot fungi, is assumed to be one of the main producers of laccases. T. versicolor produces laccase and MnP as major ligninolytic enzymes; however, the role of these enzymes in decolorization of azo dyes is not yet clear. Laccase and/or MnP activities in culture filtrate of T. versicolor were not able to decolorize azo dyes, thus indicating a role of other enzymes or cell-bound components in azo dye degradation [8].
In order to produce laccases, white-rot fungi have to be cultured under specific conditions. Two types of culture techniques are used: Solid-state fermentation (SSF) and Submerged fermentation (SmF). The SSF is defined as any fermentation process occurring in absence or near absence of free liquid, using an inert substrate or a natural substrate as a solid support [9]. The former works as an attachment place for the microorganism, whereas the latter also acts as a carbon source, which considerably reduces the production costs [10]. SSF is advantageous in obtaining concentrated metabolites and subsequent purification procedures are economical [11,12]. In SSF, the microorganisms grow under conditions close to their natural habitat. This may allow them to produce certain enzymes and metabolites, which usually would not be produced or would only be produced at a low yield in SmF [9]. Therefore, the selection of an adequate support is essential, since the success of the process depends on it.
Laccases have become important, industrially relevant enzymes that can be used for a number of diverse applications, including biocatalytic purposes such as delignification of lignocellulosics and crosslinking of polysaccharides, bioremediation applications such as waste detoxification and textile dye transformation [13], food technological uses, personal and medical care applications [14], and biosensor and analytical applications [15]. In view of the broad biotechnological applications of laccases [16-18], there is a scientific need to identify different sources of laccases having diverse properties so that suitable laccases for various applications could be identified.
The enzymatic treatment of wastewater requires the production of large amounts of enzymes, in this case laccases, at low cost. The current commercial price of laccases is high, constituting a drawback for its use. A low-cost process for the production of laccases is necessary for a sustainable enzymatic wastewater treatment. Therefore, it is necessary to establish an easy and low-cost procedure for the production of laccase. In the present study the properties of crude laccase from SSF culture of Trametes versicolor is investigated. The application of the enzyme for dye decolorization is also studied.
2. MATERIALS AND METHODS
2.1. Microorganisms and Inoculum
A fungal strain of Trametes versicolor 1A collected from hills in the city of Plovdiv, Bulgaria was used in this work. The culture belongs to the collection of the Department of “Biotechnology” at the University of Food Technologies in Plovdiv-Bulgaria. The culture is maintained on 2% lima bean agar plates and slants at 4oC. For enzyme production, a 7-day old plate culture grown on 2% potato dextrose agar (PDA) was used. Mycelial inoculum was prepared by inoculating 107 spores of fungus from agar-slant culture to 300 ml shake flask containing 50 ml beer must 7.5oB. The pH of the media was adjusted with 1M NaOH to 6.5. The inoculated flasks were incubated at 30oC and 220 rpm for 72 h.
2.2. Solid-Substrate Fermentation (SSF)
The SSF was carried out using a medium consisted of 4.0 g wheat bran, 2.5 g oats straw and 2.5 g beetroot press in 300 ml flasks. The moist of the substrate was adjusted to 60% by salt solution containing (%): (NH4)2SO4—0.14; KH2PO4—0.2; MgSO4.7H2O—0.03; CaCl2—0.03; FeSO4.7H2O, ZnSO4.7H2O, MnSO4.7H2O and CoCl2 —0.002 (pH 4.5). After autoclaving (121oC for 30 min) and cooling, the substrate was inoculated with the appropriate mycelial inoculum prepared as mentioned above. SSF fermentation with monocultures was used as reference. All flasks were cultivated at 30oC for 7 days. Triplicate flaks were set up for each experimental variation.
2.3. Enzyme Extraction
The crude enzyme extract was obtained by adding 50 ml distilled water to the fermented matter. The flasks were mixed for 30 min at room temperature (25oC) using a shaker (220 rpm). Solids were removed first by filtering and then by centrifuging at 5000 g for 5 min. The supernatant obtained was used as crude enzyme extract.
2.4. Laccase Activity Measurement
Laccase activity was assayed according to Marbah et al. [20] using syringaldazine as a substrate. One unit of laccase activity was defined as 0.001 ∆A530 for 1 min, pH 4.5 and 30oC
2.5. Determination of the Kinetic Parameters (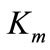 and
and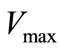 ) of the enzyme
) of the enzyme
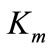 and
and 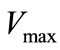 values were determined using 4-hydroxy-3,5-dimethoxybenzaldehyde azine (syringaldazine) and 2,2´-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) as substrates [20] at different concentrations (0.01 ÷ 0.1 mM) in 0.2M citrate-phosphate buffer (pH 4.5). The computing procedures were performed by using a package of applied software programs of own development in the software media of Matlab and Eureka [21,22]. For analyzing the results two approaches were used:
values were determined using 4-hydroxy-3,5-dimethoxybenzaldehyde azine (syringaldazine) and 2,2´-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) as substrates [20] at different concentrations (0.01 ÷ 0.1 mM) in 0.2M citrate-phosphate buffer (pH 4.5). The computing procedures were performed by using a package of applied software programs of own development in the software media of Matlab and Eureka [21,22]. For analyzing the results two approaches were used:
Michaelis-Menten 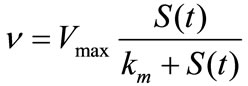 (1)
(1)
Reversible substrate inhibition
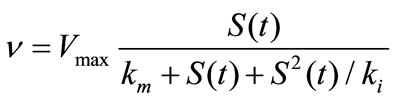 (2)
(2)
The kinetic parameters in each of the models were calculated according to the optimization procedure which minimized a criterion of the type:
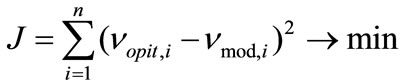 , (3)
, (3)
where 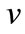 is the initial velocity of the enzyme reaction calculated by (1) and (2) and
is the initial velocity of the enzyme reaction calculated by (1) and (2) and 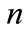 is the number of analyzing points.
is the number of analyzing points.
2.6. Effect of Ph on the Activity and Stability of the Enzyme
Laccase activity is a function of pH measured in 0.2M citrate-phosphate buffer over the range from 1.8 to 6.6. The highest activity was set as 100% of relative activity. For the stability experiments, the crude extract was incubated under initial conditions for 30 minutes at 30oC and residual laccase activity was determined at intervals according to the standard conditions cited above. The buffers used in that study were: citrate (pH 3.0, 3.5, 4.0), acetate (pH 4.0, 4.5, 5.0, 5.5), phosphate (pH 5.5, 6.0, 6.5, 7.0).
2.7. Effect of Temperature on the Activity and Stability of the Enzyme
Laccase activity was measured at pH 4.5 in 0.2M citrate-phosphate buffer at different temperatures over the range 10-70oC. The enzyme’s thermal stability was assayed by incubating the crude extract at different temperatures in the range of 30-70oC at different times using 0.2M citrate-phosphate buffer. After the incubation, the crude extract was cooled and laccase activity was determined at standard conditions.
2.8. Effect of Ions
The crude extract was incubated with different ionic solutions: KCl, ZnCl2, CuCl, Pb(NO3)2, AgNO3, ZnSO4, MgSO4, MnSO4, NaCl and CaCl2 at 10 mM; NaN3 at 1.0 mM and residual laccase activity was determined at intervals according to the standard conditions cited above with syringaldazine as substrate. The activity of the enzyme immediately after ultrafiltration was set as 100% of relative activity.
2.9. Removing of Salts (Ultrafiltration)
Removing of salts in crude enzyme extract was carried out in ultrafiltration module by pressure of nitrogen of 0.3 МРа. Cellulose acetate membrane (500 Da) was used. The crude enzyme extract was rinsed and filtered until conductivity of 56 μS was achieved (Tungsram’s “Radelkis” conductometer).
2.10. Dye Decolorization Studies
Crude enzyme extract was collected at the maximum laccase activity (day 7), filtered, clarified by centrifugation at 8000 g for 15 min, frozen, defrosted and then filtered to remove the precipitated polysaccharides. The resulting clear filtrate was concentrated by ultrafiltration (as described above). In vitro decolorization experiments were performed with the concentrated clear filtrate. For the purpose of the investigation the crude enzyme extract was diluted twice, thus we used two samples—with 2000 and 1000 U/ml laccase activity. The dyes tested for the in vitro studies were: indigo carmine (indigoid) CI 73015, phenol red (sulfonaphthalein), Remazol Brilliant Blue R, and the azo-dyes Orange II and Congo Red. All were purchased from Aldrich (St. Louis, MO, USA) and were used in a concentration of 0.01%. The reaction mixture for dye decolorization consisted of equal volumes of an aqueous solution of dye and crude laccase (2000 or 1000 U/ml) in citrate phosphate buffer (pH 5.0). The residual dye concentration was measured spectrophotometrically at 608 nm for indigo carmine, 587 nm for Remazol Brilliant Blue R, 483 nm for Orange II, 497 nm for Congo Red and 475 nm for phenol red. A control test containing the same amount of a heatdenatured laccase was performed in parallel. The assays were done thrice, the experimental error being below 3%.
3. RESULTS AND DISCUSSIONS
3.1. Effect of pH and Temperature on Laccase Activity and Stability
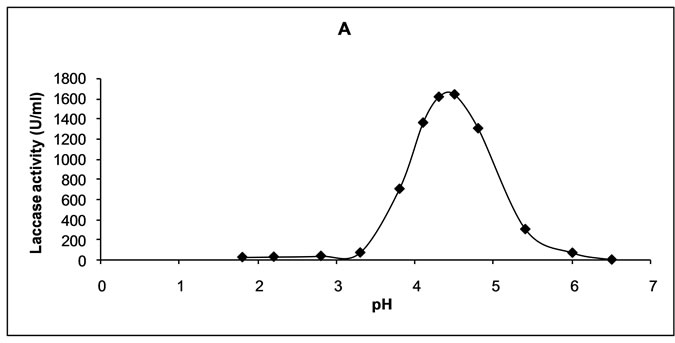
Most of the fungal laccase have optimum pH in the range of 3.0-4.0 and temperature in the range of 40-60ºC for oxidation of phenolic compounds. Crude laccase produced by Trametes versicolor 1A was found to express maximal activity at pH 4.5 (Figure 1(a)). The enzyme activity was also high in the pH range between 4.1 and 4.8, being respectively 99.3% and 95.2% of the maximum. Crude laccase was proved active in the range of pH modification from 3.3 to 6.0.
Crude laccase from the studied culture established maximal activity at 45ºC (Figure 1(b)). The enzyme retained over 90% of its activity in the temperature range 40-47ºC, the respective values being 94.2% and 93.3% of the maximum. Within the range 30-50ºC the loss of enzymatic activity was below 30 %, and in particular— 28.9% and 23.2%. At 60ºC the loss of laccase activity was 83.6%.
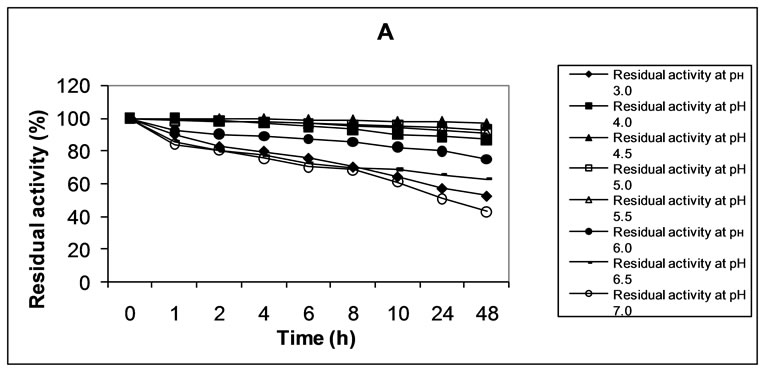
In the range of pH values between 3.0 and 7.0 the enzyme was found to be most stable at pH 4.5 (Figure 2(a)). During the 48-hour follow-up of the investigation the enzyme activity was found to decrease only by 3.4%.
 (a)
(a)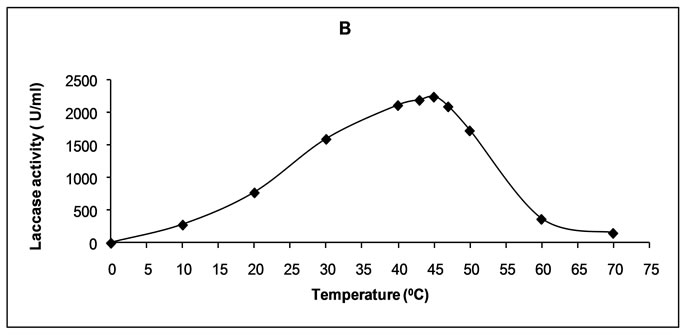 (b)
(b)
Figure 1. Effect of pH (a) and temperature (b) on the activity of the enzyme.
Minor losses of enzyme activity were registered at pH 5.0 and 5.5, as well, namely 7.1% and 9.2% for 48 h. Crude laccase was assessed as being most unstable at pH 7.0 – the reduction of enzymatic activity was by 57.2% for 48 h.
The thermal stability of crude laccase was followed within the temperature interval 30-60oC (Figure 2(b)). The greatest stability of the enzyme was observed at 30oC – the residual activity after 48 h incubation was 97.2%. The enzyme was still stable at 40oC – the loss of enzymatic activity for the same period of incubation was 9.8%. At 50oC the losses of enzymatic activity were 53.4 % for 6 h and 90.0% for 48 h incubation. After 6 h incubation at 60oC the loss of activity reached 58.0%, while after 48 h incubation no enzymatic activity was registered. At 70oC the enzyme had lost 53.5% of its initial activity in 5 min, and had been completely inactivated in 20 min (data not shown).
3.2. Kinetic Analysis
Two compounds, ABTS and syringaldazine, were used as substrates for measuring the kinetic constants of crude laccase. The results from the investigation of process dynamics in the case of ABTS and syringaldazine as substrates are presented in Table 1.
The conditions for setting in the establishment of substrate inhibition are specified in (2): (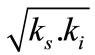
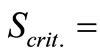 ). That correlation, as well as (3), was used as the grounds for an a priori choice of model type. The values of the kinetic parameters calculated by the two models, and the values of
). That correlation, as well as (3), was used as the grounds for an a priori choice of model type. The values of the kinetic parameters calculated by the two models, and the values of 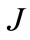 and
and 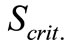 criteria for
criteria for
 (a)
(a)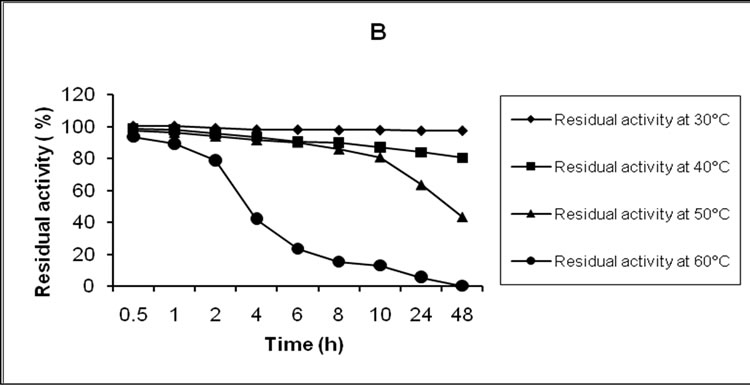 (b)
(b)
Figure 2. Effect of pH (a) and temperature (b) on the stability of crude laccase.
the substrates ABTS and syringaldazine are presented in Table 2.
For ABTS substrate the value of the parameter 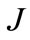 calculated by model (2) was one order below that obtained by model (1). On the other hand, So = 0.1 [mM] turned to be only 10% lower than.
calculated by model (2) was one order below that obtained by model (1). On the other hand, So = 0.1 [mM] turned to be only 10% lower than.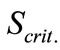 Therefore, in the case of ABTS substrate, more appropriate descriptor of process dynamics proves to be the model taking into account the presence of reversible substrate inhibition (2).
Therefore, in the case of ABTS substrate, more appropriate descriptor of process dynamics proves to be the model taking into account the presence of reversible substrate inhibition (2).
For syringaldazine, model (1), was found to be more suitable for investigating the dynamics of the enzymatic reaction, since the value of 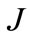 was once again an order below the value obtained by model (2) but So = 0.1 [mM] was noticeably lower than
was once again an order below the value obtained by model (2) but So = 0.1 [mM] was noticeably lower than 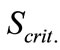 = 0.145 (by. 45 %)—i.e. in that case there was a marked substrate limitation under the conditions of the experiment.
= 0.145 (by. 45 %)—i.e. in that case there was a marked substrate limitation under the conditions of the experiment.
The kinetic constants determined are quite comparable with those reported for other Trametes spp. The value of 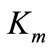 obtained for sustrate ABTS was identical with the data announced for laccase from T. trogii [23] but disagreed with the value cited for laccase from Pycnoporus sanguineus SCC 108 [24].
obtained for sustrate ABTS was identical with the data announced for laccase from T. trogii [23] but disagreed with the value cited for laccase from Pycnoporus sanguineus SCC 108 [24].
The 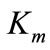 index for syringaldazine substrate was lower than the value of the index established for ABTS substrate, pointing out the greater affinity of the investigated
index for syringaldazine substrate was lower than the value of the index established for ABTS substrate, pointing out the greater affinity of the investigated
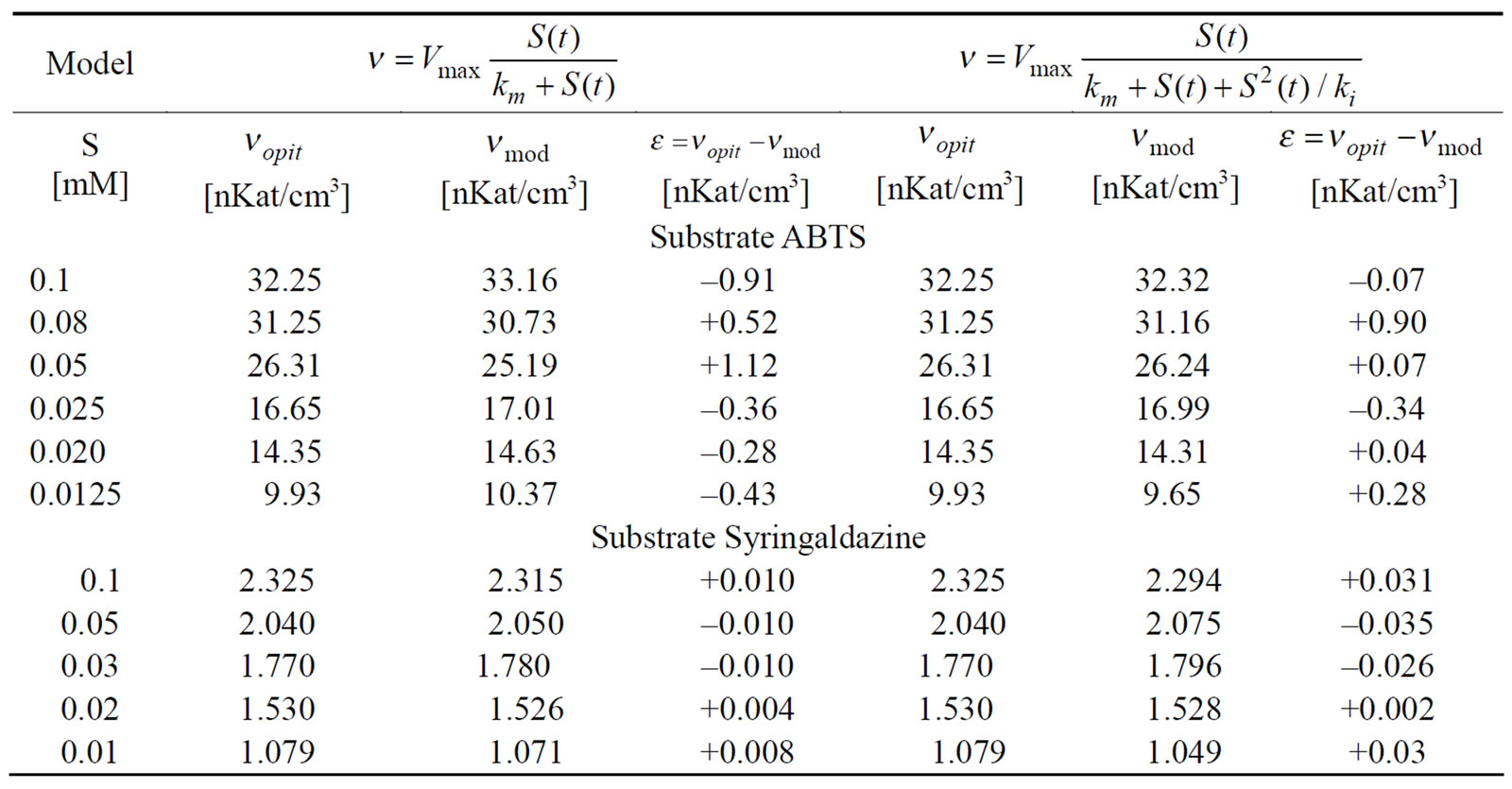
Table 1. Dynamics of the process using model (1) and (2) for ABTS and Syringaldazine.
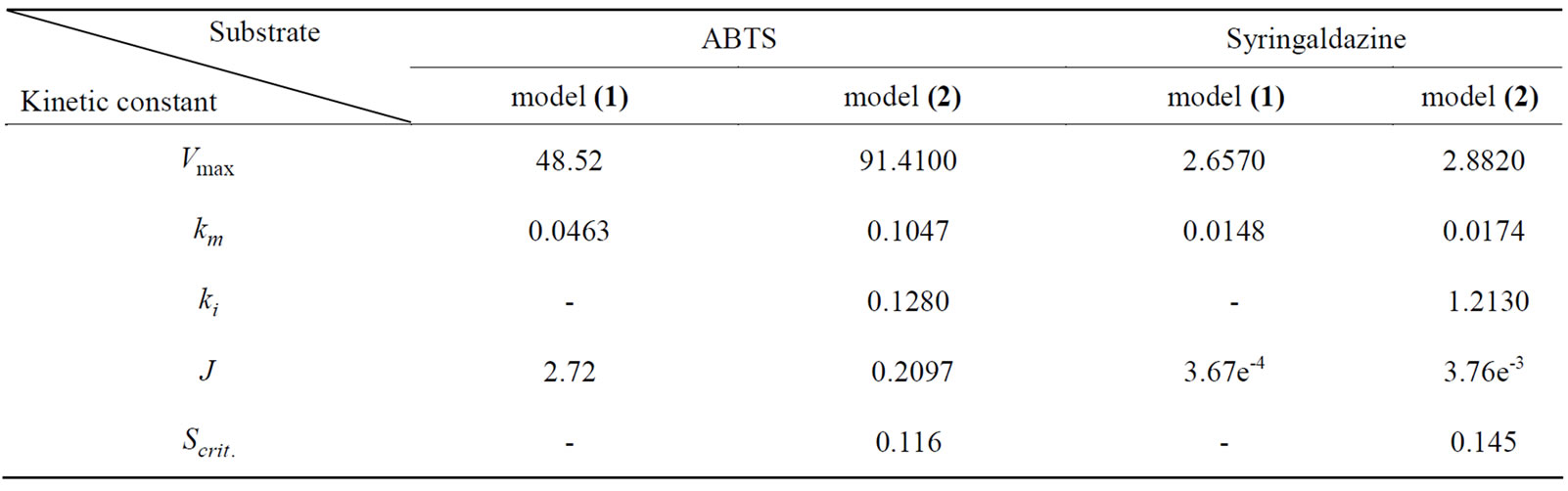
Table 2. Kinetic constant using model (1) and (2) for ABTS and Syringaldazine.
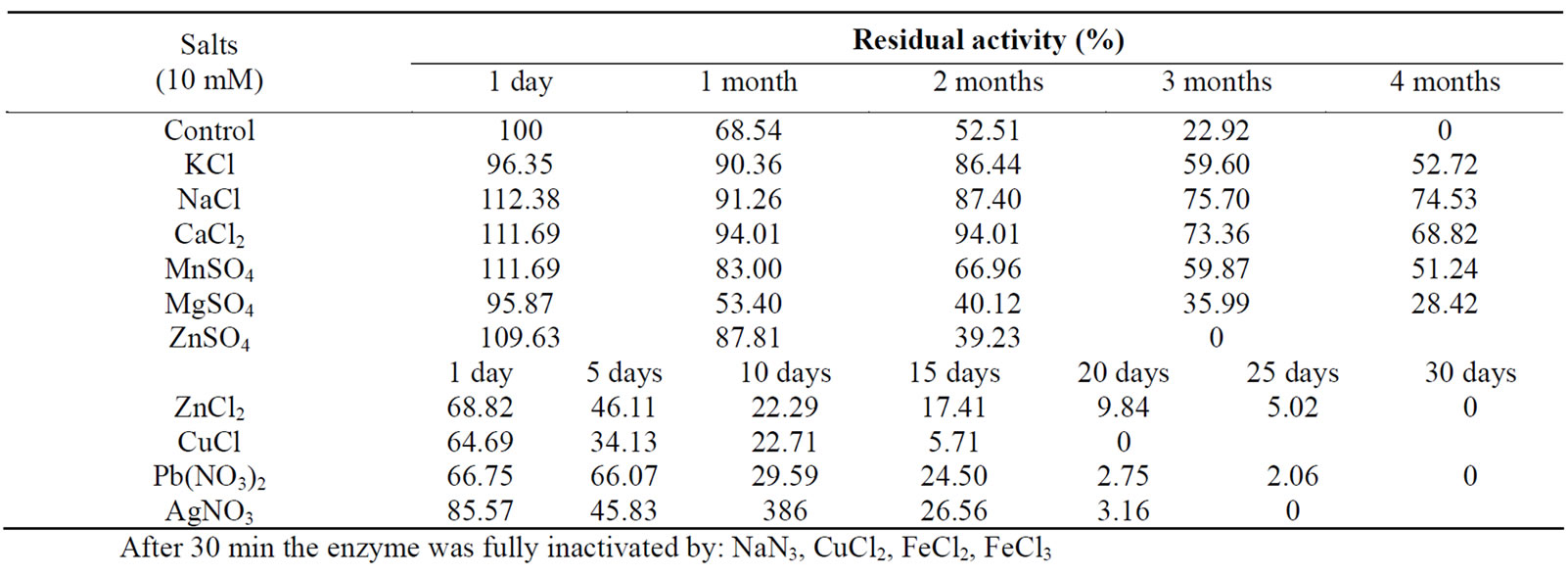
Table 3. Effect of ions on enzyme activity.
laccase to the first of the substrates.
3.3. Effect of Ions on Enzyme Activity
Laccase in crude enzyme extract (control) was found to lose its activity after an incubation period of 3 months (Table 4). In the presence of 5 of the investigated salts— KCl, NaCl, CaCl2, MnSO4 and MgSO4, laccase demonstrated high stability—over 50% of its initial activity was still retained after 4-month incubation. It has been assumed that in the presence of various salts enzymes are more substantially influenced by cations. Data from our study revealed that the strongest positive impact on enzyme’s molecule was exercised by Na(I) ions, ensuring the molecule stabilization for a period of 4 months with the least loss of enzymatic activity—25%.
On the other extreme was the effect of Zn(ІІ) and Cu(І) ions introduced into the medium as chlorides – their availability destabilized the enzyme molecule making it much more unstable than crude laccase; in the presence of the above cited salts a complete loss of enzymatic activity was registered after 25 and 15 d (in the respective case). In principle, heavy metals have been known to posses a negative impact on enzymes and this was confirmed by the results from our study. It is a clear fact, however, that their destabilizing influence does not go beyond the influence of ZnCl2 and CuCl.
The suggestion that Na(I) added in the form of NaCl have a positive effect in terms of laccase stability was not confirmed by the experiment with NaN3, in which after 30 min of treatment a complete loss of enzymatic activity was registered. It could be assumed that in the specific case dominated the influence of the respective anion. Azide is a very effective inhibitor of laccase II, and complete inhibition was observed with 1.0 mM NaN3. Azide is thought to bridge both the type 2 and type 3 Cu in laccase. Our results are with correspondence with [25,26]. They reported for full or 98% inhibition of laccase activity of some basidiomycetes and ascomycetes by 1.0 mM sodium azide but for shorter period of time to compare to our crude enzyme.
Complete loss of enzymatic activity was observed in the presence of CuCl2, FeCl2 and FeCl3 after 30 min of incubation, suggesting that Cu(І) and Cu(ІІ) had a negative impact on the enzyme, regardless of being its cofactors. Probably that finding could be attributed to the high concentration of the introduced salts.
3.4. Dye Decolorization Studies
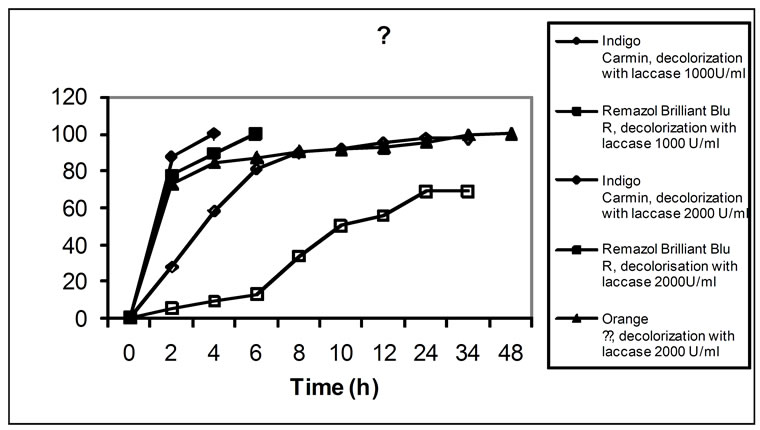
The ability of white-rot fungi to decolorize synthetic dyes has been widely studied, particularly with Phanerochaete chrysosporium and Trametes versicolor [27]. In the present study, we assessed the ability of the crude laccase (extracted fluid) from SSF culture of T. versicolor, to decolorize five structurally different synthetic dyes. The decoloration of type model dyes is a simple method to assess the aromatic degrading capability of ligninolytic enzymes [28]. Dye decolorization by laccase is a property of the crude enzyme that underlies one of its possible applications. The results concerning dye decolorization by laccase with enzymatic activities of 1000 and 2000 U/ml are presented on Figures 3(a) and (b). The application of enzyme solutions with higher activity proved better results for each of the five dyes studied. As it can be seen in Figure 3, the decoloration rate obtained was very different in each case. 100% decolorization by laccase (2000 U/ml activity) was completed in the case of Indigo Carmine for 4 h, Remazol Brilliant Blue R—for 6 h, Orange II—for 48 h and Congo Red—for 13 d. No complete decolorization of Phenol Red was achieved. Decol orization of Indigo Carmine by means of laccase with
 (a)
(a)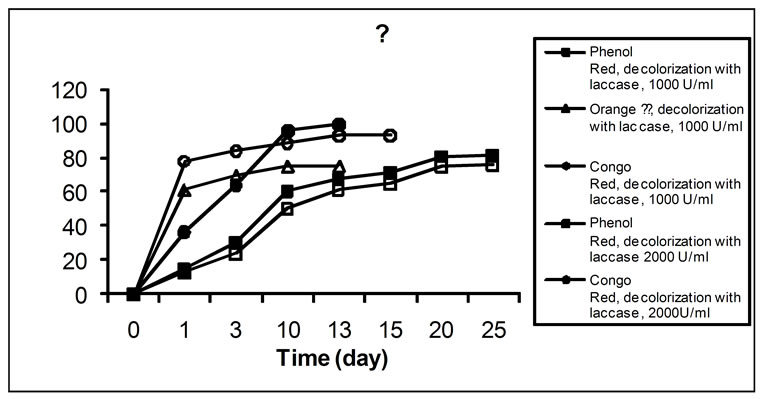 (b)
(b)
Figure 3. Decolorization (%) of various dyes (a), (b) by laccase from T. versicolor 1A
1000 U/ml activity was 97.5% for 34 h, of Remazol Brilliant Blue R—69.1% for 24 h, of Orange ІІ—75.3% for 13 d, and of Congo Red—93.4% for 13 d. In summary, laccase decolorized most readily the indigo dye Indigo Carmine, followed by the anthracene derivative Remazol Brilliant Blue R, while the decolorization of the azo-dyes Orange II and Congo Red was much slower. This could be due to either enzyme inhibition by some products generated in the decoloration process or substrate inhibition. These results differ from those found by Soares et al. [29], who reported that the addition of a redox mediator was necessary for azo-dyes decoloration by a commercial laccase from Novo Nordisk, Denmark (produced by SmF of a genetically modified Aspergillus microorganism). The discrepancy between our results and those from Soares et al. [29] could be due to the difference in fungal species from which the laccase was obtained, the different culture medium and/or the technique used. In addition, the redox potential of laccases varies depending on the laccase source [30], which could also dictate the need of a redox mediator for the decoloration of a particular dye to occur. When a commercial laccase was used, azo-dye decoloration was significantly lower than that attained by laccase from SSF cultures of T. versicolor. Since equal doses of laccases were used in the decoloration process, the difference in the decoloration efficiency of the two laccases was most likely due to the difference in laccase isoenzymes produced by the different strains as well as to the difference in specificities to different dyes of diverse structures [31]. In addition, as commented above, it could also be due to the difference in the redox potential of laccases from different microorganisms.
Decolorization of the dyes covered by our study has been reported for laccase produced by Trametes versicolor ATCC 200801, as well, but authors do not specify dye concentrations [32]. Regarding T. versicolor CC BAS614, over 50% degradation of Remazol Brilliant Blue R и Orange 16 for 14 days has been reported [33]. Our data suggests that a more active form of the enzyme allows for the achievement of a complete or at least a higher degree decolorization, as well as for a successful reduction of decolorization time. The influence of laccase activity on dye decolorization is another topic not discussed in scientific literature.
4. CONCLUSIONS
The crude enzyme laccase from SSF culture of Trametes versicolor 1A was isolated and characterized. The unpurified enzyme was very stable with very good potential for application for industrial wastewater treatment.
On one hand, the results clearly showed the enormous potential of wheat bran, oats straw and beetroot press as a support-substrates for production of laccase at low cost by T. versicolor under solid-state conditions. In addition, the laccase produced presented a highly decolorizing ability, especially for indigo and anthracene dyes. This makes laccase from this fungus very attractive for further investigations as well as for its application to different biotechnology areas.
5. ACKNOWLEDGEMENTS
This work was supported by national Science Fund, Bulgaria.
REFERENCES
- Thurston, C.F. (1994) The structure and function of fungal laccases. Microbiology, 40, 19-26.
- Gianfreda, L., Xu, F. and Bollag, J. (1999) Laccases: A useful group of oxidoreductive enzymes. Bioremediation Journal, 3(1), 1-25.
- Call, H.P. and Mucke, I. (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym(R)-process). Journal of Biotechnology, 53(2), 163-202.
- Bulter, T., Alcalde, M., Sieber, V., Meinhold, P., Schlachtbauer, C. and Arnold, F.H. (2003) Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Applied and Environment Microbiology, 69(2), 987-995.
- Eggert, C., Temp, U. and Eriksson, K.E.L. (1996) Laccase-producing white-rot fungus lacking lignin peroxidase and manganese peroxidase. ACS Symposium Series, 655, 130-150.
- Solomon, E.I., Sundaram, U.M. and Machonkin, T.E. (1996) Multicopper oxidases and oxygenases. Chemistry Reviews, 96(7), 2563-2605.
- Schlosser, D., Grey, R. and Fritsche, W. (1997) Patterns of ligninolytic enzymes in Trametes versicolor. Distribution of extraand intracellular enzyme activities during cultivation on glucose, wheat straw and beech wood. Applied Microbiology and Biotechnology, 47, 412-418.
- Swamy, J. and Ramsay, J.A. (1999) Effects of Mn2+ and NH4+ concentrations on laccase and manganese peroxidase production and Amaranth decoloration by Trametes versicolor. Applied Microbiology and Biotechnology, 51, 391-396.
- Pandey, A., Selvakumar, P., Soccol, C.R. and Nigam P. (1999) Solid state fermentation for the production of industrial enzymes. Current Science, 77(1), 149-162.
- Murthy, M.V.R., Karanth, N.G. and Raghava Rao, K.S.M.S. (1993) Biochemical engineering aspects of solid-state fermentation. Advances in Applied Microbiology, 38, 99-147.
- Ashakumary, L., Selvakumar, P.S. and Pandey, A. (1994) Column fermentor for solid state fermentation. In: Pandеy, A., Ed., Solid State Fermentation, Wiley Eastern Limited/Newage International Publishers, 33.
- Viniegra-González, G., Favela-Torres, E., Aguilar, C., Romero-Gómez, J., Díaz-Godínez, G. and Augur, C. (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering, 13(2-3), 157-167.
- Gianfreda, L., Xu, F. and Bollag, J.M. (1999) Enzymatic oxidative transformation of chlorophenol mixtures. Journal of Environmental Quality, 32(1), 63-69.
- Xu, F. (1999) Laccase. In: Michael, C.F. and Stephen, W.D., Ed., Encyclopedia of Bioprocess Technology: Fermentation, Biocatalysis, and Bioseparation, Wiley, NY, 1545-1554.
- Yaropolov, A.I., Skorobogat’ko, O.V., Vartanov, S.S. and Varfolomeyev, S.D. (1994) Laccase: Properties, catalytic mechanism, and applicability. Applied Biochemistry and Biotechnology, 49(3), 257-280.
- Rodriguez, C.S. and Herrera, J.L.T. (2006) Industrial and biotechnological applications of laccases: A review. Biotechnology Advances, 24(5), 500-513.
- Camarero, S., Lbarra, D., Marinez, A.T., Romero, J., Gutierrez, A. and Del Rio, J.C. (2007) Paper pulp delignification using laccase and natural mediators. Enzyme and Microbial Technology, 40(5), 1264-1271.
- Myasoedova, N.M., Chernykh, A.M., Psurtseva, N.V., Belova, N.V. and Golovleva, L.A. (2008) New efficient producers of fungal laccases. Applied Biochemistry and Microbiology, 44(1), 73-77.
- Marbach, I., Harel, E. and Mayer, A.M. (1985) Pectin, a second product for laccase production by Botrytis cinerea. Phytochemistry, 24(11), 2559-2561.
- Johanes, C. and Majcherczyk, A. (2000) Laccase activity test and laccase inhibitors. Journal of Biotechnology, 78(2), 193-199
- Mathews, J. and Fink, K. (2001) Numerical methods using MATLAB. Prentice Hall, Upper Saddle River, NJ.
- Stanchev, V., Stoilova, I. and Krastanov, A. (2008) Biodegradation dynamics of high catechol concentrations by Aspergillus awamori. Journal of Hazardous Materials, 154(1-3), 396-402.
- Zouari-Mechichi, H., Mechichi, T., Dhouib, A., Sayadi, S., Martínez, A.T. and Martínez, M.J. (2006) Laccase purification and characterization from Trametes trogii isolated in Tunisia: decolorization of textile dyes by the purified enzyme. Enzyme and Microbial Technology, 39(1), 141-148.
- Litthauer, D., Vuuren, M.J., Tonder, A. and Wolfaardt, F. (2007) Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108). Enzyme and Microbial Technology, 40(4), 563-568.
- Palonen, H., Saloheimo, M., Viikari, L. and Kruus, K. (2003) Purification, characterization and sequence analysis of a laccase from the ascomycete Mauginiella sp. Enztme and Microbial Technology, 33(6), 854-862.
- Jordaan, J., Pletschke, B.I. and Leukes, W.D. (2004) Purification and characterization of a thermostable laccase from an unidentified basidiomycete. Enzyme and Microbial Technology, 34(6), 549-554.
- Banat, I.M., Nigam, P., Singh, D. and Marchant, R. (1996) Microbial decolourization of textile-dye-containing effluents: A review. Bioresource Technology, 58(3), 217- 227.
- Novotný, C., Rawal, B., Bhatt, M., Patel, M., Sasek, V. and Molotoris, H.P. (2001) Capacity of Irpex lacteus and Pleurotus ostreatus for decolorization of chemically different dyes. Journal of Biotechnology, 89(2-3), 113-122.
- Soares, G.M.B., Pessoa de Amorim, M.T. and Costa-Ferreira, M. (2001) Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue R. Journal of Biotechnology, 89(2-3), 123-129.
- Li, K., Xu, F. and Eriksson, K.L. (1999) Comparison of fungal laccases and redox mediators in oxidation of a non-phenolic lignin model compound. Applied and Environmental Microbiology, 65(6), 2654-2660.
- Nyanhongo, G.S., Gomes, J., Gübitz, G.M., Zvangya, R., Read, J. and Steiner, W. (2002) Decolourisation of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Research, 36(6), 1449-1456.
- Birhanli, E. and Yesilada, O. (2006) Increased production of laccase by pellets of Funalia trodii ATTC 200800 and Trametes versicolor ATCC 200801 in repeated-batch mod. Enzyme and Microbial Technology, 39(6), 1286- 1293.
- Baldrian, P. and Šnajdr, J. (2006) Production of lignolytic enzymes by litter-decomposing fungi and their ability to decolorize synthetic dyes. Enzyme and Microbial Technology, 39(5), 1023-1029.

