Advances in Chemical Engineering and Science
Vol.4 No.3(2014), Article
ID:47856,7
pages
DOI:10.4236/aces.2014.43040
Effect of Preparation Method on Catalytic Properties of Double Perovskite Oxides LaSrFeMo0.9Co0.1O6 for Methane Combustion
Jiandong Zheng, Xiongfeng Lang, Changjiang Wang
College of Material and Chemical Engineering, Chuzhou University, Chuzhou, China
Email: zjd071@126.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 6 May 2014; revised 6 June 2014; accepted 30 June 2014
ABSTRACT
The double perovskite oxides LaSrFeMo0.9Co0.1O6 was prepared by co-precipitation method and sol-gel method. The title catalysts were calcined at 800˚C and characterized by XRD H2-TPR, SEM and TG-DTA techniques. The catalytic activity was evaluated for methane combustion. The specific surface area of them was calculated by BET model. The samples exhibit significant catalytic activity for methane combustion at 800˚C. Upon calcination at 800˚C, the LaSrFeMo0.9Co0.1O6 prepared by sol-gel method retains a specific surface area of 24 m2∙g−1 and shows an excellent activity for methane combustion (the conversion of 10% and 90% are obtained at 505˚C and 660˚C, respectively).
Keywords:Methane Combustion, Double Perovskite Oxides, Catalytic Activity

1. Introduction
We know nature gas is an important economical energy. Catalytic combustion of nature gas is a crucial technology both for energy production and for environmental pollution abatement [1] -[4] . For heat generation process, using natural gas as fuel, catalytic combustion instead of the conventional combustion has several advantages, such as high efficiency and lower temperature which effectively suppresses thermal NOx formation. For the effective and stable catalytic combustion process, suitable catalysts play a crucial role. Generally, supported noble metal oxides, particularly palladium oxide, are excellent catalysts for lower temperature combustion, but noble metals are expensive and prone to deactivation owing to sintering, decomposition and undesirable interaction with supports under hydrothermal situations encountered in combustion [5] -[7] . A variety of inexpensive transition metal oxide catalysts, such as solid solution oxides, perovskites, pyrochloresand hexaaluminates have been explored for catalytic combustion of methane [8] -[11] .
The perovskite-type oxides have been extensively investigated as catalysts for the combustion of methane with their tailored catalytic property and thermal stability by versatile substitution of Aand/or B-sites. It is well-known that the catalytic properties of perovskites are mainly determined by the nature, oxidation states and relative arrangements of B-site cations. As a subclass of perovskite oxides ABO3, the double perovskites A2B'B''O6 have attracted considerable interest due to their unique structural [12] -[15] . For the double perovskiteoxides, two different B' and B'' cations occupy the B sublattice. They have more variations than the single perovskite-type oxides. The greater variation may promote catalytic chemistry for double perovskite type catalysts. Nevertheless, very few works have been reported in the last decade on exploring the catalytic properties of double perovskites. The double perovskite-oxides A2FeMoO6 (A = Ca, Sr and Ba) were studied as catalysts for catalytic combustion of methane in 2004 [16] [17] . Now few papers had been reported the effect of B'' site substitution in the double perovskites A2B'B''O6 for methane catalytic combustion.
We report here a new double perovskites LaSrFeMo0.9Co0.1O6 prepared by co-precipitation method and solgel method to catalyze combustion of methane. The properties of these catalysts were characterized by XRD, low temperature nitrogen adsorption-desorption (BET), TPR, SEM and TG-DTA techniques. Their catalytic activities were evaluated for methane combustion in a fix bed micro-reactor.
2 Experimental
2.1. Preparation of Materials
2.1.1. Co-Precipitation Method
LaSrFeMo0.9Co0.1O6 double perovskite was prepared by co-precipitation method (carbonates route). The stoichiometric amounts of analytical grade La(NO3)3∙6H2O, Sr(NO3)2, Fe(NO3)3∙6H2O and Co(NO3)2∙6H2O solution were prepared separately by dissolving nitrates in distilled water and MoO3 in ammonia. The solutions were mixed and added into a well-stirred container by addition of (NH4)2CO3 at constant temperature (80˚C) and pH value of 7 - 8. After filtering and washing with water several times, the solid product was dried at 120˚C for 12 h and then calcined at 800˚C in Muffle furnace for 4 h under air. The sample thus prepared is referred to as C1 hereafter.
2.1.2. Sol-Gel Method
The same ingredient LaSrFeMo0.9Co0.1O6 sample was prepared by sol-gel method. The stoichiometric amounts of analytical grade La(NO3)3∙6H2O, Sr(NO3)2, Fe(NO3)3∙6H2O and Co(NO3)2∙6H2O solution were prepared separately by dissolving nitrates in distilled water and MoO3 in ammonia. The solutions were mixed and added into a well-stirred container by addition of citric acid at constant temperature (60˚C) The final solution was evaporated until a gel was formed and then the gel was kept in an oven at 120˚C to obtain powder. The resulting powder was calcined at 800˚C in Muffle furnace for 4 h under air to form mixed oxide. The sample thus prepared is referred to as S1 hereafter.
2.2. Characterization
The phase composition of the calcined samples was determined by X-ray powder diffraction (XRD) using a Ni filter and CuKα radiation, at 40 kV and 30 mA. The data were collected between 15˚ and 70˚.
The specific surface areas of the samples were measured on a Gemini V 2380 Series Instrument using N2 adsorption at liquid N2 temperature. The specific surface area was determined according to the Brunauer-Emmett-Teller theory.
H2-TPR measurements were carried out in a flow reactor equipped with a thermal conductivity detector. The samples of 0.05 g were previously pretreated in helium at 400˚C for 30 min, and then they were cooled to room temperature. A 5 vol% H2/N2 stream (20 ml/min) was passed over the sample while it was heated from 40˚C to 800˚C at the heating rate of 10˚C/min.
The micro-morphologies of testing samples were scrutinized on a field emission scanning electron microscope (SEM, JEOL JSM-6510LV).
TG and DSC were carried out on a SDT-Q600 thermal analyzer. Samples were tested over the temperature range from room temperature up to 700˚C at the constant heating rate of 10˚C/min in air.
2.3. Catalytic Activity Test
The reaction of methane combustion was carried out in a conventional microreactor under atmospheric pressure. Catalyst (300 mg, 420 - 841 μm) was loaded in a quartz reactor (i.d. 8 mm), with quartz wool sealed at both ends of the catalyst bed. A mixture of 1 vol% methane and 99 vol% air was fed into the catalyst bed at GHSV = 50,000 h−1. The output gas compositions were analyzed by an on-line gas chromatography (GC9890) with a capillary column and a flame ionization detector (Temperature of column: 150˚C, Temperature of sample injector: 200˚C, Temperature of detector: 230˚C).
3 Results and Discussion
3.1. Crystalline Phases and Specifics Surface Area
The XRD patterns of two catalysts are shown in Figure 1. It can be seen that the sample have diffraction peaks at 2θ = 25˚, 30˚, 33˚, 46˚, and 58˚, which are typical double perovskite diffraction peaks. The ordered double perovskites LaSrFeMo0.9Co0.1O6 may be regarded as a regular arrangement of alternating FeO6 and MoO6/CoO6 corner-shared octahedra, with La and Sr cations occupying the voids in between the octahedral. A single perovskites of LaCoO3, LaMoO3, SrMoO3 and SrCoO3 can be seen in the Figure 1. It is worthy of notice that those spinel can form double perovskites via solid state reactions. Compared with the sample C1, The catalyst S1 has lower intensity. This implies that the preparation method is important in the formation of double perovskites crystal. Sol-gel method renders the precursor mixture homogenous and enables facile mass transfer, resulting in a formation of pure double perovskites during subsequent calcination at 800˚C.
The specific surface areas (SSA) of the LaSrFeMo0.9Co0.1O6 samples after calcination at 800˚C are shown in Table1 We can see the SSA of S1(24 m2×g−1) is larger than that of C1(16 m2×g−1). This it is due to sol-gel method makes the precursor mixture homogenous and enables facile mass transfer.
3.2. Temperature-Programmed Reduction by Hydrogen
The H2-TPR profiles of two samples are plotted in Figure 2 to compare their redox properties. It is impossible
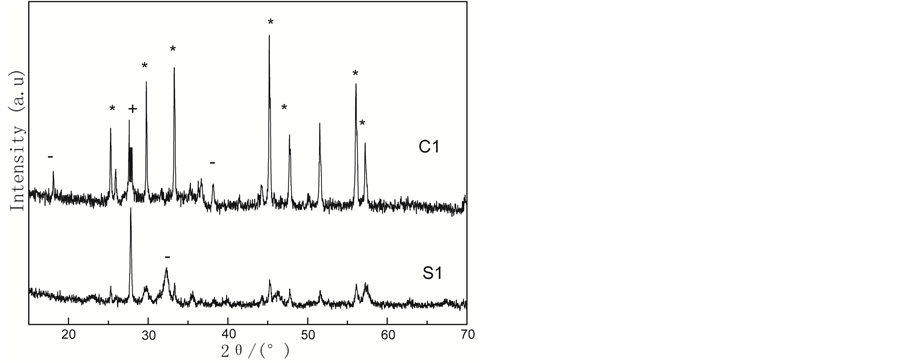
Figure 1. XRD patterns for LaSrFeMo0.9Co0.1O6 prepared by different methods. *Double perovskite-LaCoO3 SrCoO3 + LaMoO3 SrMoO3.
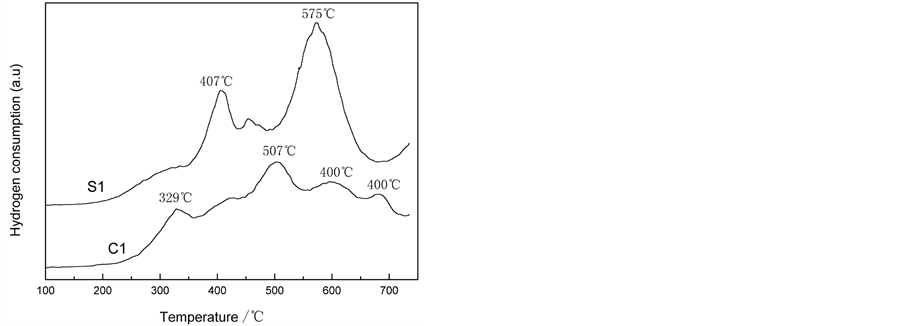
Figure 2. H2-TPR profiles of the LaSrFeMo0.9Co0.1O6 prepared by different methods.
to reduce the La3+ and Sr2+ ions at A-site under H2-TPR condition, the H2 consumption peaks displayed on the TPR diagrams of the oxides come from the reduction of B site ions [13] . The curves of LaSrFeMo0.9Co0.1O6 prepared by sol-gel method have two peaks at 407˚C, 575˚C, corresponding to Fe3+ → Fe2+, MoO3 → MoO2 and Co2+ → Co. By contrast, the peak area of C1 is smaller than that of S1. It suggests there has more active oxygen species available for the oxidation reaction of S1. It is in line with the characterization of activity.
3.3. TG-DSC Analysis
TG-DSC results of C1 and S1 after 120˚C ageing are shown in Figure 3. When the samples are heated from room temperature to 800˚C at a constant heating rate of 10˚C×min−1, C1 and S1 loose about 30% and 70% of weight, respectively. It shows that more organic compounds such as citric acid are removed during the sol-gel process. The samples of C1 and S1 behave differently. For C1 sample, DSC curve presents two lower endothermic peaks and a stronger exothermic peak before 600˚C. The first endothermic peak between 250˚C and 450˚C is due to the removal of the adsorbed water and the decomposition of SrMnCO3, MnCO3 and so on. The loss of weight is above 20% when the temperature is from 100˚C to 450˚C. The second peak appears when the temperarure is above 550˚C. However, the loss of weight is only 6.4%.
By contrast, For S1 sample, DSC curve presents three endothermic peaks before 700˚C. The first endothermic peak below 150˚C is due to the removal of the excess citric acid and adsorbed water. The second peak between 300˚C and 450˚C corresponds to the decomposition of carbonate such as MnCO3 and so on. At the same time the loss of weight is 20% when the temperature is from 300˚C to 500˚C. When the temperature is above 450˚C, there is a bigger endothermic peak. However, only 7% weight loss is observed.
So we think, in the process of transformation to form double perovskite oxide, all the losses of weight are due to the removal water, citric acid and the carbonate decomposition. When the temperature is above 550˚C, it begins to form double perovskite oxide. In the process of transformation to form double perovskite oxide, it will absorb heat, but no loss arises.
3.4. Morphology of Catalysts
Figure 4 presents the SEM images of the catalysts. The catalysts all had the layered structure which can retard the sintering of the material. It was noted that the product of this reaction displayed different particle morphologies with the change in preparation method, from flakes to irregular grains. They were composed of plate-like particles, which were 5 - 10 nm in thickness. From Figure 4, we can see the sample S1 like chrysanthemum. There are lots of honeycombs in the sample S1. It can result that the sample S1 has the higher specific surface areas.
3.5. Catalytic Activity
The catalytic behaviors for methane combustion over catalysts are shown in Figure 5 and Table1 All the cata-
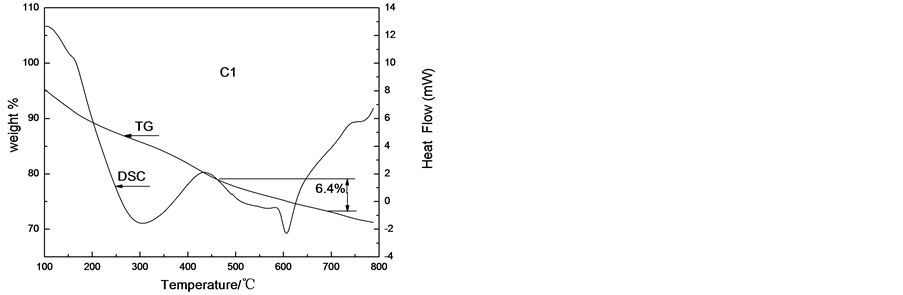
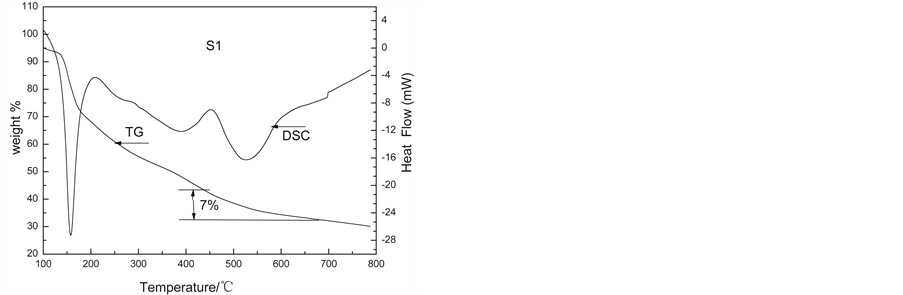
Figure 3. The TG-DSC curves of LaSrFeMo0.9Co0.1O6 after 120˚C ageing.
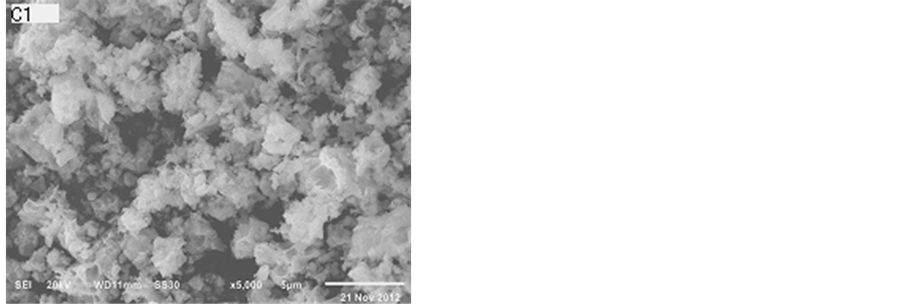
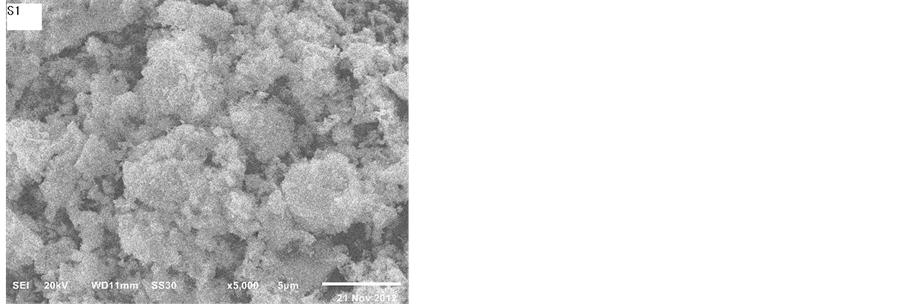
Figure 4. SEM images of catalysts after calcination at 800˚C.
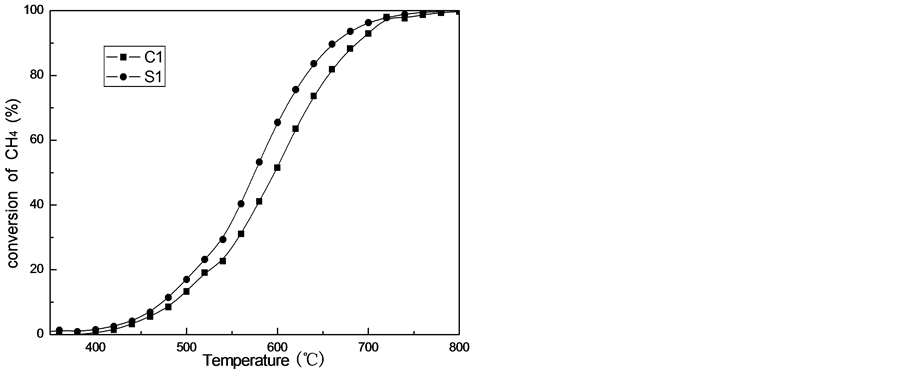
Figure 5. Catalytic activities of catalysts in the combustion of methane.
lysts, tested under identical experimental conditions, exhibit the S-shaped profiles for CH4 conversion as a function of reaction temperature. Usually, the activity of methane catalytic combustion was characterized by T10%, T50% and T90% representing the reaction temperature at methane conversion of 10%, 50% and 90% respectively. The T10% and T90% of C1 catalyst are 530˚C and 684˚C, respectively. Compared with the C1 catalyst, the S1 catalyst shows higher activity, its T10% and T90% are 505˚C and 660˚C, respectively. Its light-off temperature T10% decreases by 25˚C and total conversion temperature T90% decreases by 24˚C, respectively. It is obvious that the difference in catalytic activity is attributed to the difference in the preparation. Thus we believe that LaCoO3 and SrCoO3 is existed in C1, when the temperature is higher, it will be sintered. Sol-gel method renders the precursor mixture homogenous.
4. Conclusion
The effect of preparation method on the catalytic activity of double perovskite oxides LaSrFeMo0.9Co0.1O6 for methane combustion was investigated. The results showed that LaSrFeMo0.9Co0.1O6 prepared by sol-gel method has better catalytic activity than that prepared by co-precipitation. The catalyst LaSrFeMo0.9Co0.1O6 prepared by sol-gel method possesses the higher specific surface area (24 m2∙g−1) and has the lowest temperature for initial and complete conversion of methane than those prepared by co-precipitation method. It could be concluded that preparation method has a great impact on the catalytic activity.
Acknowledgements
We are very grateful to the Education Department Natural Science of Anhui Province (KJ2012A213), Project of Teaching Research (2012jyxm534) and the project of Chu Zhou University (2014CXXL054) for the financial support.
References
- Yan, X. and Crookes, R.J. (2010) Progress in Energy and Combustion Science. Progress in Energy and Combustion Science, 36, 651-676. http://dx.doi.org/10.1016/j.pecs.2010.02.003
- Gao, Z. and Wang, R. (2010) Catalytic Activity for Methane Combustion of the Perovskite-Type La1-xSrxCoO3 Oxide Prepared by the Urea Decomposition Method. Applied Catalysis B, 98, 147-153. http://dx.doi.org/10.1016/j.apcatb.2010.05.023
- Choudhary, T.V., Banerjee, S. and Choudhary, V.R. (2010) Catalysts for Combustion of Methane and Lower Alkanes. Applied Catalysis A, 234, 1-23. http://dx.doi.org/10.1016/S0926-860X(02)00231-4
- Fabbrini, L. and Rossetti, I. (2003) Effect of Primer on Honeycomb-Supported La0.9Ce0.1CoO3 Perovskite for Methane Catalytic Flameless Combustion. Applied Catalysis A, 44, 107-116.
http://dx.doi.org/10.1016/S0926-3373(03)00025-0 - Li, C., Wang, W. and Zhao, N. (2011) Structure Properties and Catalytic Performance in Methane Combustion of Double Perovskites Sr2Mg1-xMnxMoO6. Applied Catalysis B, 102, 78-84.
http://dx.doi.org/10.1016/j.apcatb.2010.11.027 - Li, C. and Wang, W. (2011) Double Perovskite Oxides Sr2Mg1-xFexMoO6 for Catalytic Oxidation of Methane. Journal of Natural Gas Chemistry, 20, 345-349.
http://dx.doi.org/10.1016/S1003-9953(10)60211-3 - Li, S. and Wang, X. (2006) Catalytic Combustion of Methane over Mn-Substituted Ba-La-Hexaaluminate Nanoparticles. Journal of Alloys and Compounds, 432, 333-337.
http://dx.doi.org/10.1016/j.jallcom.2006.06.022 - Todd, H., Gardner, J.J. and Spivey, A.C. (2013) CH4-CO2 Reforming over Ni-Substituted Barium Hexaaluminate Catalysts. Applied Catalysis B, 157, 129-136.
- Hu, R.S., Ding, R.R., Chen, J., Hu, J.N. and Zhang, Y.L. (2012) Preparation and Catalytic Activities of the Novel Double Perovskite-Type Oxide La2CuNiO6 for Methane Combustion. Catalysis Communications, 21, 38-41. http://dx.doi.org/10.1016/j.catcom.2012.01.008
- Ren, X., Zheng, J. and Song, Y. (2008) Catalytic Properties of Fe and Mn Modified Lanthanum Hexaaluminates for Catalytic Combustion of Methane. Catalysis Communications, 9, 807-810.
http://dx.doi.org/10.1016/j.catcom.2007.09.004 - Machej, T. and Serwicka, E.M. (2014) Cu/Mn-Based Mixed Oxides Derived from Hydrotalcite-Like Precursors as Catalysts for Methane Combustion. Applied Catalysis A: General, 47, 487-494.
- Xiong, Y. and Liang, H. (2009) Partial Oxidation of Methane to Syngas over the Catalyst Derived from Double Perovskite (La0.5Sr0.5)2FeNiO6. Applied Catalysis A: General, 371, 153-160.
http://dx.doi.org/10.1016/j.apcata.2009.09.044 - Baylet, A., Royer, S. and Mare, P. (2008) Effect of Pd Precursor Salt on the Activity and Stability of Pd-Doped Hexaaluminate Catalysts for the CH4 Catalytic Combustion. Applied Catalysis B: Environmental, 81, 88-96.
- Zhang, K., Zhou, G.D. and Li, J. (2009) Effective Additives of A (Ce, Pr) in Modified Hexaaluminate LaxA1-xNiAl11O19 for Carbon Dioxide Reforming of Methane. Catalysis Letters, 130, 246-253. http://dx.doi.org/10.1007/s10562-009-9876-3
- Wang, Y., Ouyang, J.H. and Liu, Z. (2010) Influence of Dysprosium Oxide Doping on Thermo Physical Properties of LaMgAl11O19 Ceramics. Materials and Design, 31, 3353-3357.
http://dx.doi.org/10.1016/j.matdes.2010.01.058 - Falcon, H. and Barbero, J. (2004) Double Perovskite Oxides A2FeMoO6 (A = Ca, Sr and Ba) as Catalysts for Methane Combustion. Applied Catalysis B, 53, 37-45.
http://dx.doi.org/10.1016/j.apcatb.2004.05.004 - Zheng, J.D. and Ren, X.G. (2008) Catalytic Properties of a (A = Ba, Ca, Sr, and Y) Modified Lanthanum Hexaaluminates for Catalytic Combustion of Methane. Reaction Kinetics and Catalysis Letters, 93, 3-9. http://dx.doi.org/10.1007/s11144-007-5165-6


