Journal of Environmental Protection
Vol.4 No.5(2013), Article ID:31422,6 pages DOI:10.4236/jep.2013.45056
Insecticidal Activity of Bacillus thuringiensis on Larvae and Adults of Bactrocera oleae Gmelin (Diptera:Tephritidae)
![]()
1Laboratory of Ecology and Management of Natural Ecosystems, Tlemcen University, Tlemcen, Algeria; 2Faculty of Science, SaintJoseph University, Beirut, Lebanon.
Email: faizahope@yahoo.fr
Copyright © 2013 Faiza Ilias et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 10th, 2013; revised April 11th, 2013; accepted May 8th, 2013
Keywords: Bacillus thuringiensis; Bactrocera oleae; Insecticidal Activity; Olive fly; Diptera
ABSTRACT
The olive fruit fly Bactrocera oleae is a major olive pest in Algeria and other Mediterranean countries. Its attacks, in some cases, seriously compromised production. Bacillus thuringiensis is a spore-forming soil bacterium which produces a protein crystal toxic to some insects. The objective of this study was to search for isolates toxic to larvae and adults of B. oleae. Four doses test were performed on neonate larvae, second instar larvae and third instar larvae. Seven Bt strains examined, showed highest toxicity levels against adults. 86% mortality was observed against neonate larvae after 7 days of application by Bt toxin isolate. The mortality of the second instar larvae was 65% mortality at dose of 108 UFC/mL. The third instar larvae was very susceptible to Bt strain with 70% of mortality. This study demonstrated that some local Algerian B. thuringiensis isolates exhibit toxic potential that could be used to control B. oleae.
1. Introduction
The olive fruit fly Bactrocera oleae (Gmel.) (Dipt. Tephritidae), is a major olive pest in Algeria and other Mediterranean countries. Its attacks, in some cases, seriously compromised production. Its control relies mainly on chemical treatments, which posses ecological and toxicological side effects. These include: environmental contamination, destruction of nontarget organisms, severe outbreaks of other secondary pests, and the presence of insecticide residues in the olive oil, caused mainly by lipophilic pesticides concentrating in this product [1]. The preference for biological pesticides over chemicals has been widely accepted in different parts of the world, in crop and forest protection and in insect vector control. The greatest successes in microbiol pesticides have come from the use of commercial preparations of Bacillus thuringiensis. These have been the most succeful biological pest control products worldwide, and 90% - 95% of the sales of microbial pesticides are of this bacterial agent [2,3].
B. thuringiensis is a gram-positive, spore-forming soil bacterium which produces a protein crystal during sporulation (known as Cry proteins or known as δ-endotoxins) [4,5], toxic to some insects. Many crystalline proteins characterized by their entomopathogenic activity are highly specific for several insect orders (Lepidoptera, Diptera, Coleoptera, Hymenoptera and Orthoptera). Nevertheless, for several crystal-producing B. thuringiensis strains, no toxic activity has yet been demonstrated [4-6]. This has been intense interest in recent years in collecting, analyzing and screening B. thuringiensis strains isolated from environmental samples. Worldwide analyzing researches have been based on the possible existence of new strains with new pathogenic spectra or host ranges [7-11] and many dipteran species have been found to be susceptible to B. thuringiensis; these include tephritidae, such as the Mexican fruit fly Anastrepha ludens [12,13], the Mediterranean fruit fly Ceratitis capitata [14-16] and the olive fruit fly B. oleae [8-17].
According to our knowledge, there is no available data on the effect of Algerain Bt strains on the fruit fly B. oleae.
The aim of this study was to evaluate the biological entomopathogenic activity of Bt strains isolated from Tlemcen regionin Algeria. We test the biological effects of Bt strains against larvae and adults of B. oleae.
2. Materials and Methods
2.1. Insect Rearing
The insects used in the bioassays came from the laboratory colony of B. oleae, maintained at the Laboratory of Ecology and Management of Natural Ecosystems, Tlemcen University (Algeria).
The laboratory conditions used for rearing and bioassays were 25˚C ± 1˚C, 60% ± 10% relative humidity (RH) and 12 h of light and 12 h of darkness. Larvae from 0.5 ml of eggs were raised on an artificial diet containing 800 cm3 carrot pulp slurry, 16 g of yeast powder, antifungal 1.04 and 15 cm3 2 N HCl [18].
Third-instar larvae were transferred into a pupation chamber with sand and were maintained there for at least 1 week before they were collected using a sieve. Adult flies were fed an artificial diet comprised of 20% (wt/wt) yeast autolysate and 80% (wt/wt) sucrose. Water was provided to the flies via a damp yellow sponge.
2.2. B. thuringiensis Strains
Samples were collected from different areas of Tlemcen region (Table 1).
Bt isolation was carried out as described previously by Bel et al. [9]. Colonies with characteristic Bt morphology and presence of parasporal inclusions in the sporangium were selected and plated again for single colony isolation. After a second microscopic observation to verify presence of protein inclusions, strains were cultured in GN (nutrient agar) medium, for 2 days until sporulation. Vegetative cells were eliminated by heating the sample at 70˚C for 20 min.
2.3. Preparation of the Doses Suspensions
We incubated each bacterium in Petri dishes containing medium GN for 24 h. We used the method of standardization of the inoculums to prepare the concentration of the strain that consisted to put the inoculum into a tube containing 9 ml of sterile distilled water and measure bacterial colonies using spectrophotometer absorbance at
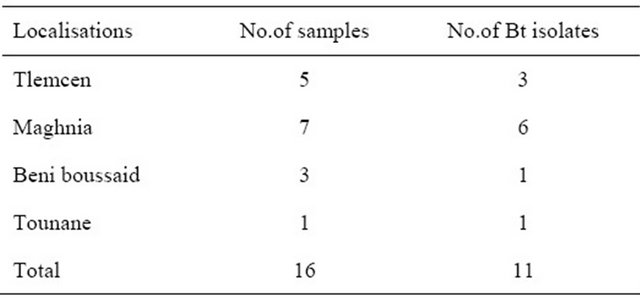
Table 1. Local Bt strains isolated in tlemcen (Algeria).
580 nm between 0.08 and 0.1 to give a concentration of 108 UFC/mL, and in fact in the dilution tubes to come up 105 UFC/mL.
2.4. Bioassays
2.4.1. Activity against First and Second Instar Larvae
Four doses test was performed (108 UFC/mL, 107 UFC/ mL, 106 UFC/mL, 105 UFC/mL,) added onto 2 g of the artificial diet surface contained in 24-well polystyrene plates. One-first instar larvae of B. oleae was added per well, and the mortality was recorded after 7 days of incubation at 25˚C in dark conditions.
Three replicates of 24 larvae were conducted for each Bt strain. Sterile distilled water was used as a negative control.
Vectobec, a commercial product based on Bti and reported to be active against dipterans, were used as standard controls.
2.4.2. Activity against Third Instar Larvae
Eight third instar larvae were transferred to plastic recipients (30 mm diam × 70 mm depth) containing 10 g of diet (see insect rearing) mixed with the toxin, except for the control group, which had just received water with the diet. Four doses of Bt suspensions were used in three repetitions for each strain to confirm the results. The recipients were covered with nylon mesh, and fixed around the edges with a rubberband.
After 48 h of exposure, the number of dead larvae was counted and the pupae were separated from diet and then transferred to Petri dish (9 cm), until adult emergence.
Bioassay tests were carried out to determine LC50 values of the Bt strains against B. oleae larvae.
2.4.3. Activity against B. oleae Adults
10 adults newly emerged (1 - 2 days old) were kept in the cage (25 cm by 25 cm) containing artificial diet (see insect rearing) mixed with the suspensions of Bt strains. The sweet substances (hydrolyzed protein and sugar) was used in the bioassay to attract the adults recently emerged to feed Bt suspensions. Water was provided to the flies via a damp yellow sponge. For each dose, a negative control was prepared with distilled water and fly feeding. Three replicates per assay were carried out. Fly mortality was recorded daily for 7 days.
2.5. Statistical Analysis
The mortality assay was calculated according to the Abbot’s Mortality corrected % = (1 − n in T after treatment/n in Co after treatment) * 100.
Where: n = Insect population, T = treated, Co = control.
The LC50 regression equation and the 95% confidence limit were calculated by using Probit analysis. Percentages of mortality obtained from bioassay tests were analyzed using one-way analysis of variance (ANOVA, p < 0.05).
3. Results
3.1. Activity against First and Second Instar Larvae
Results of the larvicidal effectiveness of Bt strains are shown in Tables 2 and 3.
The Bt strains isolated showed significantly larvicidal activity (F: 25.28, p < 0.000 at 95%). The mortality rate was ranging from 5% to 92%, obtained after 7 days of treatment with the dose 108 UFC/mL. The toxin produced by Bt 1 strain showed the highest effect, with 86% of corrected mortality. The effect of Bt 7 strain was the lowest, with 5% as percentage of larval mortality. The Vectobec product (55%) caused mortality to the same degree with some of our strains or low.
3.2. Activity against Third Instar Larvae
In Table 4, the results of bioassays on the biological activity of Bt strains tested against B. oleae third instar larvae are presented.
The susceptibility of third instar of B. oleae, evaluated as percentage of larvae that did not survive to adulthood, varied significantly between 0% - 12%, 10% - 35%, 38% - 70% at doses of 105 UFC/mL, 106 UFC/mL and 108 UFC/mL respectively. At 108 UFC/Ml, Bt 1 strain proved most effective activity (70%).
The LC50 determined after 48 h of application were 1.69 × 106 UFC/mL for Bt 1. Significant differences were noted with tested doses (F: 16.07, p < 0.000 at 95%).
3.3. Activity against B. oleae Adults
Table 5 shows biological activity of Bt strains in newly emerged adults of B. oleae.
The difference of fly’s mortality was significantly higher between those exposed to Bt strains mixtures and the ones exposed to the water control.
The majority of Bt strains killed more than 50% of B. oleae adults. The mortality values varied significantly between strains. They produced significant mortalitiesfrom 49 to 100% (F: 2.33, p < 0.000 at 95%); in the concentration of 108 UFC/mL after 10 days exposure time. The more toxic Bt strains caused adult mortality starting from the 4th day of ingestion.
The commercial product based on Bti (Vectobec) and was also tested against B. oleae adults. In general, this produc was an important mortality ranged betwwen 55% and 89% (Table 5).
4. Discussion
During sporulation, the Gram-positive bacterium, B. thuringiensis forms crystalline protein inclusions, which possess insecticidal activity. These parasporal inclusions differentiate this species from other related species such as Bacillus cereus and Bacillus mycoides [19]. The crystals are generally smaller than the spores and can represent ±30% of the dry weight of the cell [20].
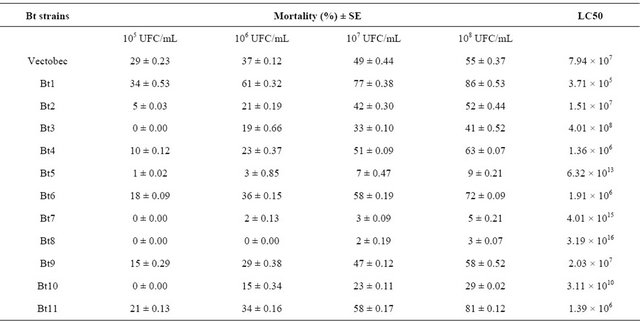
Table 2. Entomopathogenic activityof Bt strains against neonate larvae of B. oleae.
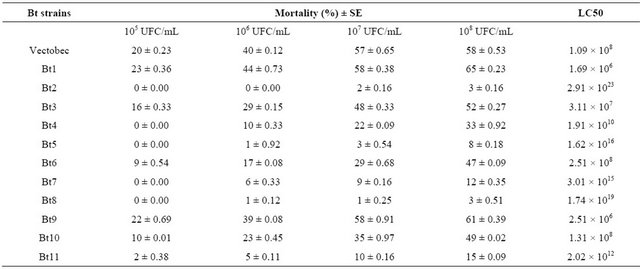
Table 3. Entomopathogenic activity of Bt strains against the second instar larvae of B. oleae.
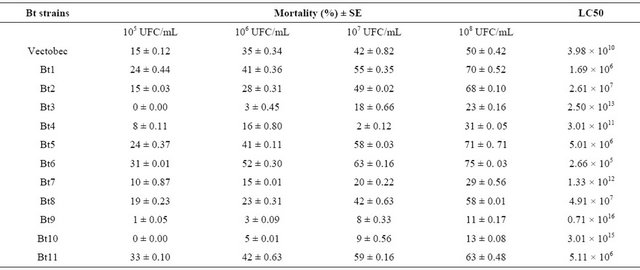
Table 4. Entomopathogenic activity of Bt strains against the third instar larvae of B. oleae.
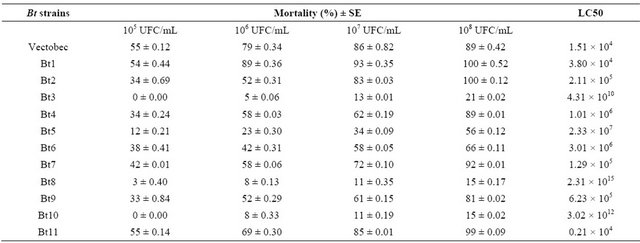
Table 5. Entomopathogenic activity of Bt strains against adults of B. oleae.
A total of eleven Bt strains were tested on larvae of B. oleae; and it was found that 80% of strains were toxic against neonate larvae, the second instar larvae 32%, and 78% on the third instar larvae. Neonate larvae were more susceptible than second or third instar larvae of B. oleae; in fact, several strains produced high mortality only after 3 days of exposure.
The biological activity of Bt strains has been studied by many authors. Our results are similar to that reported by Alberola et al. [17] who tested the Bt activity against second instar of B. oleae larvae and new emerged adults. These authors have reported that both spore-crystal mixtures (109/mL) caused 70% mortality for larvae in 72 hours and 80% of mortality for adult flies in 6 to 10 days of application. This author have tested 23 strains of Bt and found that 15 of them killed at least 70% of B. olaea larvae.
Karamanlidou et al. [8] and Yamvrias et al. [21], have reported a mortality >80% when they used various Bt strains against old larvae of the dipteran olive fruit fly, B. oleae (Gmelin).
Hassani and Gaouar Benyelles [22] have tested the effect of the preparation of Bti on wild third instar larvae and adults of C. capitata isolated from Citrus fruit orchards in Algeria, and observed toxicity in high doses (100 mg/g) with a reduction in average emergence (84.62%), concluding that the stage L3 and adults of the pest are very susceptible at this dose of Bti product.
Robacker et al. [12] evaluated the action of 55 Bt strains on larvae of Mexican fruit fly, Anastrepha ludens (Loew). Only 7 strains were found toxic on adults; the centrifuge pellets or precipitates of Bt strains have killed >50% of larvae.
Molina et al. [23] have tested sporulated cultures of 115 bacterial strains of Bacillus pumilus (4.65 × 108 to 1.45 × 107 CFU/mL) against adults and neonate larvae of C. capitata. None of these strains were caused significant mortality of C. capitata adults compared with the negative controls. The mortality rates with the 115 bacterial strains at the end of the experiment ranged from 0% to 40%, while the average mortality rates with the negative controls ranged from 5% to 30%. Similar results were obtained in bioassays with larvae, the maximal corrected mortality rates with the 115 bacterial isolates ranged from 0% to 36%, while the average mortality rates with the negative controls ranged from 1% to 12% after 15 days, at the end of the experiment. After this toxicity screening, they obtained a novel Bacillus pumilus strain, which is highly toxic to C. capitata larvae. The mortality rate for C. capitata larvae ranged from 68 to 94% depending on the conditions under which the culture was kept before the bioassay.
Aboussais et al. [16] have tested 26 strains of Bt on both larvae and adults of C.capitata; and it was found that 100% of strains were toxic against adults of C. capitata, 92% on neonate larvae on adults, and 60% on third instar larvae. Neonate larvae and adults were more susceptible than third instar larvae of C. capitata; in fact, several strains produced high mortality only after 3 days of exposure.
The obtained results during our study showed that some Bt strains were most toxic against adults than on larvae and vice versa. For example, the insecticidal activity of Bt 5 against larvae of B. oleae is 9%, whereas the activity of this strain towards B. oleae adults is 56% (Tables 3 and 5). The genes which coding for insecticidal activity against the two stages of the medfly, could be different [12,17]. Alberola et al. [17] have also reported that there are some different chemical conditions in the gut of the two stages of the insect that can control the activation or activity of the protoxin.
5. Conclusion
B. oleae is the most important problem for olive producers. The present study indicates that it is possible to find naturel strains of B. thuringiensis which are toxic for larvae of B. oleae. A number of our collection Bt strains showed high insecticidal activity against B. oleae in comparison to that noted for the commercial product Vectobec previously used in the control of harmful fruit flies. Some of our Bt strains can be used in the biological control system to fight against C. capitata and may contribute to reduce the use of chemical insecticides harmful to the consumers and the environment.
REFERENCES
- G. Delrio, “Integrated Control in Olive Groves. In Biological Control and Integrated Crop Protection: Towards Environmentally Safer Agriculture,” Proceedings of the IOBC/WPRS, Veldhoven, 8-13 September 1991, pp. 67- 76.
- B. Spear, “Genetic Engineering of Bacterial Insecticides,” In: H. Lebaron, R. Mumma, R. Honeycutt, J. Duesing, J. Phillips and M. Hass, Eds., Biotechnology in Agricultural Chemistry, American Chemical Society, Washington DC, 1987, pp. 204- 214.
- B. Carlton, “Development of Genetically Improved Strains of Bacillus thuringiensis,” In: P. Hedin, J. Menn and R. Hollingrowth, Eds., Biotechnology for Crop Protection, American Chemical Society, Washington DC, 1988, pp. 260-279.
- H. Hofte and H. R. Whitely, “Insecticidal Crystal Proteins of Bacillus thuringiensis,” Microbiological Reviews, Vol. 53, No. 2, 1989, pp. 242-255.
- E. Shnepf, N. Crickmore, J. Van Rie, D. Lereclus, J. Braun, J. Feitelson, D. R. Zeigler and D. H. Dean, “Bacillus thuringiensis and Its Pesticidal Crystal Proteins,” Microbiology and Molecular Biology Reviews, Vol. 62, No. 3, 1998, pp. 775-806.
- M. Ohba and K. Aizawa, “Distribution of Bacillus thuringiensis in Soils of Japon,” Journal of Invertebrate Pathology, Vol. 47, No. 3, 1986, pp. 277-282. doi:10.1016/0022-2011(86)90097-2
- P. A. W. Martin and R. S. Travers, “Worldwide Abundance and Distribution of Bacillus Thuringiensis Isolates,” Applied and Environmental Microbiology, Vol. 55, No. 10, 1989, pp. 2437-2442.
- G. Karamanlidou, A. F. Lambropoulos, S. I. Koliais, T. Manousis, D. Ellar and C. Kastritsis, “Toxicity of Bacillus thuringiensis to Laboratory Populations of the Olive Fruit Fly (Dacus oleae),” Applied and Environmental Microbiology, Vol. 57, No. 8, 1991, pp. 2277-2282.
- C. N. Chilcott and P. J. Wigley, “Isolation and Toxicity of Bacillus thuringiensis from Soil and Insect Habitats in New Zealand,” Journal of Invertebrate Pathology, Vol. 61, No. 3, 1993, pp. 244-247. doi:10.1006/jipa.1993.1047
- Y. Bel, F. Granero, T. M. Alberola, M. J. MartínezSebastian and J. Ferré, “Distribution, Frequency and Diversity of Bacillus thuringiensis in Olive Tree Environments in Spain,” Systematic and Applied Microbiology, Vol. 20, No. 4, 1997, pp. 652-658. doi:10.1016/S0723-2020(97)80038-0
- J. Iriarte, Y. Bel, M. D. Ferrandis, R. Andrew, J. Murillo, J. Ferré and P. Caballero, “Environmental Distribution and Diversity of Bacillus thuringiesnis in Spain,” Systematic and Applied Microbiology, Vol. 21, No. 1, 1998, pp. 97-106. doi:10.1016/S0723-2020(98)80012-X
- D. C. Robacker, A. J. Martínez, J. A. García, M. Díaz and C. Romero, “Toxicity of Bacillus thuringiensis to Mexican Fruit Fly (Diptera: Tephritidae),” Journal of Economic Entomology, Vol. 89, No. 1, 1996, pp. 104-110.
- J. Toledo, P. Liedo, T. Williams and J. Ibarra, “Toxicity of Bacillus thuringiensis β-Exotoxin to Three Species of Fruit Flies (Diptera: Tephritidae),” Journal of Economic Entomology, Vol. 92, No. 5, 1999, pp. 1052-1056.
- R. E. Gingrich, “Demonstration of Bacillus thuringiensis as a Potential Control Agent for the Adult Mediterranean Fruit Fly, Ceratitis capitata (Wied.),” Journal of Applied Entomology, Vol. 104, No. 1-5, 1987, pp. 378-385.
- J. C. Vidal-Quist, P. Castañera and J. González-Cabrera, “Diversity of Bacillus thuringiensis Strains Isolated from Citrus Orchards in Spain and Evaluation of Their Insecticidal Activity against Ceratitis capitata,” Journal of Microbiology and Biotechnology, Vol. 19, No. 8, 2009, pp. 749-759.
- H. Aboussaid, L. El Aouame, S. El Messoussi and K. Oufdou, “Biological Activity of Bacillus thuringiensis (Berliner) Strains on Larvae and Adults of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae),” Journal of Environmental Protection, Vol. 1, No. 4, 2010, pp. 337- 345.
- T. M. Alberola, S. Aptosoglou, M. Arsenakis, Y. Bel, G. Delrio, D. J. Ellar, J. Ferre, S. P. Gash, F. Granero, S. Koliais, M. J. Martinez-Sebastian, R. Prota, S. Rubino, A. Satta, G. Scarpellini, A. Sivropoulou and E. Vasara, “Insecticidal Activity of Strains of Bacillus thuringiensis on Larvae and Adults of Bactrocera oleae Gmelin (Dipt. Tephritidae),” Journal of Invertebrate Pathology, Vol. 74, No. 2, 1999, pp. 127-136. doi:10.1006/jipa.1999.4871
- G. L. Finney, “Afortified Carrot Medium for Mass-Culture of the Oriental Fruit Fly and Certain Other Tephritidis,” Journal of Economic Entomology, Vol. 49, No. 1, 1956, p. 134.
- G. S. A. B. Stewart, K. Johnstone, E. Hagelberg and D. J. Ellar, “Commitment of Bacterial Spores to Germinate,” Biochemistry Journal, Vol. 198, No. 1, 1981, pp. 101- 106.
- J. A. Baum and T. Malvar, “Regulation of Insecticidal Crystal Protein Production in Bacillus thuringiensis,” Molecular Microbiology, Vol. 18, No. 1, 1995, pp. 1-12. doi:10.1111/j.1365-2958.1995.mmi_18010001.x
- C. Yamvrias and M. Anagnou, “Preliminary Tests on the Sensitivity of the Larvae of Dacus oleae to Bacillus thuringiensis var. israelensis, in Fruit Flies of Economic Imprtance,” In: R. Cavalloro, Ed., Balkema, Rotterdam 1989, pp. 345-348.
- F. Hassani and N. Gaouar Benyelles, “Application of Bacillus thuringiensis (Bti) Struggling Microbiological Control of the Fruit Fly Ceratitis capitata (wied) (Diptera: Tephritidae),” IBSCientific Journal of Science, Vol. 3, No. 1, 2008, pp. 10-13.
- C. A. Molina, J. F. Cana-Roca, A. Osuna and S. Vilchez, “Selection of a Bacillus pumilus Strain Highly Active against Ceratitis capitata (Wiedemann) Larvae,” Applied and Environmental Microbiology, Vol. 76, No. 5, 2010, pp. 1320-1327. doi:10.1128/AEM.01624-09

