Journal of Diabetes Mellitus
Vol.2 No.2(2012), Article ID:19356,7 pages DOI:10.4236/jdm.2012.22034
Four-hour evaluation of a medical food in subjects with type 2 diabetes receiving oral hypoglycemic medication
![]()
1Abbott Nutrition, Columbus, USA; *Corresponding Author: menghua.luo@abbott.com
2Kuban State Medical University Based on City Clinical Hospital of Emergency Care, Krasnodar, Russia
3Bashkir State Medical University Based on City Clinical Hospital #21, Ufa, Russia
4Russian State Medical University of Federal Agency of Healthcare and Social Development Based on City Clinical Hospital #68, Moscow, Russia
5Novosibirsk State Medical University Based on City Clinical Hospital #1, Novosibirsk, Russia
6Tyumen State Medical Academy, Tyumen, Russia
Received 21 February 2012; revised 27 March 2012; accepted 30 April 2012
Keywords: type 2 diabetes; diabetes specific formula; glucose control
ABSTRACT
Background: Postprandial hyperglycemia is an independent risk factor for diabetes-associated complications in individuals with type 2 diabetes. Dietary modification plays an important role in glycemic control. This study was to examine the efficacy of a diabetes specific formula (DSF) during a 4-hour postprandial meal tolerance test (MTT) in Russian subjects with type 2 diabetes receiving oral hypoglycemic medication. Methods: In a randomized, cross-over design, 168 eligible subjects from 11 study centers consumed, in a random order, the DSF (230 mL) or a common light hospital breakfast (i.e. standard meal) on two different occasions. The amounts of macronutrients were similar between the two meals providing ~200 kcal, 11 g protein, 26 g carbohydrate and 8 g fat. Capillary glucose levels were measured at baseline (before meal consumption), and post-meal consumption at 30, 60, 90, 120, 180 and 240 min. Results: The DSF was well tolerated in all subjects. There were 111 subjects completed the study per protocol (mean ± SEM: age: 58.6 ± 0.8 yr, BMI: 31.8 ± 0.42 kg/m2, waist circumference: 101 ± 1.3 cm, HbA1c: 8.0% ± 0.1%). Glucose levels reached peak values at 60 min (median) and the lowest levels at the end of the 240-min MTT test. The mean positive area under the curve (PAUC), the primary outcome, was significantly smaller after DSF consumption (mean ± SEM: 183.02 ± 18.74, median: 132.55) than the PAUC after consumption of the standard meal (mean ± SEM: 239.95 ± 23.11, median: 166.89; p = 0.027). The actual and adjusted peak glucose concentrations were similar between the two treatments. Conclusions: In patients with type 2 diabetes receiving oral hypoglycemic agents, compared to a hospital meal, the DSF improves postprandial glucose control. Combining results from earlier studies, long-term use of DSF may be beneficial to improve glucose management and decrease diabetes-associated complications.
1. INTRODUCTION
Incidence and prevalence of type 2 diabetes are increasing globally; the World Health Organization estimated that in 2000, 171 million people had diabetes, representing 2.8% of the world’s population, and predicts that this number will increase to 366 million (4.4%) by 2030 [1]. Per Diabetes Atlas (2009) [2], Russia was one of the “top 10” countries with the highest number of people with diabetes in the world in 2007.
Diabetes is marked by elevated blood glucose levels, which are the result of defects in insulin production, insulin action or both. Poorly controlled diabetes can lead to development of serious complications such as nephropathy, neuropathy, retinopathy, and peripheral and central vascular diseases. Improving glycemic control can significantly reduce and delay the occurrence of these complications [3,4]. Intensive treatment with pharmacologic glucose-lowering agents can be of great benefit in controlling diabetes; however, these therapies have been associated with adverse effects including weight gain and an increased risk of hypoglycemia. The risk of such adverse effects may be reduced through the concomitant use of diabetes-specific diet as an adjunctive therapy for post-meal glycemic control.
Attenuating post-meal glucose excursions is important for achieving HbA1c goals and preventing diabetes-associated retinopathy, cognitive dysfunction and macrovascular events [5,6]. The International Diabetes Federation (IDF) recommends that two-hour post-meal glucose levels should not exceed 7.8 mmol/L or 140 mg/dL, while at the same time avoiding hypoglycemia [5]. It has been shown that dietary modification is an effective way to help glycemic control in diabetes patients. Diabetes-specific nutritional products are designed to deliver balanced nutrients and minimize postprandial blood glucose excursions in people with diabetes. A diabetes-specific formula (DSF) (Glucerna Shake®, Abbott Nutrition, Columbus, Ohio, USA) was developed to provide a complete balance of high quality protein, a fat blend rich in monounsaturated-fatty acids (MUFAs) and low in saturated fatty acids (no trans fat), and a blend of slowlydigested and low glycemic carbohydrates and fiber. Compared to standard enteral formulas, the DSF has been shown to attenuate postprandial glucose spikes while providing high quality nutrition (protein, fat carbohydrate, vitamins, and minerals) that meets American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) dietary guidelines [7]. A 24-week, structured diabetes management program that included this formula as a partial meal replacement showed a decrease in HbA1c in patients with diabetes [8]. However, the effect of feeding the DSF compared to regular food in patients taking oral hypoglycemic medication is not clear. Therefore, the aim of this study was to assess the effect of the commercially available DSF in patients receiving oral hypoglycemic medication compared to a standard breakfast meal.
2. STUDY DESIGN AND METHODS
2.1. Overview of Study Conduct
Before the initiation of the multicenter study, an investigator meeting was held to discuss the study design and procedures with all principle investigators and study coordinators. All study personnel were trained on the protocol, informed consent procedures, case report forms (CRFs), maintenance of essential study documents, and all other study procedures at, or before, study initiation. During the study, a study monitor visited clinical centers on a regular basis to assure that: 1) All study procedures were correctly followed and in accordance with International Conference on Harmonization (ICH), Good Clinical Practice (GCP) and applicable local regulatory guidance; 2) Equipments were calibrated correctly; and 3) Data were collected and recorded accurately on CRFs.
All completed CRFs were transmitted to and managed at Abbott Nutrition by a data management team who were unaware of the randomization scheme. Once CRFs were received, data were validated by a multilevel review process and were entered in a format that was suitable for statistical analyses.
2.2. Human Subjects
This randomized, un-blinded, two-treatment, crossover study was conducted in 11 study centers in the Russian Federation in 2009-2010. Adult subjects (age ≥ 18 years old) with type 2 diabetes receiving stable doses of oral hypoglycemic medication for at least two months were eligible. Additional inclusion criteria were that a subject’s BMI was 20 - 40 kg/m2, screening HbA1c level was 6.5% - 11% and weight was stable for the past two months prior to screening visit. Subjects were excluded from the study if one of the following conditions existed: 1) Subject had been using exogenous insulin, Byetta® (by Amylin Pharmaceuticals, Inc.) or alpha-glucosidase inhibitor for glucose control; 2) Subject had confirmed type 1 diabetes and/or had history of diabetic ketoacidosis; 3) Subject currently had infection (requiring medication), inpatient surgery or received systemic corticosteroid treatment (injected, oral, inhaled or nasal) in the last 3 months; or received antibiotics in the last 3 weeks; 4) Subject had an active malignancy excluding dermal malignancies; 5) Subject had significant cardiovascular event within 6 months prior to study entry or history of congestive heart failure; 6) Subject had organ dysfunction, end stage organ failure (such as end stage renal disease) or was post organ transplant; 7) Subject had a chronic, contagious, infectious disease, such as active tuberculosis, Hepatitis B or C, or HIV; 8) Subject had been taking daily medications (excluding allowed medications) at doses that would interfere with nutrient absorption, metabolism, excretion, gastric motility, or blood glucose, as determined by the study physician; and 9) Subject was known to be allergic or intolerant to any ingredient found in the test meal.
Subjects were protocol evaluable if they 1) met inclusion and exclusion criteria; 2) prepared for the tests correctly; 3) received and consumed a study meal as planned; 4) had blood glucose level at baseline and 240 min and no more than one intermediate test result missed or outside of the allowed window during a test.
Prior to participation of the study, all subjects provided written informed consent, approved by an independent Ethics Committee and local Ethics Committee as applicable, and applicable privacy authorization.
2.3. Study Procedures
Eligible subjects were invited to a study center two times, preferably within 7 - 14 days apart, to receive either 1 serving of the DSF (230 mL) or a common light hospital breakfast (i.e. standard meal, composed of 230 mL 1.5% milk, 30 g white bread, and 4 g fat spread) by a randomization schedule generated by a computer program. Each study site had a set of sealed envelopes containing the subject treatment group assignment which were opened and used in ascending numerical order. The amounts of macronutrients were similar between the two meals providing ~200 kcal, 10 g protein, 26 g carbohydrate and 8 g fat (Table 1). Before each study visit, subjects were asked to consume at least an average of 150 g carbohydrates per day for 3 days and be fasted for 8 - 14 hours prior to each visit.
On the study day morning, subjects’ medications were reviewed to ensure no changes in hypoglycemic agents. Then, subjects whose capillary blood glucose level was 3.3 - 16.7 mmol/L (60 - 300 mg/dL) were instructed to take their routine oral hypoglycemic medications. Fifteen (±5) minutes after the medication was consumed, subjects were provided one of the study meals per randomization schedule. Capillary glucose levels by finger stick were determined at baseline (right before meal consumption), and post-meal consumption at 30, 60, 90, 120, 180 (±5) and 240 (±10) min. Snacks or meals were provided and consumed if there was a hypoglycemic symptom and after the test was completed.
Measuring plasma glucose levels for a meal tolerance
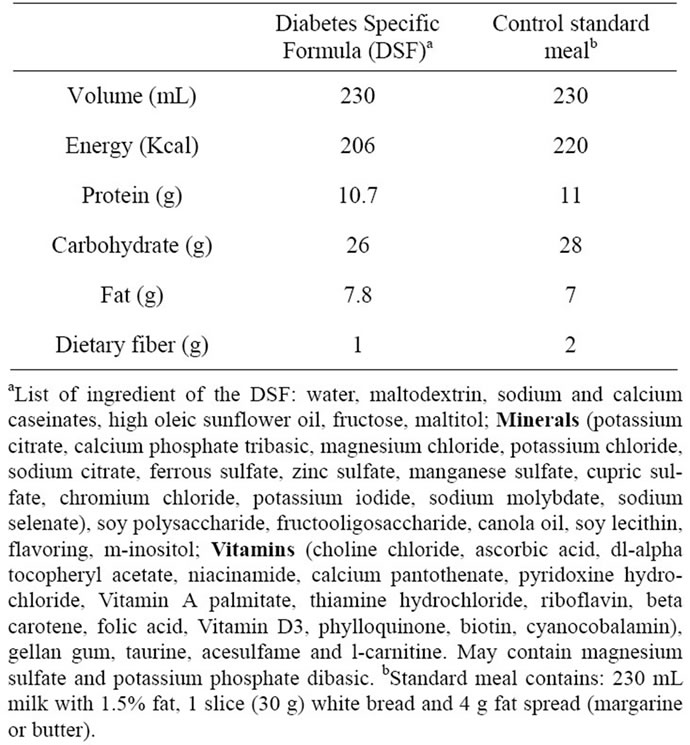
Table 1. Macronutrient composition.
test is not common practice in Russia Federation; therefore, glucometers (Precision Xtra™ Plus, Abbott Laboratories, Chicago, IL, USA) were provided to and used by all study sites. Each time a new box of test strips was opened, the glucometers were calibrated and used according to the manufacturer’s recommendation. The test strips were calibrated against YSI Glucose Analyzer (YSI Life Sciences) with typical variation less than 3.8% to 5.2%.
2.4. Statistical Analysis
The methodology for measuring glycemic response was adapted from previous studies [9,10]. The primary variable was the positive area under the curve (PAUC) for capillary glucose concentration over 0 to 240 minutes. Secondary variables included peak and adjusted peak glucose concentration, and peak time over 0 to 240 minutes. These variables were first examined with the mixed model approach for cross-over trials via SAS Proc Mixed. For all variables, there was evidence for the residuals to not be normally distributed. Therefore, all variables were analyzed with the non-parametric approach to the cross-over model utilizing the Wilcoxon Rank Sum Test. Data are presented as mean ± SEM.
3. RESULTS
A total of 168 subjects were enrolled in this study. A total of seven subjects did not complete the study due to 1) fasting glucose levels that were ≥16.7 mmol/L on the test day (n = 3) or 2) voluntary withdrawal or not returning for the 2nd visit (n = 4). Of the 161 subjects who completed the protocol, 111 subjects (25 males and 86 females) were considered to be protocol evaluable (age 58.6 ± 0.8 years old, weight 86.0 ± 1.5 Kg, BMI 31.8 ± 0.4 Kg/m2, waist circumference 101.1 ± 1.3 cm, hemoglobin A1c 8.0% ± 0.1%). At the screening, a majority of subjects (n = 76, 68.5%) were on one medication and 31 subjects (27.9%) were on 2 medications for glucose control. Sulfonylureas (such as Glibenclamide, Glimepiride and Diabeton MB) and biguanides (such as Metformin and Glucophage) were used most frequently in these patients (46.8% and 76.6%, respectively). A few patients received Glibomet (n = 4, 2.7%), Avandia (n = 2, 1.8%), Dipeptidyl peptidase IV inhibitor (n = 3, 2.7%), and Novoform (n = 4, 3.6%).
Mean actual and adjusted capillary glucose concentrations at individual time points are presented in Table 2 and Figure 1. The glucose values after consumption of the breakfast meal and the DSF appeared to be similar at each individual time point. Glucose levels reached peak values at 60 min (median) and nadir values at the end of the 240 minute of the MTT test. The peak glucose values were 11.35 ± 0.25 and 10.90 ± 0.27 mmol/L after consumption of the breakfast meal and the DSF, respectively
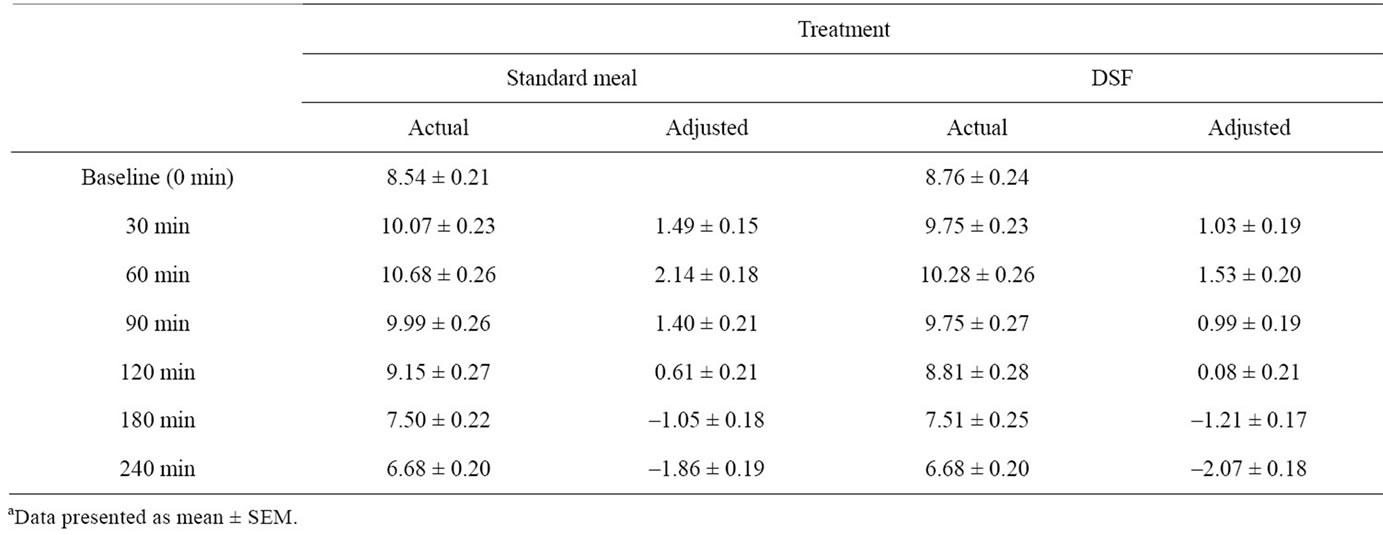
Table 2. Actual and adjusted capillary glucose concentrations (mmol/L) at individual time pointsa.
(p > 0.05). The area under the curve (AUC) was similar between the two treatments (data not shown).
Figure 2 illustrates the observation that the mean PAUC was significantly lower after consumption of the DSF (mean ± SEM: 183 ± 19, median: 133) than the standard meal (mean ± SEM: 240 ± 23, median: 167; p = 0.027).
Both study treatments were well tolerated. There were
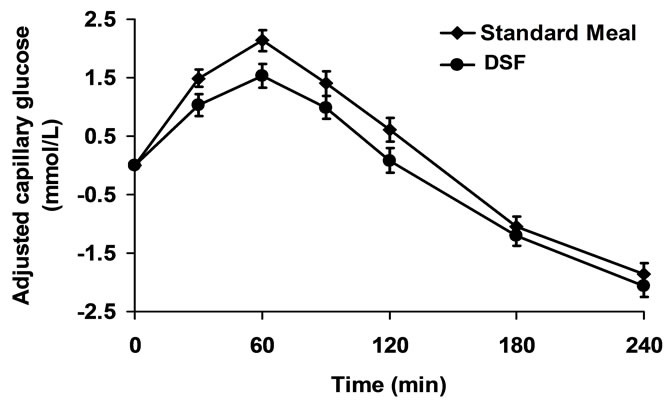
Figure 1. Adjusted capillary glucose levels over time.
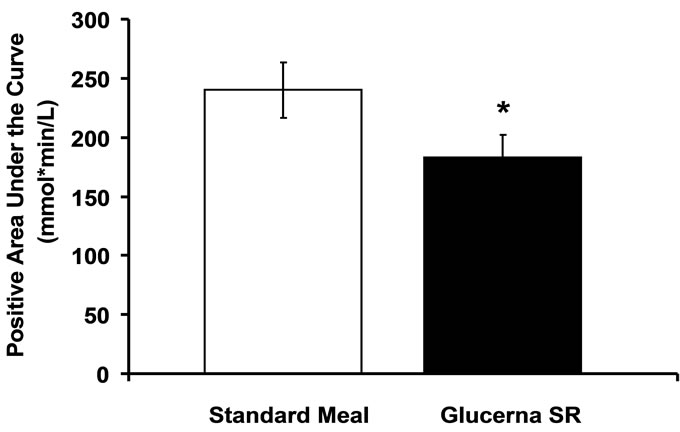
Figure 2. Positive area under the curve from baseline to 240 min. *p < 0.05 vs. standard meal.
three mild adverse events reported by 3 subjects. One reported flatulence following consumption of the DSF; one experienced hypoglycemia symptoms 240 minutes following consumption of the standard meal; and one developed a viral infection that did not have a temporal association with study products. Therefore, no safety concerns were noted for either study treatment.
4. DISCUSSION
Improving postprandial glycemic control is particularly important to prevent diabetes complications in patients with type 2 diabetes. In hospitalized patients, nutrition support is a critical component of medical care: adequate nutrition slows catabolism and can impact outcomes such as infection, complications, and lengths of hospital stay [11]. In patients with diabetes, nutrition support also must pay careful attention to the amount and type of carbohydrate and its resulting impact on glycemic control and diabetes complications. Formulas are specifically-designed for patients with diabetes to provide good tasting and high quality nutrition in a calorieand portion-controlled manner. Previous studies have shown that the DSF, a calorically-dense, diabetes-specific formula, is safe and effective in decreasing postprandial glucose and improving insulin sensitivity in healthy subjects [12] as well as in patients with impaired glucose tolerance in inpatient and outpatient settings [13, 14]. This study is the first to show that DSF also provides good post-prandial glycemic control when compared to a typical hospital breakfast.
Because patients with type 2 diabetes are likely to be overweight/obese, the ADA recommends lifestyle change for diabetes prevention and management, which includes decreased caloric consumption and increased energy expenditure via physical activity [15]. However, adherence to a dietary regimen to decrease caloric intake is often hard to achieve. Calorie replacement for high-calorie, low nutrient-value meals/snacks has become an effective component of diabetes management programs, particularly for those who lack the ability to self-select appropriate food choices and maintain portion control [15]. In a 24-week, structured diabetes-intervention program including exercise and diabetes education, the DSF, when used to replace liquid breakfast food items (such as milk, soymilk, rice soup, or congee), helped patients with type 2 diabetes to lose weight, reduce HbA1c level, and improve insulin sensitivity [8]. Similar beneficial effects of the DSF were also reported by Tatti et al. [16]. Thus, replacing a part of daily meals or snacks with the DSF can be a good strategy for glycemic control and weight management in patients with type 2 diabetes.
Numerous studies have shown that postprandial glycemic response is closely correlated with glycosylated hemoglobin [17], which contributes to complications of type 2 diabetes [18]. This study confirms that the DSF attenuated postprandial glucose response in patients with type 2 diabetes whose previous glucose control had been stable. The control treatment in this study was a common light hospital breakfast containing milk, white bread and fat spread. Macronutrient (energy, protein, carbohydrate, and fat) contents were similar between the DSF and the breakfast meal; however, the type and amount of carbohydrate and fat in the DSF are important elements in the management of blood glucose levels and prevention of diabetes complications. The carbohydrates from the standard meal contained simple sugar, which spikes postprandial glucose level to a great extent. In contrast, the DSF contains a blend of slowly-digested, low glycemic carbohydrates, which include fructose and maltitol. Fructose functions to facilitate glucose clearance through the formation of fructose-1-phosphate. This fructose intermediate metabolite reduces the inhibition of glucokinase in the liver, thus attenuating postprandial rise in blood glucose levels [19,20]. Maltitol is a low glycemic polyol. It is incompletely digested and absorbed and contributes to the reduced postprandial glycemic response [21]. In addition, the fiber contained in the DSF slows the gastric release and digestion of carbohydrates and contributes to the attenuated glycemic response.
The American Heart Association and ADA recommend an increased intake of omega-3 polyunsaturated fatty acid (PUFA) to decrease the risk of vascular disease in high-risk populations, including individuals with type 2 diabetes [15,22]. The fat source in the DSF is from high oleic sunflower oil and canola oil, both of which are high in monoand poly-unsaturated fatty acids and low in saturated fatty acids. Sanz-Paris et al. observed decreased glycemic response to the DSF formula which was high in MUFA compared to an enteral formula which was high in complex carbohydrate and low in fat
[23]. It has also been shown that a MUFA-rich diet with high plant-based omega-3 PUFA is beneficial for metabolic control and vascular function versus MUFAs alone [24]. Therefore, the unique combination of the carbohydrates and fat in the DSF provides an advantage in improving overall health status in patients with type 2 diabetes.
This study focused on the 240-minute glycemic response after consumption of the DSF or the breakfast meal. In contrast to a standard meal tolerance test, in which glucose level peaks at 90 to 120 minutes and returns to baseline/premeal levels by 240 minutes, we observed the peak glucose level at 60 minutes after both test meals. In addition, it was observed that the mean glucose level appeared to be the lowest at 240 minutes which was about 2 mmol/L lower than the baseline levels. The actual and adjusted glucose levels at individual time points were similar between the treatments. These may be explained in part by the administration of hypoglycemic agent(s) before the consumption of study meal. In fact, despite the use of hypoglycemic medication, the PAUC after the DSF consumption was statistically lower than that after the standard meal, indicating that replacing the hospital breakfast meal with the DSF has an additional effect of managing postprandial glucose levels in patients with type 2 diabetes taking oral hypoglycemic medication.
A high rate of unevaluable subjects was observed in this study (34%), mainly due to mistimed sample collection. All subjects in the study received stable doses of oral hypoglycemic medications for at least 2 months prior to screening and the medication did not change during the study. However, baseline capillary glucose levels between the two study visits appeared to be different in twelve evaluable subjects, defined as the difference between the two MTT was ≥5mmol/L. In addition, the highest glucose levels were observed at baseline in ten subjects. After contacting study sites, it was discovered that those subjects acknowledged consuming larger amount of carbohydrates (e.g., bread, cookies, etc.) on the day before one of their study visits than the day before the other visit. Because there was no exclusion criterion for the upper limit of carbohydrate intake, those subjects were included in the final evaluable analysis. However, data from these subjects may potentially confound the final results of the 4-hour meal tolerance tests.
5. CONCLUSION
In conclusion, this study shows the DSF is well-tolerated in patients with type 2 diabetes. Compared to the hospital breakfast meal, the DSF improves postprandial glucose control in patients with type 2 diabetes, in addition to the effects observed from hypoglycemic agents.
Combining results from earlier studies, long-term use of the DSF will be beneficial to improve glucose management and decrease diabetes-associated complications.
6. ACKNOWLEDGEMENTS
Abbott Nutrition (Columbus, Ohio, USA) provided funding this study and was responsible for study design, study monitoring, data analysis and manuscript preparation and submission.
![]()
![]()
REFERENCES
- Wild, S., Roglic, G., Green, A., Sicree, R. and King, H. (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care, 27, 1047-1053. doi:10.2337/diacare.27.5.1047
- Diabetes Atlas. (2009) International Diabetes Federation. http://www.eatlas.idf.org/index1397.html
- The Diabetes Control and Complications Trial Research Group. (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England Journal of Medicine, 329, 977-986. doi:10.1056/NEJM199309303291401
- UK Prospective Diabetes Study (UKPDS) Group. (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet, 352, 837-853. doi:10.1016/S0140-6736(98)07019-6
- Ceriello, A. and Colagiuri, S. (2008) International Diabetes Federation guideline for management of postmeal glucose: A review of recommendations. Diabetes Medicine, 25, 1151-1156. doi:10.1111/j.1464-5491.2008.02565.x
- Woerle, H.J., Neumann, C., Zschau, S., Tenner, S., Irsigler, A., Schirra, J., et al. (2007) Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Research Clinical Practice, 77, 280-285. doi:10.1016/j.diabres.2006.11.011
- Pastors, J.G., Warshaw, H., Daly, A., Franz, M. and Kulkarni, K. (2002) The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care, 25, 608-613. doi:10.2337/diacare.25.3.608
- Sun, J., Wang, Y., Chen, X., Chen, Y., Feng, Y., Zhang, X., et al. (2008) An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pacific Journal of Clinical Nutrition, 17, 514-524.
- Climt, C.R., Prout, T.E., Bradley, R.F., Dolger, H., Fisher, G., Gastineau, C.F., et al. (1969) Standardization of the oral glucose tolerance test. Diabetes, 18, 299-307.
- Wolever, T.M., Jenkins, D.J., Jenkins, A.L. and Josse, R.G. (1991) The glycemic index: Methodology and clinical implications. American Journal of Clinical Nutrition, 54, 846-854.
- Via, M.A. and Mechanick, J.I. (2011) Inpatient enteral and parenteral (corrected) nutrition for patients with diabetes. Current Diabetes Reports, 11, 99-105. doi:10.1007/s11892-010-0168-5
- Gonzalez-Ortiz, M., Martinez-Abundis, E., HernandezSalazar, E., Kam-Ramos, A.M. and Robles-Cervantes, J.A. (2006) Effect of a nutritional liquid supplement designed for the patient with diabetes mellitus (Glucerna SR) on the postprandial glucose state, insulin secretion and insulin sensitivity in healthy subjects. Diabetes Obesity Metabolism, 8, 331-335. doi:10.1111/j.1463-1326.2005.00512.x
- Coulston, A.M. (1998) Clinical experience with modified enteral formulas for patients with diabetes. Clinical Nutrition, 17, 46-56. doi:10.1016/S0261-5614(98)80017-4
- McCargar, L.J., Innis, S.M., Bowron, E., Leichter, J., Dawson, K., Toth, E. and Wall, K. (1998) Effect of enteral nutritional products differing in carbohydrate and fat on indices of carbohydrate and lipid metabolism in patients with NIDDM. Molecular Cellular Biochemistry, 188, 81-89. doi:10.1023/A:1006872506658
- Bantle, J.P., Wylie-Rosett, J., Albright, A.L., Apovian, C.M., Clark, N.G., Franz, M.J., et al. (2008) Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care, 31, S61-S78. doi:10.2337/dc08-S061
- Boj-Carceller, D., Bocos-Terraz, P., Moreno-Vernis, M., Sanz-Paris, A., Trincado-Aznar, P. and Albero-Gamboa, R. (2011) Are proton pump inhibitors a new antidiabetic drug? A cross sectional study. World Journal of Diabetes, 2, 217-220. doi:10.4239/wjd.v2.i12.217
- Monnier, L. and Colette, C. (2006) Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocrine Practice, 12, 42-46.
- Stolar, M. (2010) Glycemic control and complications in type 2 diabetes mellitus. American Journal of Medicine, 123, S3-S11. doi:10.1016/j.amjmed.2009.12.004
- Nuttall, F.Q., Gannon, M.C., Burmeister, L.A., Lane, J.T. and Pyzdrowski, K.L. (1992) The metabolic response to various doses of fructose in type II diabetic subjects. Metabolism, 41, 510-517. doi:10.1016/0026-0495(92)90210-2
- Wolf, B.W., Humphrey, P.M., Hadley, C.W., Maharry, K.S., Garleb, K.A. and Firkins, J.L. (2002) Supplemental fructose attenuates postprandial glycemia in Zucker fatty fa/fa rats. Journal of Nutrition, 132, 1219-1223.
- Wakabayashi, S., Kishimoto, Y. and Matsuoka, A. (1995) Effects of indigestible dextrin on glucose tolerance in rats. Journal of Endocrinology, 144, 533-538. doi:10.1677/joe.0.1440533
- Lichtenstein, A.H., Appel, L.J., Brands, M., Carnethon, M., Daniels, S., Franch, H.A., et al. (2006) Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation, 114, 82-96. doi:10.1161/CIRCULATIONAHA.106.176158
- Sanz-Paris, A., Calvo, L., Guallard, A., Salazar, I. and Albero, R. (1998) High-fat versus high-carbohydrate enteral formulae: Effect on blood glucose, C-peptide, and ketones in patients with type 2 diabetes treated with insulin or sulfonylurea. Nutrition, 14, 840-845. doi:10.1016/S0899-9007(98)00124-5
- West, S.G., Hecker, K.D., Mustad, V.A., Nicholson, S., Schoemer, S.L., Wagner, P., Hinderliter, A.L., Ulbrecht, J., Ruey, P. and Kris-Etherton, P.M. (2005) Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia, 48, 113-122. doi:10.1007/s00125-004-1600-7

