Journal of Environmental Protection
Vol. 4 No. 6 (2013) , Article ID: 33143 , 10 pages DOI:10.4236/jep.2013.46068
Complexation with ATP as a Cause of Pollutants Toxicity to Aquatic Life
![]()
Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, Russia.
Email: easar@icp.ac.ru
Copyright © 2013 Elena A. Saratovskikh. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 31st, 2013; revised May 2nd, 2013; accepted June 1st, 2013
Keywords: Pesticides; Lontrel Complexes with Metals; Biotesting; Toxicity; Correlation with Complexes of ATP
ABSTRACT
The toxicity of some pesticides, namely, Lontrel, Sencor, Roundup, Bentazon, and Hymexazol, and also Lontrel complexes with Cu, Co, Mn, Mg, Mo, Ni, and Zn that are being widely used in agriculture and, hence, abundant in the environment were characterized by biotesting. The variation in the enzymatic activity of the luminous bacteria Beneckea harveyi and in the reproductive function of the infusoria Tetrahymena pyriformis was measured and used to determine toxicity factors. The toxicity of the above compounds was found to correlate with their ability to form stable complexes with adenosine triphosphate (ATP).
1. Introduction
Nowadays, different kinds of chemicals originating from the agricultural use of pesticides eventually become deposited in soil, water basins, and ground water. Ever increasing human impact to the nature puts forward an acute problem of environment protection [1-3]. Proper nature-conservative measures can be suggested only on the basis of deep insight into the mechanism of effect of toxic compounds at the cellular and sub-cellular level. We have to know a relationship between the mechanism of the toxicant action and the effect produced by a pollutant (Plt) on the entire organism. Most adequately, such a relationship can be established by the methods of biotesting using either entire test-objects or their functional fragments.
Adenosine triphosphate (ATP) is the only biomolecule that supports the processes of energy storage/release in living organisms. Previously, we explored the effect of Plt on biological objects on the molecular level: the complexation of technogenic toxicants with ATP mononucleotide, its structural analog (ε-ATP), metals, and di/ polynucleotides [4-7]. Binding of Plt with ATP gives rise to energy shortage in cells, which may block the functioning of tricarboxylic acids cycle, interrupt electron transfer processes, and, as a result, suppress photosynthetic processes.
In this work, we investigated the toxicity of some pesticides, viz., Lontrel, Sencor, Roundup, Bentazon, and Hymexazol, and also Lontrel complexes with Cu, Co, Mn, Mg, Mo, Ni, and Zn by the methods of biotesting. We aimed at elucidating a relationship between the toxicity of the above compounds and their ability to form stable complexes with ATP.
2. Material and Methods
2.1. Used Substances, Concentrations and Replicates
In experiments, we used the pesticides characterized in Table 1. Their isolation from commercially available chemicals and purification were carried out as described elsewhere [4]. The Lontrel complexes with metals were synthesized as reported previously [8,9].
ε-ATP was synthesized as proposed by [4]. Equilibrium constants Kc for the complexation of Plt with ATP and ε-ATP were estimated from the fluorescence quenching curves using a mathematical model suggested by us previously [4]. Fluorescence measurements were performed with an Aminco-Bowman spectrofluorimeter. The excitation of ATP at λexc = 290 nm gave rise to a fluorescence band with a maximum at λmax = 380 nm; for ε-АТP, λexc = 312 nm and λmax = 420 nm.
Toxicity of the pesticides under study was detected over a wide range of their concentration, from 10−3 up to 10−1 М, in view of the fact that the concentration rec-
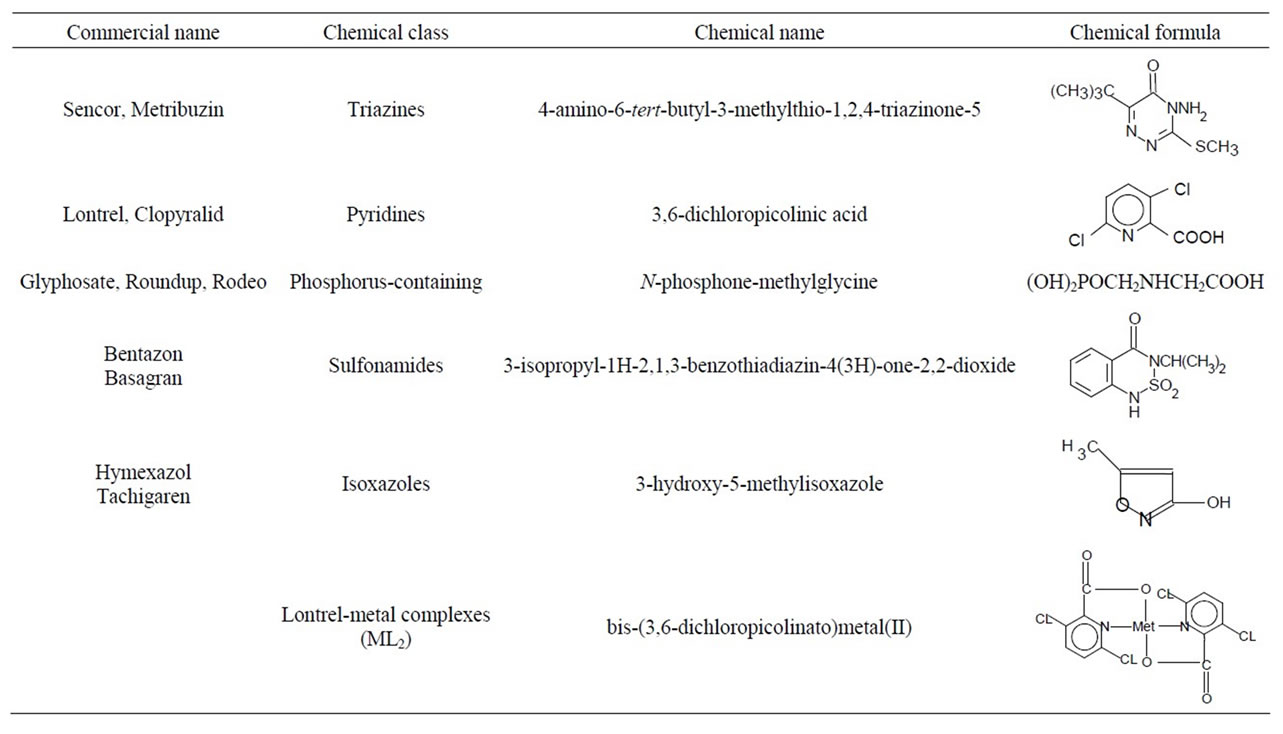
Table 1. Commercial names and chemical formulae of the studied pesticides.
ommended for agricultural use is known to have a value of 10−2 М. Stronger toxicity of Lontrel complexes with metals was explored within the concentration range 10−2 - 10−8 М.
2.2. Toxicological Testings
2.2.1. The Biotest on Beneckea harveyi
In experiments, we monitored a change in the enzymatic activity of the luminous bacteria Beneckea harveyi, which becomes noticeable already in 30 min after incubation [3,10,11].
NaCl (reagent grade, from Agat-Med, Russia) was additionally purified by double recrystallization. Water was used after bidistillation. The culture of luminous marine bacteria Beneckea harveyi, viz., Photobacterium phosphoreum, was prepared at the Institute of Biophysics (Siberian Branch, Russian Academy of Sciences, Krasnoyarsk).
For experiments, lyophilized cells of the bacteria B. harveyi (normally kept in a deep-freezer) were suspended in a 3% aqueous solution of NaCl. To 0.5 ml of such a solution, 0.3 - 0.5 ml of suspended bacteria was added. A 0.85% solution of NaCl was used as a reference sample. The luminescence intensity I of the enzyme luciferase present in the cells (λexc = 290 nm, λmax = 340 nm) was monitored with a BLM-8801 luminometer. A decrease in I (compared to that of the reference sample) measured in the presence of added pesticide was regarded as a measure of toxicity T for a given compound [3,10,11].
A decrease in I can be associated either with the inhibition of luciferase or with the effect of the toxicants on some other units of the metabolism chain. The toxicity index T was determined from the expression
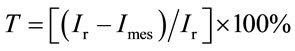 , (1)
, (1)
where Ir is the luminescence intensity of the reference sample, and Imes is the value measured for a solution containing a given pesticide. For Т £ 19%, the pesticide is regarded as nontoxic. For 19 < T £ 50%, the pesticide is considered as toxic, while for T > 50%, it is regarded as strongly toxic.
2.2.2. The Biotest on Tetrahymena pyriformis
In another set of experiments, we measured the effect of pesticides on the reproductive function of the infusoria Tetrahymena pyriformis upon a 24-h exposure as described in [10,11]. Similarly, the toxicity factor K was calculated using the expression
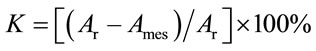 , (2)
, (2)
where Аr is an increment in the total number of infusoria cells in the reference sample, and Аmes is that measured for the sample containing a given pesticide. A pesticide was regarded as toxic when K > 50%. The reliability of the data was estimated using the Student criterion.
In both cases, the ЕС50 values, toxicant concentrations ensuring a 50% effect, were determined graphically using the procedure described in [10].
3. Results and Discussion
3.1. Toxicity of Pesticides with Respect to Luminous Bacteria and Infusoria
Toxicity factors T towards the luminous bacteria B. harveyi as a function of pesticide concentration C are shown in Figure 1. It follows that K increases proportionally to C, although the slopes of the linear plots differ: the largest slope is observed for Sencor and the smallest one is observed for Hymexazol. Even at C = 10−1 М Hymexazol remains nontoxic (Т < 19%). Bentazon is seen to be lowly toxic over the whole range of C (24% < Т ≤ 40%). In the range from 10−3 to 3 × 10−3 М, all of the compounds under study are lowly toxic (Т ≤ 50%). Sencor becomes highly toxic at C = 5 × 10−3 М, Lontrel becomes highly at 10−2 М, while Roundup is highly toxic at 10−1 М.
Toxicity factors K towards the infusoria T. pyriformis vs. C are presented in Figure 2. Again, the plots are seen to be linear, the toxicity of Sencor is the largest, while that of Hymezazol is the lowest. Even at C = 10–3 М all of the compounds under consideration are highly toxic (K > 50%). The slope of the linear plots is seen to decrease in the order Sencor > Lontrel > Roundup > Bentazon > Hymexazol. With increasing C, the toxicity of Hymexazol increases more slowly than that of Sencor by a factor of five.
Our data on the ЕС50 values are collected in Table 2. It follows that these are markedly close in their magnitude and increase in the order Sencor < Lontrel < Roundup < Bentazon < Hymexazol from 2.63 × 10–3 М (0.56 g/l) to 9 × 10–1 М. Generally, the ЕС50 values measured for B. harveyi are somewhat lower than those for T. pyriformis.
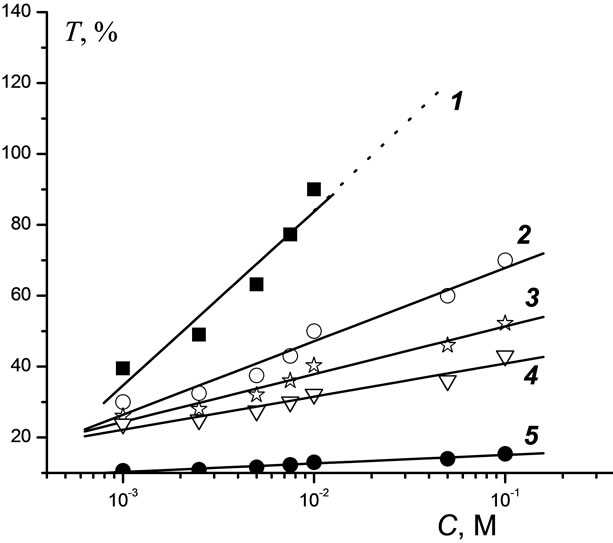
Figure 1. Dependence of the toxicity of pesticides with respect to luminescent bacteria on pesticide concentrations: 1—Sencor; 2—Lontrel; 3—Glyphosate; 4—Bentazon; 5— Hymexazol.
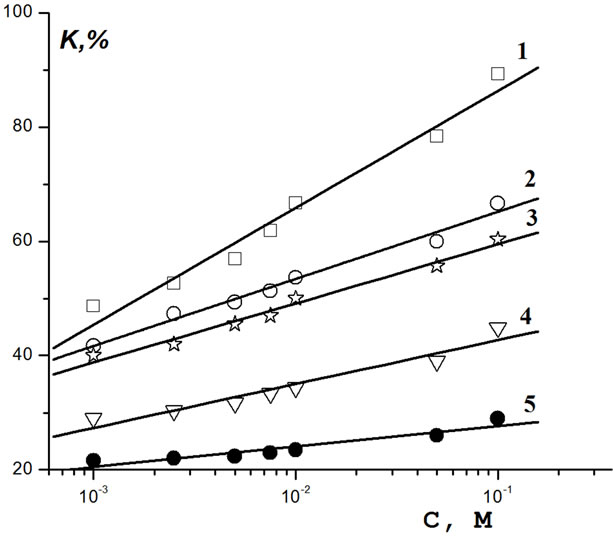
Figure 2. Dependence of the toxicity of pesticides with respect to infusoria on pesticide concentrations: 1—Sencor; 2 —Lontrel; 3—Glyphosate; 4—Bentazon; 5—Hymexazol.
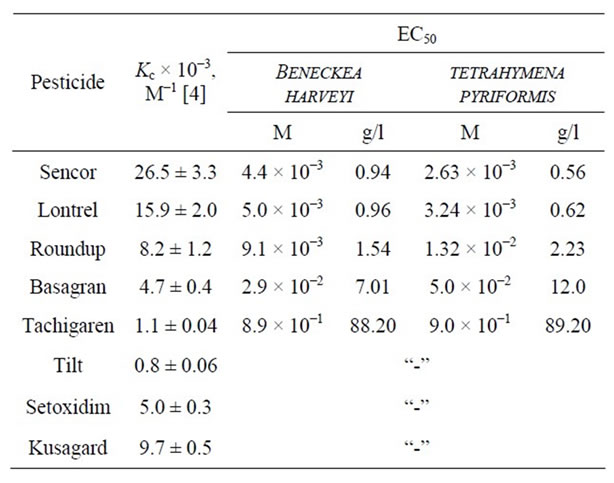
Table 2. Values of EC50 measured for pesticides by the biotesting method with the use of fluorescent bacteria Beneckea harveyi and infusoria Tetrahymena pyriformis.
3.2. Determination of Toxicity for the Metal Complexes
As known [8,9], some Plt are capable of forming various complexes that stable in soil and aquatic environment even under the action of microorganisms and UV irradiation. In addition, these complexes were found [3] to exhibit high reactivity with respect to biomolecules. However, any quantitative data on the toxicity of such Pltmetal complexes are still lacking in the literature. In this work, we explored the toxicity of the MetL2 complexes towards the bacteria B. harveyi, where ligand L is Lontrel (3,6-dichloropicalinic acid) and Met = Cu, Co, Mn, and Mg. As follows from Figure 3, these complexes exhibit toxicity even at C = 10–7 М. Since curves 1 - 4 are parallel,
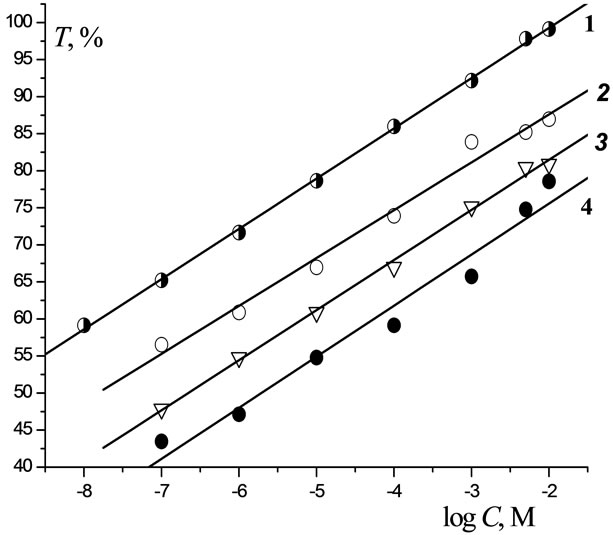
Figure 3. Dependence of the toxicity of the Lontrel-metal complexes with respect to luminescent bacteria on the logarithm of the concentration of complexes: 1—MnL2; 2— CuL2; 3—CoL2.
for different complexes T increases with increasing C with an identical rate. For all C, the values of T decrease in the order CuL2 > CoL2 > MnL2 > MgL2, as well as the EC50 values (Table 3). Note that the toxicity of Lontrel complexes turned out to be higher than that of Lontrel itself. Compared to Lontrel, the ЕС50 value for MgL2 is lower by a factor of two, while that for CuL2 is lower by three orders of magnitude.
As seen in Figure 4, CuL2, CoL2, NiL2, and MoL2 (C = 10–5 М) also exhibit toxicity towards the infusoria T. pyriformis (K > 50%). For C < 10–5 М, MnL2, ZnL2, and MgL2 are nontoxic (K < 50%). The above complexes are always toxic for C > 10–4 М. Again, curves 1 - 7 are nearly parallel. At any C, the K values decrease in the order CuL2 > CoL2 > NiL2 > MoL2 > MnL2 > ZnL2 > MgL2. MgL2 was the only complex that exhibited a lower toxicity compared to that of Lontrel (at any C). The K values for CuL2, CoL2, and NiL2 were higher than that for Lontrel by a factor of two, while those for MoL2, MnL2, and ZnL2 were higher by a factor of 1.5.
As follows from Table 3, the ЕС50 value for MnL2 is lower than that for Lontrel by a factor of five. Those for NiL2, MoL2, and CoL2 are lower by a factor of ten, where that for CuL2 is lower by a factor of 16. For the infusoria T. pyriformis, the respective ЕС50 values turned out to be higher that those for B. harveyi by 1 - 2 orders of magnitude. Tentatively, this can be associated with the fact that in experiments in vitro with B. harveyi we are dealing with the deactivation of a sole enzyme (luciferase) by contrast to in vivo experiments with T. pyriformis.
A living organism is known to synthesize metallothioneines, low-molecular-weight cysteine-rich proteins,
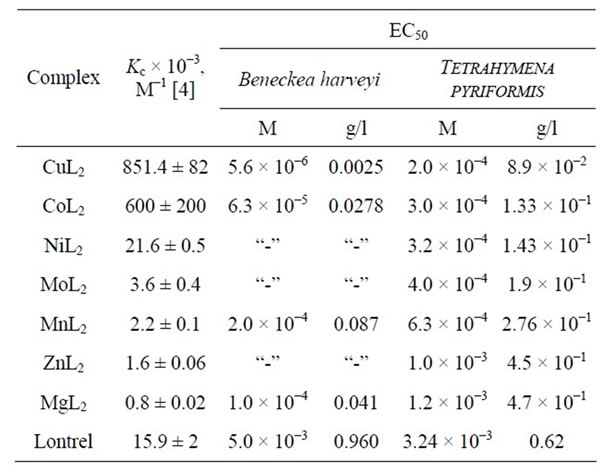
Table 3. Values of EC50 measured for Lontrel-metal complexes by the biotesting method with the use of fluorescent bacteria Beneckea harveyi and infusoria Tetrahymena pyriformis.
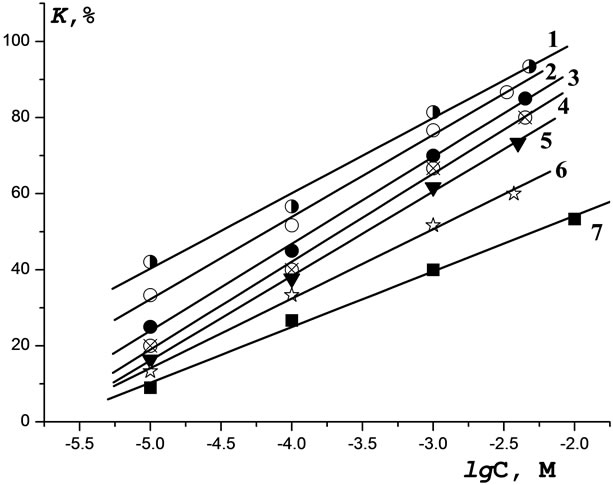
Figure 4. Dependence of the toxicity of the Lontrel-metal complexes with respect to infusoria on the logarithm of the concentration of complexes: 1—MnL2; 2—CuL2; 3—CoL2.
designed for binding metal cations [12] and some organic xenobiotics, such as pesticides [13] for their subsequent elimination from the organism. Such a self-protecion system is present in all living organisms, including representatives of aquatic life. Probably, this can explain the higher resistivity of the cells in T. pyriformis to the action of metal complexes with Lontrel.
As follows from Tables 2 and 3, the metal complexes become toxic in concentrations C two orders of magnitude lower than those of free pesticides. Accordingly, the toxicity of MetL2 complexes is two orders of magnitude higher than that of Lontrel.
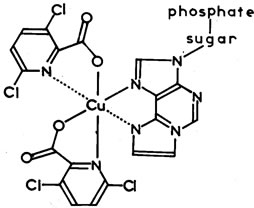
As known [4], some pollutants (L) are capable of forming complexes with nucleotides, in particular, with ATP and its structural analog ε-АТP
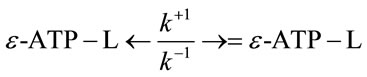
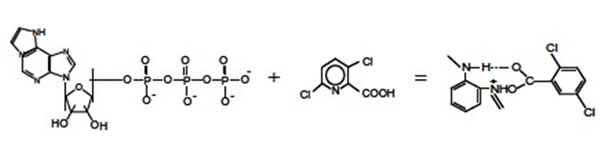
Ternary complexes of ATP with metals are formed similarly
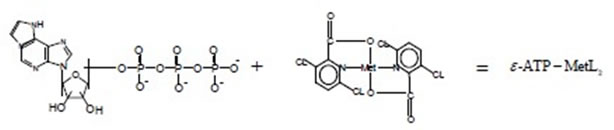
The structure of the ε-АТP—CuL2 complex [4] is illustrated below
|
3.3. Correlation between the Energy Shortage and Toxicity
Since energy metabolism is one of the key processes taking place in living organisms and ATP is the only biomolecule responsible for assimilation and dissimilation of energy, the above complexation can be expected to infringe energy metabolism and, hence, lead to energy shortage in cells [7]. In turn, the energy shortage blocks the functioning of the tricarboxylic acid cycle, electron transport, and photosynthetic processes. As a result, this was found to cause the death of plant organisms, as it has been demonstrated for pea and wheat crops [6].
Similar correlation between the complex formation with ATP leading to energy shortage in cells and the toxicity to the soil-living microarthropods Xenylla grisea (Hypogastruridae) and Folsomia candida (Isotomidae) was established by [14]. In this context, it seemed interesting to correlate the energy shortage in cells of aquatic organisms caused by a given pesticide to the equilibrium constant Kc for complexation with ATP. Therefore, the values of Kc given in Tables 2 and 3 will be used to characterize the above energy shortage in cells.
In Figure 5, the toxicity T towards the bacteria B. harveyi as a function of Kc for complexation with ε-ATP at various C (from 10–1 to 10–3 М) is seen to be directly proportional.
The toxicity K towards the cells of T. pyriformis as a function of Kc is shown in Figure 6. Again, the K values
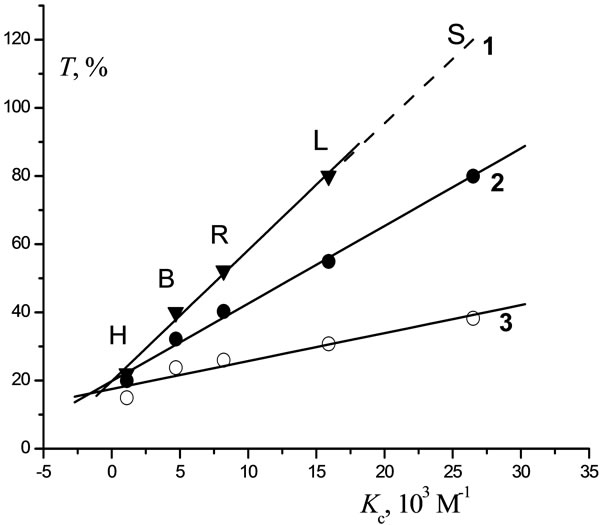
Figure 5. Correlation of the toxicity of pesticides with respect to luminescent bacteria with the values of constant of [e-АТP-pesticide] complex formation (Kc) for pesticide concentrations: 1—10−1 М; 2—10−2 М; 3—10−3 М. S—Sencor; L—Lontrel; B—Bentazon; R—Glyphosate; H—Hymexazol.
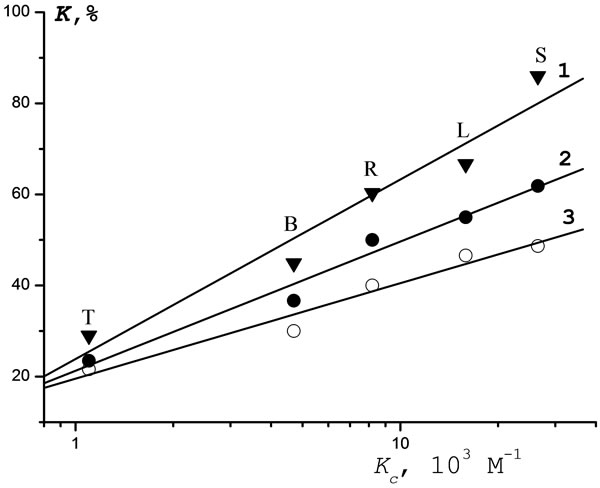
Figure 6. Correlation of the toxicity of pesticides with respect to infusoria with the values of constant of [e-АТPpesticide] complex formation (Kc) for pesticide concentrations: 1—10−1 М; 2—10−2 М; 3—10−3 М; S—Sencor; L— Lontrel; B—Bentazon; R—Glyphosate; H—Hymexazol.
are seen to be proportional to Kc over the entire range of C (from 10–1 to 10–3 М).
The toxicity of metal complexes towards B. harveyi and T. pyriformis as a function of Kc are shown in Figures 7 and 8, respectively, as a series of parallel linear plots. With increasing Kc, the toxicity of the complexes under study is seen to increase proportionally over the entire range of C (10–1 - 10–7 М).
The ЕС50 values as a function of Kc for our pesticides are given in Figure 9. Using this plot, we attempted to predict the ЕС50 values based on a known magnitude of
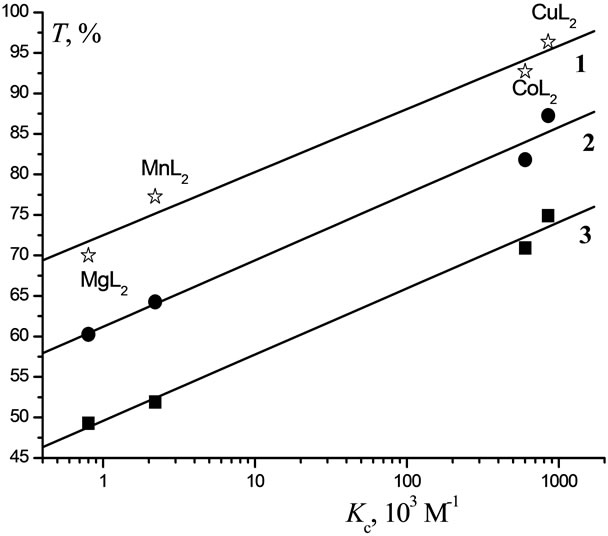
Figure 7. Correlation of the toxicity of Lontrel-metal complexes with respect to luminescent bacteria with the values of constant of [e-АТP-MetL2] complex formation (Kc) for Lontrel-metal complexes concentrations: 1—10−1 М; 2— 10−2 М; 3—10−3 М.
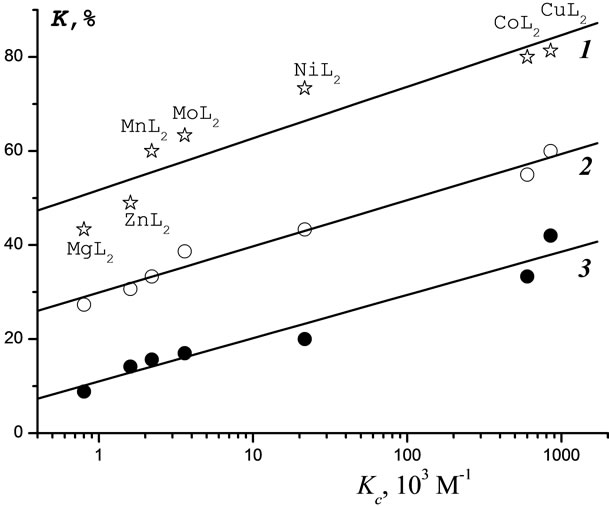
Figure 8. Correlation of the toxicity of Lontrel-metal complexes with respect to infusoria with the values of constant of [e-АТP-MetL2] complex formation (Kc) for Lontrelmetal complexes concentrations: 1—10−1 М; 2—10−2 М; 3— 10−3 М.
Kc. For this purpose, we drew the reported values of Kc for Kuzagard, Setoxydime, and Tilt onto curve 1 in Figure 9. Our curve 1 predicts that for the above compounds the ЕС50 values must attain the values of 1.0 × 10–2, 3.4 × 10–2, and 1.1 М, respectively.
Just as for pesticides, we compared the behavior of the complexes at the same ЕС50 value and plotted the de pendence of this ЕС50 on Kc (Figure 10). Apparently, this plot holds true for the metal complexes with a given ligand (L). This dependence is universal: it relates the
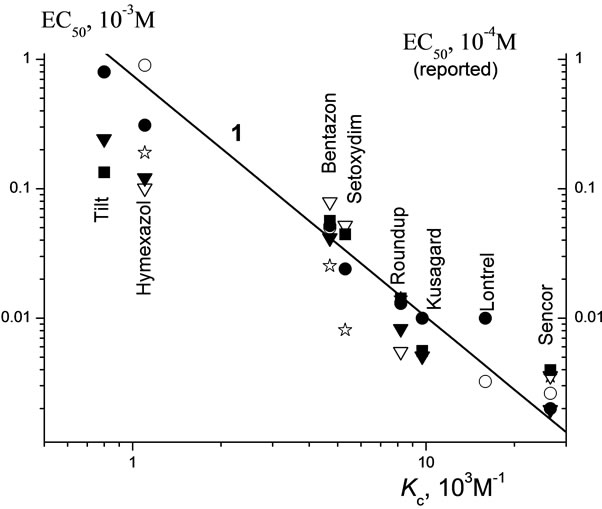
Figure 9. Regularity of the dependences of the values ЕС50 of pesticides with respect to infusoria on values of constant of complex formation (Kc): (○) our data points for the infusoria Tetrahymena pyriformis and the reported data for (·) zooplankton Daphnia magna [21]; (☆) juvenile fish Lepomis macrochirus up to 0.4 cm in size [21], ( ) juvenile fish Oncorhynchus mykiss [21,34], (
) juvenile fish Oncorhynchus mykiss [21,34], ( ) adult fish Oncorhynchus mykiss [21,24,28-30], and (■) adult fish Cyprinodon variegates [21,22,28,32,33,35].
) adult fish Oncorhynchus mykiss [21,24,28-30], and (■) adult fish Cyprinodon variegates [21,22,28,32,33,35].
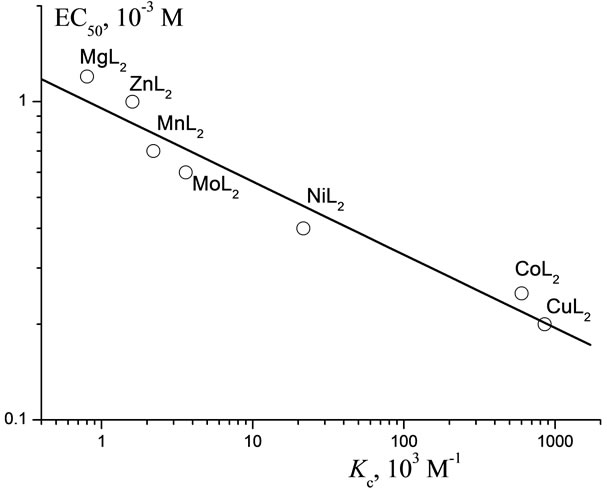
Figure 10. ![]() Regularity of the dependences of the toxicity of Lontrel-metal complexes with respect to infusoria on values of constant of complex formation (Kc).
Regularity of the dependences of the toxicity of Lontrel-metal complexes with respect to infusoria on values of constant of complex formation (Kc).
energy shortage in cell caused by that or another Plt to the acute toxicity of a given Plt. and “principle”.
3.4. Relationship between Available Experimental and Literature Data
It seemed interesting to check out the universality of the above relationship on the available set of relevant data (Table 4). For the water flea Scapholeberis kingi, the LC50 value for Roundup is seen to be lower than Bentazon. However, for the opossum Americamysis bahia, the LC50 values well correlate with Kc for the complexation of ATP with Roundup, Setoxydime, Bentazon, and Tilt.
Despite a wide use of Lontrel in agriculture, information about its toxicity is virtually lacking in the literature. The available data for the simplest ciliated T. pyriformis [15] and T. thermophila [16-18] indicate that the toxicity of Lontrel is higher than that of Hymexazol by a factor of 5 - 10 (EC50 = 104.6 × 10–6 g/l = 0.54 × 10–6 М and 500 × 10–6 g/l = 5.05 × 10–6 М, respectively). According to our data, this difference is only 2 - 2.5 times. Our data for the infusoria demonstrate the ability of the pesticides under study to complexation with ATP. The reported data for zooplankton Daphnia magna are summarized in Figure 9. Our curve 1 and the EC50 values reported by different workers for six pesticides are seen to correlate.
The reported LC50 values for Crustacea are collected in Table 5. The toxicity of Roundup (to Orconectes nais), Hymexazol (to Decapoda), and Tilt (to Procambarus sp.) are seen to decrease in the above order according to our Kc values for these compounds.
The LC50 values for different fishes are summarized in Table 6 and Figure 9. As follows from Figure 9, our curve 1 well agrees with the available data for zooplankton and fish. These data also confirm a correlation between the Plt toxicity and the molecular mechanism of their action, namely, ATP binding in living cells.
As known, on the one hand, all living organisms, including fish, possess a unique system for excretion of foreign chemical compounds [12]. On the other hand, the pollutants under study influence not only on luciferase but also on the entire system of redox enzymes [19,20], free pool of metals [9], and nucleotides [4,5]. The whole set of biochemical reactions proceeds simultaneously, so that homeostasis is completely suppressed. The processes suppressing all functions of the organism increase in a geometric progression. All this leads to an increase in the toxicologic effect in vivo, i.e., to lower effective concentrations of pollutants. This circumstance can explain a different order for a decrease in Kc for the pesticides under study and ATP and in the toxicity of these compounds. Nevertheless, a correlation between the toxicity and Kc values was found to be well pronounced for all representatives of aquatic life.
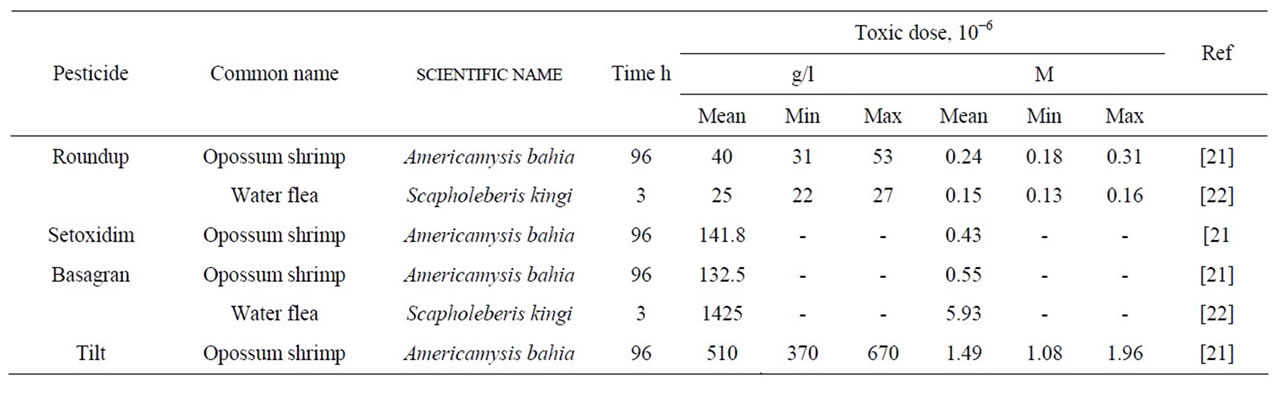
Table 4. Toxic effect of pesticides on different zooplankton species (LC50, from literary data).
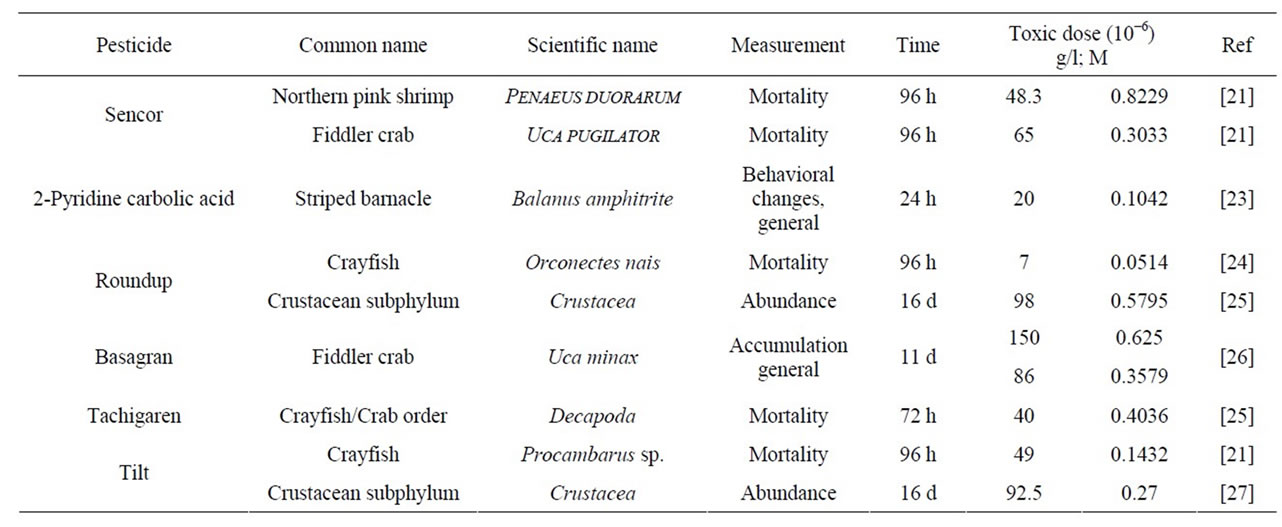
Table 5. Toxic effects of pesticides on crustacea (LC50, from literary data).
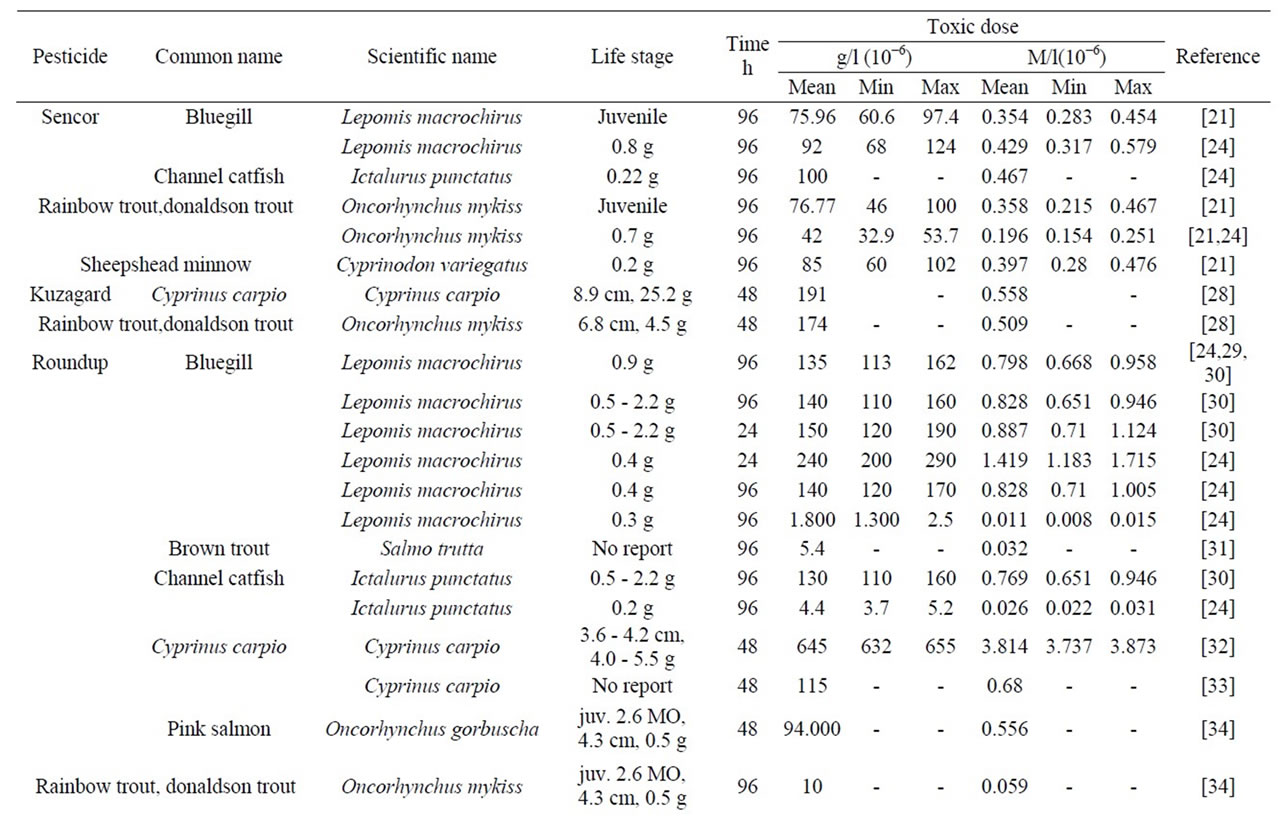
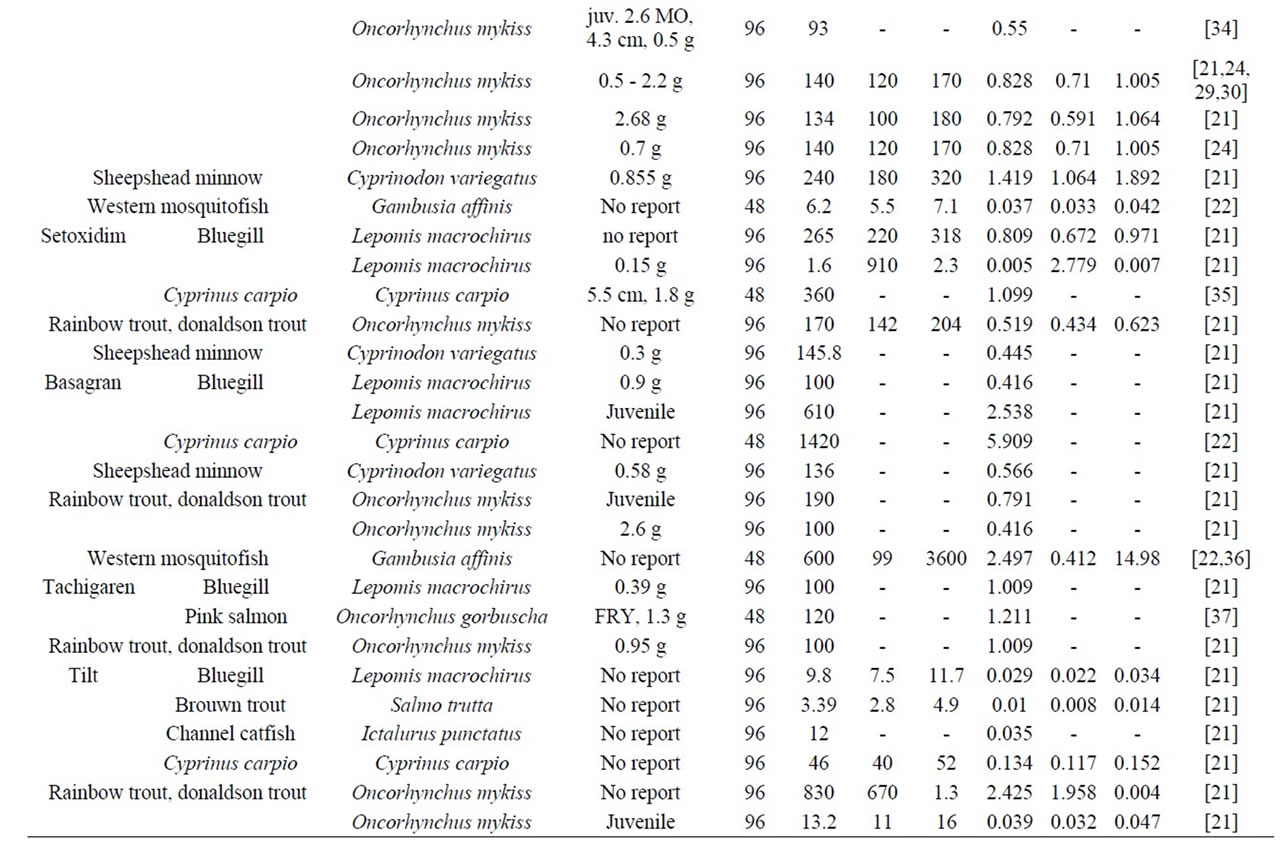
Table 6. Toxicity of pesticides with respect to fishes (mortality—LC50, from literary data).
Therefore, our data suggest that, upon ingress of Plt, pesticides, and their metal complexes in living organism, these compounds undergo chemical binding with ATP to give stable Plt-ATP complexes with their own Kc value. Such bioorganic complexes infringe energy metabolism in a living organism. The cells suffer from energy shortage, which leads to the death of the cells and sequentially of the entire organism. Such a mechanism of action manifests itself as a decrease in the enzymatic activity of bacteria or in the reproductive function of infusoria.
The established toxicity—Kc relationship was found to hold true for a wide variety of multicellular organisms. This relationship can be used for express-evaluation of pollutant toxicity towards representatives of aquatic life.
4. Conclusions
1) The toxicity factors of pesticides, viz., Lontrel, Sencor, Roundup, Bentazon, and Hymexazol and also Lontrel complexes with Cu, Co, Mn, Mg, Mo, Ni, and Zn towards the bacteria Beneckea harveyi and infusoria Tetrahymena pyriformis were measured.
2) The concentration dependence of the above toxicity factors was determined.
3) At any concentration, the toxicity of the pesticides was found to increase in the following order: Sencor > Lontrel > Roundup > Bentazon > Hymexazol, while that of the MetL2 complexes is as follows: CuL2 > CoL2 > NiL2 > MoL2 > MnL2 > ZnL2 > MgL2. The toxicity of MetL2 complexes is higher (except for MgL2) than that of free Lontrel.
4) Toxicity of that or another man-caused pollutant was found to be directly related to the energy shortage arising in cells under the action of pollutant complexes with ATP at the molecular level.
REFERENCES
- Yu. I. Skurlatov, G. G. Duka and A. Misiti, “Vvedenie v Ekologicheskuyu Khimiyu (Introduction to Ecological Chemiscry),” Vysshaya Shkola, Moscow, 1994, 400 p.
- E. A. Saratovskikh, N. B. Kozlova and V. V. Goncharov, “Pollution of Volga Water with Sewage of Kazan City,” Vodnye Resursy (Moscow), Vol. 24, No. 1, 1997, pp. 56- 60.
- E. A. Saratovskikh, N. B. Kozlova, V. G. Papin and E. V. Shtamm, “Biochemical and Photochemical Degradation of the Herbicide Lontrel,” Applied Biochemistry and Microbiology, Vol. 42, No. 1, 2006, pp. 38-44. doi:10.1134/S0003683806010042
- E. A. Saratovskikh, T. A. Kondrat’eva, B. L. Psikha, R. I. Gvozdev and V. G. Kartsev, “Complex-Formation of Some Pesticides with Adenosine Triphosphoric Acid,” Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science, Vol. 37, No. 11, 1988, pp. 2252-2258. doi:10.1007/BF00959872
- E. A. Saratovskikh, M. V. Lichina, B. L. Psikha and R. I. Gvozdev, “Character of the Reaction of Dinucleotides and Polynucleotides with Some Pesticides,” Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science, Vol. 38, No. 9, 1989, pp. 1822-1827. doi:10.1007/BF00957770
- E. A. Saratovskikh, R. I. Papina and V. G. Kartsev, “Influence of Some Pesticides on Dicotyledonous and Cereals,” Sel’skokhoziaystvennaya Biologia, Biologia Rastenii, Seria, Vol. 5, 1990, pp. 152-159.
- E. A. Saratovskikh, R. I. Papina and T. A. Kondrat’eva, “Deranged Metabolism of Cells during Germination of Dicotyledonous and Cereals under the Action of Pesticides and Metal Complexes,” Tsitologiya, Vol. 41, No. 3- 4, 1999, pp. 308-309.
- Z. G. Aliev, L. O. Atovmyan, E. A. Saratovskikh, V. I. Krinichnyi and V. G. Kartzev, “Synthesis, Structure, and Spectral Characteristics of Copper Complexes with Picolinic-Acid Derivatives,” Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science, Vol. 37, No. 11, 1988, pp. 2246-2252. doi:10.1007/BF00959871
- E. A. Saratovskikh, “Synthesis of Bidentate Complexes of 3,6-Dichloropicolinic Acid,” Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science, Vol. 38, No. 10, 1989, pp. 2140-2141. doi:10.1007/BF00962125
- “Method of Determining the Toxicity of Water and Water Extracts from the Soil, Sludge, Waste on Mortality and Fertility Change Daphnids,” Biological Control Methods, Akvaros, Moscow, 2001.
- “Manual for Determining the Toxicity of Waters, Bottom Deposits, and Drilling Fluids by Biotesting,” NIA-Priroda, Moscow, 2002.
- I. L. Tsvetkov and A. S. Konichev, “Ecological Biochemistry of Aquatic Life,” Publishing of Moscow State University, Moscow, 2006, 104 p.
- D. M. Pampanin, I. Marangon, E. Volpato, G. Campesan and C. Nasci, “Stress Biomarkers and Alkali-Labile Phosphate Level in Mussels (Mytilus Galloprovincialis) Collected in the Urban Area of Venice (Venice Lagoon, Italy),” Environmental Pollution, Vol. 136, No. 1, 2005, pp. 103-107. doi:10.1016/j.envpol.2004.12.010
- E. A. Saratovskikh and A. I. Bokova, “Influence of Herbicides on the Population of Soil-Dwelling Collembolan,” Toxicological Review, Vol. 5, 2007, pp. 17-23.
- T. W. Schultz, F. M. Applehans and G. W. Riggin, “Structure-Activity Relationships of Selected Pyridines. III. Log Kow Analysis,” Ecotoxicology and Environmental Safety, Vol. 13, No. 1, 1987, pp. 76-83. doi:10.1016/0147-6513(87)90044-3
- W. Pauli, S. Berger and S. Schmitz, “Validierung Toxikologischer Prufparameter an Tetrahymena: Membranfunktionen, Chemotaxis, Rotation im Elektrischen Drehfeld,” FU-Berlin, Inst. für Biochemie und Molekularbiologie, UFO-Plan, F+E-Vorhaben, 106 03 083 (OECDG Data File), 1993.
- W. Pauli, S. Berger, L. Jaskulka and S. Schmitz, “A Case for the Inclusion of a Protozoan Test in Aquatic Toxicity Assessment Using Tetrahymena,” Science of The Total Environment, Vol. 134, No. S1, 1993, pp. 779-786.
- W. Pauli, S. Berger, S. Schmitz and L. Jaskulka, “Chemosensory Responses of Ciliates: A Sensitive End Point in Xenobiotic Hazard Assessment,” Environmental Toxicology and Water Quality, Vol. 9, No. 4, 1994, pp. 341- 346. doi:10.1002/tox.2530090414
- E. A. Saratovskikh, L. A. Korshunova, R. I. Gvozdev and A. V. Kulikov, “Inhibition of the Nicotinamide Adenine Dinucleotide-Oxidoreductase Reaction by Herbicides and Fungicides of Various Structures,” Russian Chemical Bulletin, Vol. 54, No. 5, 2005, pp. 1322-1327. doi:10.1007/s11172-005-0401-6
- E. A. Saratovskikh, L. A. Korshunova, O. S. Roschupkina and Yu. I. Skurlatov, “Inhibition of the Nicotinamide Adenine Dinucleotide-Oxidoreductase Reaction by Metal Complexes,” Advances in Chemical Physics, Vol. 26, No. 8, 2007, pp. 40-45.
- “Pesticide Ecotoxicity Database [Formerly: Environmental Effects Database (EEDB)],” Environmental Fate and Effects Division, US EPA, Washington DC, 2000.
- F. Sun, “Evaluating Acute Toxicity of Pesticides to Aquatic Organisms: Carp, Mosquito Fish and Daphnids,” Plant Protection Bulletin, Vol. 29, No. 4, 1987, pp. 385-396.
- M. Kawamata, K. Kon-Ya and W. Miki, “Trigonelline, an Antifouling Substance Isolated from an Octocoral Dendronephthya sp.,” Fisheries Science, Vol. 60, No. 4, 1994, pp. 485-486.
- F. L. J. Mayer and M. R. Ellersieck, ‘Manual of Acute Toxicity: Interpretation and Database for 410 Chemicals and 66 Species of Freshwater Animals,” Resource Publications, No. 160, US Department Interior, Fish and Wildlife Service, Washington DC, 1986.
- H. Okudaira, “Hymexazol. A New Plant Protecting Agent, V. Safety Tests,” Sankyo Kenkyusho Nempo, Vol. 73, No. 25, 1973, pp. 48-51.
- G. M. Booth, C. C. Yu and D. J. Hansen, “Fate, Metabolism, and Toxicity of 3-Isopropyl-1H-2,1,3-Benzo-Thiadiazin-4(3H)-1-2,2-Dioxide in a Model Ecosystem,” Journal of Environmental Quality, Vol. 2, 1973, pp. 408-411. doi:10.2134/jeq1973.00472425000200030025x
- D. O. Hessen, T. Kallqvist, M. I. Abdel-Hamid and D. Berge, “Effects of Pesticides on Different Zooplankton Taxa in Mesocosm Experiments,” Norwegian Journal of Agricultural Sciences, No. 13, 1994, pp. 153-161.
- Z. Svobodova, J. Machova, R. Faina, A. Kocova and P. Kunce, “Acute Toxicity of Selected Pesticides to Aquatic Organisms,” Buletin Vyzkumny Uztav Rybarsky Hydrobiologicky Vodnany (Prague), Vol. 22, No. 1, 1986, pp. 8- 20.
- W. W. Johnson and M. T. Finley, “Handbook of Acute Toxicity of Chemicals to Fish and Aquatic Invertebrates,” Resource Publications, Fish and Wildlife Service, USDI, Washington DC, 1980.
- L. C. Folmar, H. O. Sanders and A. M. Julin, “Toxicity of the Herbicide Glyphosate and Several of Its Formulations to Fish and Aquatic Invertebrates,” Archives of Environmental Contamination and Toxicology, Vol. 8, No. 3, 1979, pp. 269-278. doi:10.1007/BF01056243
- K. J. Aanes, “Some Pesticides Used in Norwegian Agriculture and Their Environmental Effects on Common Inhabitants in Freshwater Ecosystems. Tolerance Limits,” In: A. Helweg, Ed., Pesticides in the Aquatic Environment. Appearance and Effects, 12-14 November 1991, Tune Landboskole, Denmark, pp. 108-131.
- N. K. Neskovic, V. Poleksic, I. Elezovic, V. Karan and M. Budimir, “Biochemical and Histopathological Effects of Glyphosate on Carp, Cyprinus carpio L.,” Bulletin of Environmental Contamination and Toxicology, Vol. 56, No. 2, 1996, pp. 295-302. doi:10.1007/s001289900044
- G. C. Li and C. Y. Chen, “Study on the Acute Toxicities of Commonly Used Pesticides to Two Kinds of Fish,” K’O Hsueh Fa Chan Yueh K’an, Vol. 9, No. 2, 1981, pp. 146-152.
- W. T. Wan, R. G. Watts and D. J. Moul, “Effects of Different Dilution Water Types on the Acute Toxicity to Juvenile Pacific Salmonids and Rainbow Trout of Glyphosate and Its Formulated Products,” Bulletin of Environmental Contamination and Toxicology, Vol. 43, No. 3, 1989, pp. 378-385. doi:10.1007/BF01701872
- Y. Nishiuchi, H. Iwamura and K. Asano, “Toxicity of Pesticides to Some Aquatic Animals. VI. Acute Toxicity of Latest Registered Pesticides to Some Aquatic Animals,” Seitai Kagaku, Vol. 8, No. 1, 1985, pp. 13-15.
- T. S. Leung, S. M. Naqvi and C. LeBlanc, “Toxicities of Two Herbicides (Basagran, Diquat) and Algicide (Cutrine-Plus) to Mosquito Fish Gambusia affinis,” Environmental Pollution Series A, Ecological and Biological, Vol. 30, No. 2, 1983, pp. 153-160. doi:10.1016/0143-1471(83)90012-0
- K. Kuroda, “Lethal Effect of Pesticides on Saghalien Trout Fry,” Water Purification Liquid Wastes Treatment, Vol. 16, No. 5, 1975, pp. 441-448.