Food and Nutrition Sciences
Vol.4 No.2(2013), Article ID:27726,12 pages DOI:10.4236/fns.2013.42025
Preliminary Evaluation for Comparative Antioxidant Activity in the Water and Ethanol Extracts of Dried Citrus Fruit (Citrus unshiu) Peel Using Chemical and Biochemical in Vitro Assays
![]()
Department of Convergence Industry, Health Industry Program, The Seoul Venture University, Seoul, South Korea.
Email: coffee670@naver.com
Received December 16th, 2012; revised January 16th, 2013; accepted January 23rd, 2013
Keywords: Antioxidant; Citrus unshiu Peel; Total Phenolics; Flavonoid; Tannin
ABSTRACT
Comparative antioxidant activities of the water and ethanol extracts obtained from dried citrus fruit (Citrus unshiu) peel were determined using chemical and biochemical in vitro assays. Chemical assays were used for evaluation of 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging, hydrogen peroxide scavenging, and reducing power of both extracts and their total phenolic, flavonoid and tannin contents and antioxidant activities were investigated. Biochemical assays were performed to evaluate the inhibition activities of AAPH-induced rat RBC hemolysis and Fe2+-induced lipid peroxidation using rat brain tissue cells. Linear correlation between the antioxidant activities of both extracts were determined by chemical assays, and total phenolic, flavonoid and tannin contents was observed in concentration-dependent mode. Both extracts showed >95% DPPH radical scavenging and >85% hydrogen peroxide scavenging, and higher reducing capacity at the same level of 3200 ppm. In the inhibition activity of AAPH-induced hemolysis, water extracts showed a strong activity in concentration-dependent mode up to 1600 ppm with no statistical difference found between 1600 and 3200 ppm (P > 0.05). In the inhibition activity of Fe2+-induced lipid peroxidation, ethanol extracts showed the higher inhibition percentage of lipid peroxidation than those of water extracts at the same concentration with no significant difference (P > 0.05) found in the range of 800 to 3200 ppm. The extracts of dried Citrus unshiu peel may be considered as potential antioxidant ingredients of functional food depending on the conditions at which reactive oxygen species are implicated.
1. Introduction
Presently, as humans are expressing more and more concerns on their health, many health-designing foods in nature, which provide health benefits over and above basic nutrition, are attracting increasing attention, among which citrus fruits are one of the major topics. Because citrus fruits are among the most abundant crops in the world [1] and have high content of nutrients such as vitamin C, carotenoids, and minerals as well as phenolic compounds, which have important role in the nutritional, organoleptic and commercial properties of agricultural foodstuffs by contributing to color, astringency, bitterness and flavor [2,3]. In particular, phenolic compounds including phenolic acids, flavonoids, and hydrolysable tannins present in citrus fruits have gained much attention due to their strong antioxidant capacity and free radical scavenging abilities, which potentially have beneficial implications in human health [4-6].
The modern life style of individuals causes over production of free radicals or reactive oxygen species such as superoxide anion radicals ( ), hydroxyl radicals (OH×), and hydrogen peroxide (H2O2). Our body has a natural defense mechanism to cope with this oxidative stress, but problems arise when the free radicals outweigh the defense mechanism, thereby damaging essential biomolecules such as proteins, DNAs and lipids, which eventually causes atherosclerosis, aging, cancer, diabetes mellitus, inflammation, and several degenerative diseases in humans [7-10]. Recently interest has been growing in obtaining natural dietary antioxidants from plant-based foods. Natural antioxidants rich in phenolic compounds play an important role in health care as they provide protection from oxidative stress and associated diseases [11,12]. Epidemiological studies on dietary citrus fruits have shown a strong correlation between the antioxidant properties of phenolic compounds and their health-promoting and diseasepreventing effects [13-16]. Citrus peel is the main waste fraction of citrus fruits, which represents roughly half of the fruit mass and contains a high concentration of phenolic compounds [17]. Reports have been made that citrus peel contained more bioactive compounds such as phenolic acid, flavonoids, tannins, and limonoids than the citrus juice [18,19], also a main source of several flavonoid compounds such as flavanones, flavanone glycosides, and polymethoxylated flavones unique to citrus, which are rare in other plants [20,21].
), hydroxyl radicals (OH×), and hydrogen peroxide (H2O2). Our body has a natural defense mechanism to cope with this oxidative stress, but problems arise when the free radicals outweigh the defense mechanism, thereby damaging essential biomolecules such as proteins, DNAs and lipids, which eventually causes atherosclerosis, aging, cancer, diabetes mellitus, inflammation, and several degenerative diseases in humans [7-10]. Recently interest has been growing in obtaining natural dietary antioxidants from plant-based foods. Natural antioxidants rich in phenolic compounds play an important role in health care as they provide protection from oxidative stress and associated diseases [11,12]. Epidemiological studies on dietary citrus fruits have shown a strong correlation between the antioxidant properties of phenolic compounds and their health-promoting and diseasepreventing effects [13-16]. Citrus peel is the main waste fraction of citrus fruits, which represents roughly half of the fruit mass and contains a high concentration of phenolic compounds [17]. Reports have been made that citrus peel contained more bioactive compounds such as phenolic acid, flavonoids, tannins, and limonoids than the citrus juice [18,19], also a main source of several flavonoid compounds such as flavanones, flavanone glycosides, and polymethoxylated flavones unique to citrus, which are rare in other plants [20,21].
Citrus unshiu, one of the genus Citrus plants and belonging to the family Rutaceae, is a seedless and easy peeling fruit known as Satsuma mandarin. It is also one of the citrus varieties widely planted and consumed in East-Asia including Korea [22]. The Citrus fruit has been used for culinary purposes and the dried peel of Citrus fruit, which is known as Chenpi [23], has been widely used as a traditional medicine for centuries to treat indigestion and inflammatory syndromes of the respiratory tract such as bronchitis and asthma [22,24]. The dried peels of Citrus fruit have been traditionally consumed as tea or medicinal liquor, which is infused by hot water or alcohol respectively, and is supposed to be beneficial to the health. Use of peel of various citrus fruit species has recently drawn much attention, not only due to its economic value as a food product, but also for its healthpromoting properties [24-26]. However, limited information is available on baseline of the dried peel extracts of Citrus unshiu species for its use as a functional food as well as on the possible correlation between the chemicals (phenolics, flavonoids, and tannins) and biological properties such as antioxidant activity of dried peel extracts of Citrus unshiu. The objective of the present study was 1) to evaluate the antioxidant activity in the water and ethanol extracts obtained from dried peel of Citrus unshiu using in vitro chemical assays; 2) to measure the total phenolic, flavonoid and tannin contents in the water and ethanol extracts of dried peel; 3) to identify a correlation between the antioxidant activity and phenolic content of dried peel extracts of Citrus unshiu; 4) to compare the antioxidant powers of the water and ethanol extracts of dried Citrus unshiu peel using in vitro biochemical assays.
2. Materials and Methods
2.1. Plant Materials and Chemicals
Dried citrus fruit (Citrus unshiu) peels, collected in the Chejudo, South Korea, were obtained from the Jegidong herb market in 2009. The dried samples were ground into coarse powder using a National Model MX-T2GN blender (National, Taipei, Taiwan) at medium speed. The sample was placed in the polyethylene bags and kept at -20˚C until analyzed. All chemicals were of analytical grade and were obtained from Sigma-Aldrich Chemical Company (St. Louis, MO, USA). Cross-linked insoluble polymer PVPP (polyvinyl-polypyrrolidlone) was purchased from BASF Chemical Company (Ludwigshafen, Germany).
2.2. Preparation of the Water and Ethanol Extracts
For water extract, 20 g of the dried sample was extracted by refluxing for 1 h with 200 ml of distilled water according to the method described by Tang et al. [27]. The obtained extract was cooled and filtered over Whatman No. 1 paper. The residue was re-extracted and filtered by the same method as mentioned above. The filtrates were pooled and concentrated under vacuum at 40˚C and then lyophilized to obtain a water extract. A part of the water extract obtained was diluted and prepared with distilled water at different concentrations for analysis. For ethanol extract, 20 g of the dried sample was extracted with 200 ml of ethanol (95%, v/v) by the same method used for water extract, and the obtained ethanol extract was diluted and prepared in a mixture of ethanol and water (7:3) at different concentrations for analysis.
2.3. Antioxidant Activity of the Water and Ethanol Extracts Using Chemical Assays
2.3.1. Determination of DPPH Free Radical Scavenging Activity
The 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging activities of the water and ethanol extracts of dried citrus fruit peel were determined at different concentrations of 5 to 4000 ppm (mg/ml) according to a method outlined by Kim [28] based on the method of Hatano et al. [29]. Four milliliter of the water and ethanol extract dissolved in water and ethanol mixture (Etoh:H2O = 7:3), respectively was mixed with 1 ml of 0.2 mM DPPH in methanol. The mixture was shaken vigorously and reacted for 30 min prior to the analysis. The absorbance of reaction mixture (0.1 ml) was measured at 517 nm. The scavenging activity of extracts was calculated using the following Equation (1): 1,1-diphenyl- 2-picryl hydrazyl (DPPH)
 (1)
(1)
where A517 of control is the absorbance of blank sample treated with no added extract and A517 of sample is the absorbance in the presence of extract.
2.3.2. Determination of H2O2 Scavenging Activity
The hydrogen peroxide (H2O2) scavenging activities of the water and ethanol extracts of dried citrus fruit peel were determined at different concentrations of 50 to 3200 ppm (mg/ml) by the method of Atmani et al. [30] with a slight modification. Two milliliters of extract was mixed with 1.2 ml of H2O2 (20 mM) in phosphate buffer (pH 7.4), and the mixture was incubated for 10 min. After incubation of the mixture, the absorbance was measured at 230 nm. A blank solution was prepared by the same way but without H2O2. The scavenging activity of extracts was calculated using Equation (2):
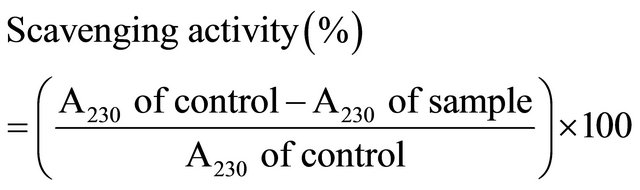 (2)
(2)
where A230 of control is the absorbance of blank solution with no added extract and A230 of sample is the absorbance in the presence of extract.
2.3.3. Determination of Reducing Power
The reducing powers of the water and ethanol extracts of dried citrus fruit peel were measured at 0, 50, 200, 800, 1600, and 3200 ppm (µg/ml) using the method of Kim [28] based on the method described by Oyaizu [31]. Extract solution (0.1 ml), phosphate buffer (0.25 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (0.25 ml, 1%) were mixed, and then incubated at 50˚C for 20 min. Aliquots of 0.25 ml of trichloroacetic acid (10%) were added to the mixture and centrifuged for 10 min at 1700 g (25˚C). The upper layer of solution (0.25 ml) was mixed with distilled water (0.25 ml) and ferric chloride [FeCl3] (0.05 ml, 0.1%). The absorbance was measured at 700 nm. A blank solution with no added extract was used as a control. Increased absorbance indicates stronger reducing power.
2.4. Determination of Total Phenolics
Total phenolic contents in the water and ethanol extracts of dried citrus fruit peel were determined spectrophotometrically according to the method of Lim et al. [32] with a slight modification based on the use of FolinCiocal teu reagent [33]. Aliquots of 0.1 ml of extracts at different concentrations of 50, 200, 800, 1600, and 3200 ppm (µg/ml) were transferred into the test tubes, and their volumes were made up to 8 ml with distilled water (7.9 ml). Folin-Ciocalteu phenol reagent (0.5 ml) was added, and the contents were mixed thoroughly. After 1 min, 1.5 ml of 20% sodium carbonate solution was added and the mixture was mixed thoroughly and then allowed to stand for 1 h. The absorbance of the blue color produced was measured at 750 nm. Phenolic content was expressed in micrograms per mililiter of extract at different concentrations based on a standard curve of tannic acid (TA), which was expressed as milligrams per liter of tannic acid equivalent (TAE).
2.5. Determination of Flavonoids
Flavonoid contents in the water and ethanol extracts of citrus fruit peel were determined using a method described by Marksimović et al. [34] with a slight modification. Aliquots of 10 ml of extract solution prepared at different concentrations of 50, 200, 800, 1600, and 3200 ppm (µg/ml) were mixed with 2 ml of distilled water and 5 ml of AlCl3 reagent (133 mg crystalline aluminum chloride and 400 mg crystalline sodium acetate dissolved in 100 ml of water). The absorbance was recorded at 430 nm. The flavonoid contents were expressed in micrograms per milliliter of extract at different concentrations based on a standard curve of quercetine, which was expressed as milligrams per liter of quercetine equivalent.
2.6. Determination of Tannins
Tannin contents in the water and ethanol extracts of citrus fruit peel were determined using a method described by Marksimović et al. [34] with a slight modification after removal of tannins by their absorption on insoluble matrix (polyvinylpolypyrrolidlone, PVPP). Insoluble, crosslinked PVPP (Kollidon CL, BASF, Germany; 100 mg) was weighed into the test tubes, and 1 ml aliquots of extract solution prepared at different concentrations of 50, 200, 800, 1600, and 3200 ppm (µg/ml) was added. The test tubes were incubated for 15 min at 4˚C, and the tubes were vortexed and centrifuged for 10 min at 4300 g. Aliquots of supernatant (0.1 ml) were transferred into test tubes and nonabsorbed phenolics were determined like that of total phenolic content estimation. Calculated values were subtracted from total phenolic contents and total tannin contents were expressed as micrograms per milliliter of extract at different concentrations based on a standard curve of tannic acid, which was expressed as milligrams per liter of tannic acid equivalent (TAE).
2.7. Antioxidant Activity of the Water and Ethanol Extracts Using Biochemical Assays
2.7.1. Determination of AAPH-Induced Rat RBC Hemolysis Inhibition
The inhibition activity of the water and ethanol extracts of dried citrus fruit peel at different concentrations of 0, 50, 200, 800, 1600, & 3200 ppm (µg/ml) against free radical-induced oxidative hemolysis using rat red blood cells (RBC) was determined according to the method described by Ng et al. [35] with a slight modification. Blood obtained from the posterior vena cava of Male Sprague-Dawley rats (200 g) was collected into a heparinized tube. The RBCs were centrifuged at 1000 g for 5 min. The RBCs separated from the plasma and buffy coat were washed three times with 10 ml of 10 mM phosphate-buffered saline (PBS) at pH 7.4. After the 3rd wash, the RBCs were centrifuged at 1000 g for 10 min to obtain an evenly packed cell preparation, and the washed RBCs were finally resuspended in PBS to obtain a 20% suspension of RBC. Twenty percent RBC suspension at 0.1 ml was added to 0.2 ml of 200 mM 2,2’-azo-bis (2-amidinopropane) dihydrochloride (AAPH; a peroxyl radical initiator) solution in PBS and 0.1 ml of extract solution of different concentrations. The reaction mixture was shaken gently during incubation in a water bath at 37˚C for 3 h. The reaction mixture was diluted with 8 ml of PBS and centrifuged at 1000 g for 10 min; the absorbance of its supernatant was then read at 540 nm (Aabs). Similarly, the reaction mixture was treated with 8 ml of distilled water to achieve a complete hemolysis, and the absorbance of the supernatant obtained after centrifugation was measured at 540 nm (Babs). The inhibition ratio (%) of hemolysis was calculated using Equation (3). Ferulic acid and a-tocopherol (152.35 mg/ml for water extract and 159.86 mg/ml for ethanol extract) were used as positive controls.
 (3)
(3)
2.7.2. Determination of Fe2+/Ascorbate-Induced Lipid Peroxidation Inhibition
The inhibition activities of the water and ethanol extracts of dried citrus fruit peel at 0, 50, 200, 800, 1600, & 3200 ppm (µg/ml) against lipid peroxidation of the rat brain tissue induced by Fe2+/ascobate were determined using the method of Lim et al. [32] based on the method of Liu et al. [36]. Lipid peroxidation was assayed using the formation of malondialdehyde (MDA) as an indicator. The brains of Sprague-Dawley (SD) rats (200 g) were dissected and washed with ice-cold 20 mM Tris-HCl buffer (pH 7.4). The brain was homogenized in 2 volumes of ice-cold 20 mM Tris-HCl buffer with a Polytron (Brinkmann Instruments, Westbury, NY) and centrifuged at 3000 g for 10 min. The supernatant was used for study of lipid peroxidation. Aliquot (0.1 ml) of the supernatant was incubated at 37˚C for 1h with 0.2 ml of extract solution in the presence of 0.1 ml of 10 mM FeSO4 and 0.1 ml of 0.1 mM ascorbic acid. The reaction mixture was stopped by adding trichloroacetic acid (TCA) and thiobarbituric acid (TBA). The reaction mixture was then heated at 80˚C for 20 min. After cooling, centrifugation was carried out at 3000 g for 10 min to remove the precipitated protein. The color intensity of the MDA-TBA complex in the supernatant was measured at 532 nm. The inhibition ratio (%) of lipid peroxidation was calculated using Equation (4). Ferulic acid and a-tocopherol (152.35 mg/ml for water extract and 159.86 mg/ml for ethanol extract) were used as positive controls.
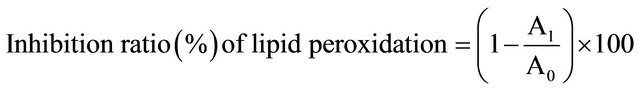 (4)
(4)
where A0 is the absorbance of control, and A1 is the absorbance of the test sample.
2.8. Statistical Analysis
All determinations for antioxidant activity in the samples were carried out in triplicate (n = 3), and all values were reported as means ± standard deviation. All experimental data were analyzed with the analysis of variance (ANOVA) using General Linear Model and significant differences (P < 0.05) among means at each treatment were determined using Least Significant Difference (LSD) at a level of a = 0.05 using Minitab computing system (Minitab Inc., State College, PA).
3. Results and Discussion
3.1. The Yield of Extracts
The percent yields of the water and ethanol extracts obtained from 20 g of dried Citrus unshiu fruit peel corresponded to 39.607% (7.959 ± 0.281 g, n = 3) and 55.486% (11.156 ± 0.348 g, n = 3), respectively.
3.2. Evaluation of Antioxidant Activity Using in Vitro Chemical Assays
3.2.1. Scavenging Activity on DPPH Free Radical
DPPH× is a stable organic free radical with most strong adsorption at 517 nm, the color of which turns from purple to yellow followed by the formation of DPPH upon absorption of hydrogen from an antioxidant [31]. Because it can accommodate many samples in a short period and is sensitive enough to detect active ingredients at low concentrations [37], it has been extensively used as a reagent to evaluate free radical scavenging activities of plant-based extracts. Free radical scavenging potentials of the water and ethanol extracts obtained from dried Citrus unshiu peel at different concentrations were detected by the DPPH assay (Figure 1). Both extracts showed a similar trend for scavenging DPPH radicals in a concentration-dependent manner, which indicated that in both extracts the increase in scavenging activity depended on the increasing concentration of the extracts.
The scavenging activities of the water extracts against DPPH radicals were 1.464%, 18.869%, 55.962%, 85.779% and 95.853%, whereas those of ethanol extracts were 1.336%, 13.149%, 44.034%, 74.286% and 97.714% at 50, 200, 800, 1600 and 3200 ppm, respectively. The scavenging activities of both extracts were significantly different (P < 0.05) in the range of 50 to 3200 ppm. The antioxidant activities of both extracts could be attributed to their hydrogen-donating ability by phenolic compounds present in extracts [38]. It is well-known that free radicals cause autoxidation of unsaturated lipid, whereas antioxidants intercept the free radical chain reaction of oxidation by donating hydrogen from the phenolic hydroxyl groups, thereby forming a stable end-product, which does not initiate or propagate further oxidation of unsaturated lipid [39]. However, as shown in Figure 1, the radical scavenging activities of water extracts were relatively weaker than those of ethanol extracts up to 2400 ppm, whereas water extracts showed opposite result. The difference in antioxidant power of both extracts could be due to different chemical structure of phenolic compounds present in both extracts [40], supported by several studies on the relationship between chemical structure and antioxidant activity of phenolic compounds by the DPPH× method [40-42]. The data described in present study indicate that the extracts obtained from dried Citrus unshiu peel could be free radical inhibitors and primary antioxidants that react with DPPH free radicals, which may be attribute to its hydrogenor electrondonating ability.
3.2.2. Scavenging Activity on Hydrogen Peroxide
Hydrogen peroxide is a non-radical reactive agent as well as one of reactive oxygen species (ROS), which can be generated in biological and food systems [43]. In fact, H2O2 by itself is not very reactive, but it may form hydroxyl radical, known to be highly reactive oxygen radical in the presence of transition metal ion and participate
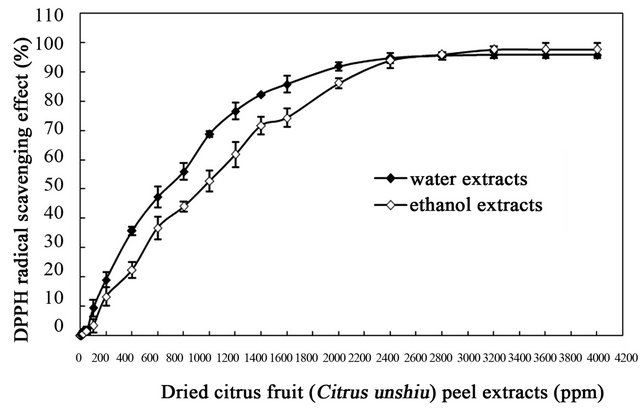
Figure 1. DPPH free radical scavenging activities of the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations.
in free-radical reaction in biological and food systems [43,44]. Therefore, removing H2O2 in biological and food systems is very important for antioxidant defense. The H2O2 scavenging activities of the water and ethanol extracts obtained from dried Citrus unshiu peel at different concentrations are shown in Figure 2. All tested water and ethanol extracts of dried Citrus unshiu peel were capable of scavenging H2O2 in a concentration-dependent manner. The H2O2 scavenging activities were 2.927%, 4.567%, 7.670%, 72.892%, and 91.335% for water extracts and 1.568%, 1.784%, 10.487%, 75.730%, and 84.973% for ethanol extracts at 50, 200, 800, 1600 and 3200 ppm, respectively. The scavenging activities of both extracts were significantly different (P < 0.05) in the concentration range of 50 to 3200 ppm. The scavenging of H2O2 by both extracts may be attributed to their phenolic compounds present in extracts, which can donate electrons to H2O2, thus neutralizing it to water [45,46]. However, H2O2 scavenging of both extracts by increasing concentrations showed a slightly different pattern depending on concentration (Figure 2). For example, as shown in Figure 2, the scavenging activities of water extracts showed slightly stronger power from 50 to 400 ppm, slightly weaker power from 800 to 1600 ppm, and slightly stronger power from 2400 to 3200 ppm than those of ethanol extracts. This difference in the activity may be influenced by chemical structure and elements of phenolic compounds, composition profile of phenolic compounds present in extracts or concentrations of phenolic compounds in the reaction test environment depending on extraction procedure [16,40,47]. The result of the present study reveals that water and ethanol extracts obtained from dried Citrus unshiu peel are a good source of electron donors, which may accelerate the conversion of H2O2 to H2O, eventually preventing the formation of hydroxyl radicals [48].
3.2.3. Reducing Power Activity
The reducing power has been widely used as a significant marker of the antioxidant activity [49]. In general, the
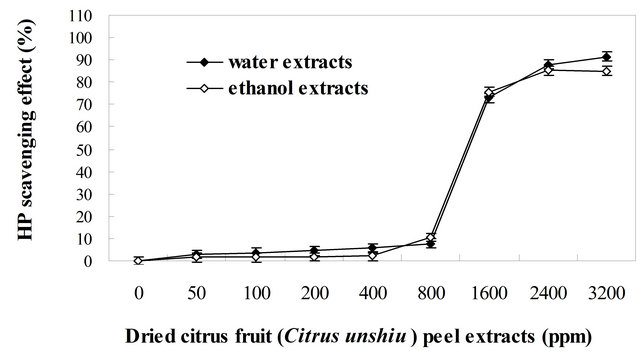
Figure 2. Hydrogen peroxide scavenging activities of the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations.
reductones in the oxidizable substrate play an important role in the reducing properties, including exertion of antioxidant action by breaking the free radical chains via donation of a hydrogen atom [50]. Furthermore, in the assay for determination of the reducing power, yellowness of the test solution changes to various shades of green and blue depending on the reducing power of compound. The presence of antioxidants causes the reduction of Fe3+ in the presence of ferricyanide complex to the ferrous (Fe2+) form by donating an electron. Therefore, measuring the formation of Perl’s Prussian blue at 700 nm can be used to monitor the Fe2+ concentration [48]. The greater the intensity of the color, the higher is the antioxidant activity of the sample. The reducing powers of the water and ethanol extracts of dried Citrus unshiu peel at different concentrations were determined by the iron (III) to iron (II) reduction assay. The data indicated a concentration-dependant mode for the reducing powers of both extracts (Figure 3). In addition, the reducing powers of both extracts also increased with the increasing concentrations. Furthermore, statistical differences in the reducing power were found among the different concentrations of both extracts (P < 0.05). Both extracts showed a relatively strong reducing power at 3200 ppm; however, the ethanol extract indicated a slightly higher reducing activity than that of water extract. This difference in reducing powers of both extracts obtained from dried Citrus unshiu peel could be due to their hydrogenor electron-donating ability [51]. Accordingly, both extracts at 3200 ppm could contain relatively higher amount of reductones, which could react with free radicals to stabilize and block radical chain reactions.
3.3. Total Phenolic, Flavonoid, Tannin Contents and Their Correlation with Antioxidant Activities
In order to establish a potential relationship between water and ethanol extracts, antioxidant activities based on the data obtained from DPPH free radical scavenging, hydrogen peroxide scavenging, and reducing power pa-
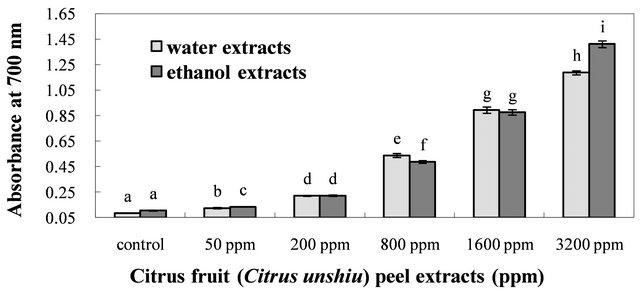
Figure 3. Reducing powers of the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations. Bars with different letters are significantly different (P < 0.05); n = 3.
rameters of both water and ethanol extracts as well as total phenolic, flavonoid, and tannin contents of both extracts at different concentrations were determined and summarized in Table 1. Total phenolic, flavonoid, and tannin contents of the water and ethanol extracts of dried Citrus unshiu peel at different concentrations showed a similar trend in a concentration-dependent mode, which indicated the increasing of total phenolic, flavonoid, and tannin contents depending on the increasing concentration of the extract. All data of each parameter investigated in water and ethanol extracts were significantly different (P < 0.05) (Table 1). Total phenolic contents were measured by Folin Ciocalteu reagent in terms of tannic acid equivalent by reference to standard curve (y = 0.001x − 0.008, r2 = 0.998). The total phenolic contents of the water and ethanol extracts at different concentrations ranged from 1.844 to 159.861 mg/ml. The flavonoid contents of both extracts at different concentrations ranged from 0.038 to 15.950 mg quercetin equivalent/ml of the extract solution by reference to standard curve (y = 0.036x + 0.015, r2 = 0.999). Finally, tannin contents of the water and ethanol extracts were determined by subtracting non-tannin phenolic contents from total phenolic contents and the tannin contents of both extracts at different concentrations ranging from 1.816 to 59.296 mg/ ml extract solution in terms of tannic acid equivalent by reference to standard curve (y = 0.001x + 0.001, r2 = 0.998). Total phenolic contents of the water and ethanol extracts were similar at the same concentration levels of extracts, whereas flavonoid contents of the ethanol extracts were approximately 1.5-fold higher than those of water extract, and tannin contents of the water extracts were approximately 2-fold higher than those of ethanol extract in the range of 800 to 3200 ppm (Table 1).
A clear association was observed between the extracts in total phenolic content and the antioxidant activities investigated by three different parameters including DPPH free radical-scavenging, hydrogen peroxide scavenging, and reducing capacity of Citrus unshiu peel. Strong linear correlations were observed between extract total phenolic content and the antioxidant activities with correlation coefficients of r2 = 0.8704 for DPPH free radical scavenging, r2 = 0.8744 for hydrogen peroxide scavenging, and r2 = 9671 for reducing capacity (Figures 4-6). As expected, DPPH radical scavenging, hydrogen peroxide scavenging, and reducing capacity showed increasing antioxidant power depending on the increase of phenolic content, an indication that the phenolic content in dried Citrus unshiu peel could be highly correlated with antioxidant activity. Thus, the content of phenolic compounds could be an important indicator of the antioxidant capacity, which could be used preliminary to screen natural sources for antioxidants [52]. Previous
Table 1. Total phenolics, flavonoids and tannins in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations.
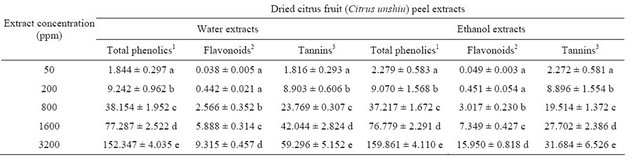
1µg tannic acid equivalent/ml of extract at different concentrations; 2µg quercetin equivalent/ml of extract at different concentrations; 3µg tannic acid equivalent/ml of extract at different concentrations: total phenolics − nontannins = tannins; Values with different letters in each column are significantly different (P < 0.05); Each value is the mean ± standard deviation; n = 3.
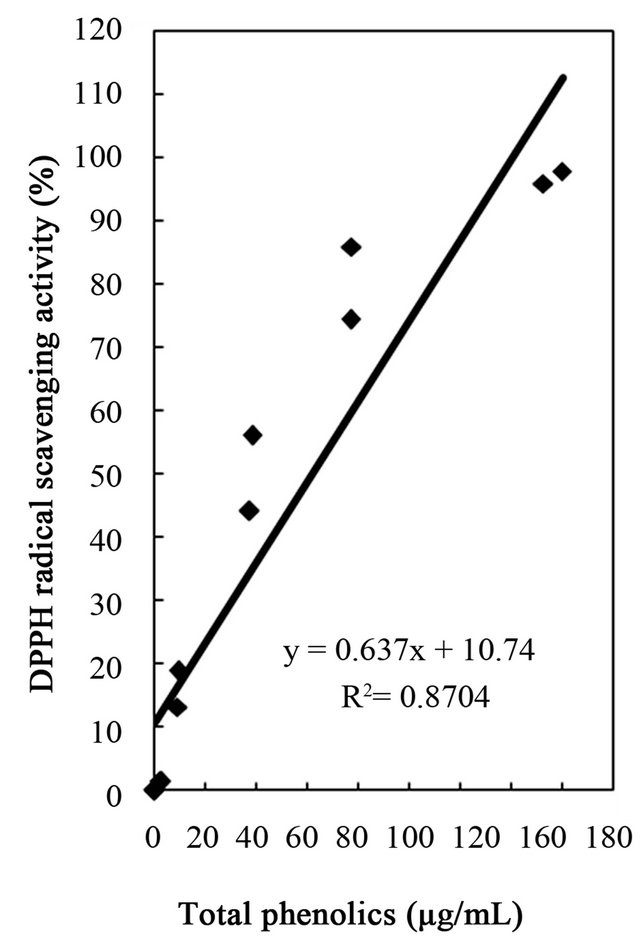
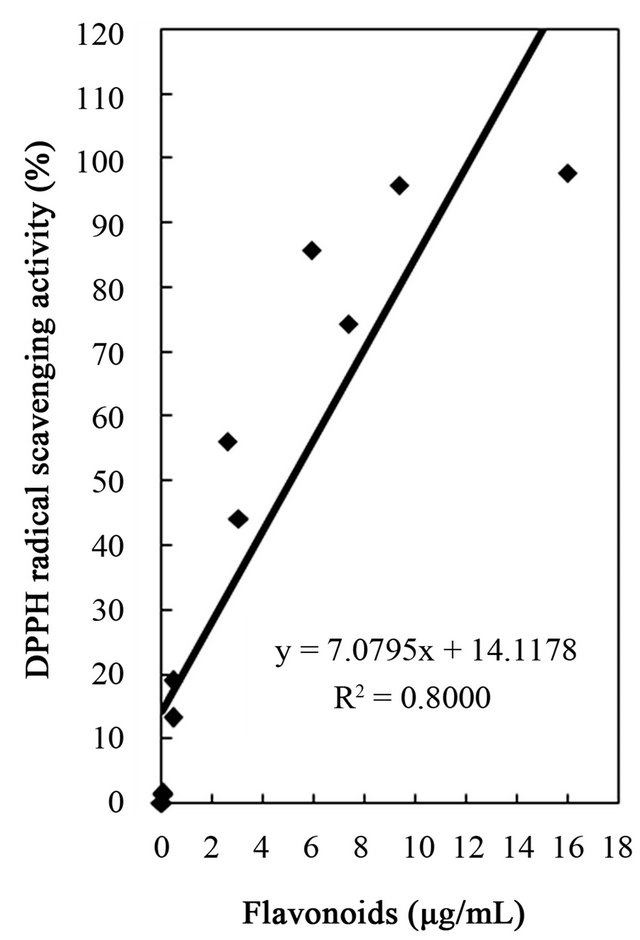
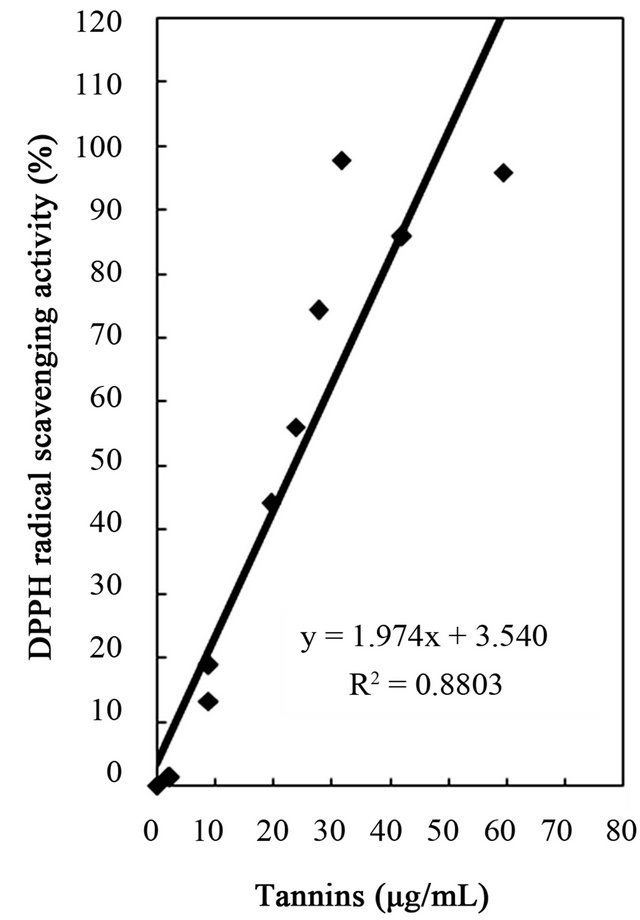
Figure 4. Correlation between free radical scavenging activity and total phenolics, flavonoids, and tannins of water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel.
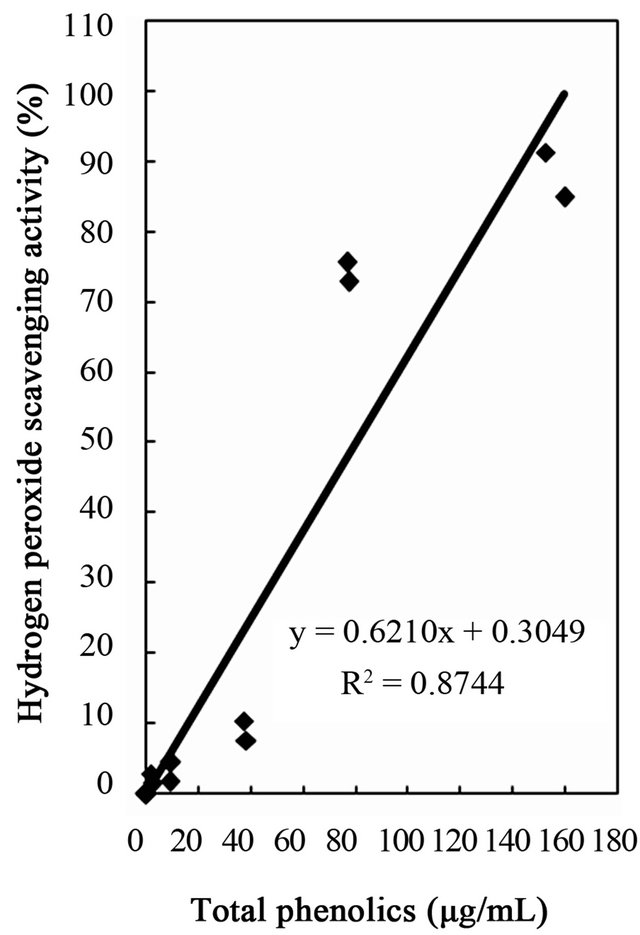
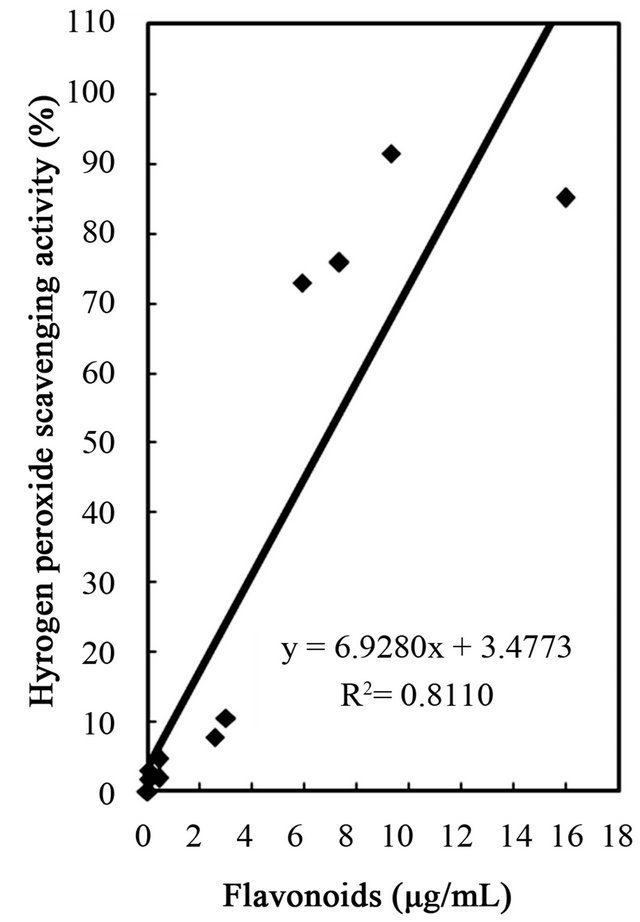
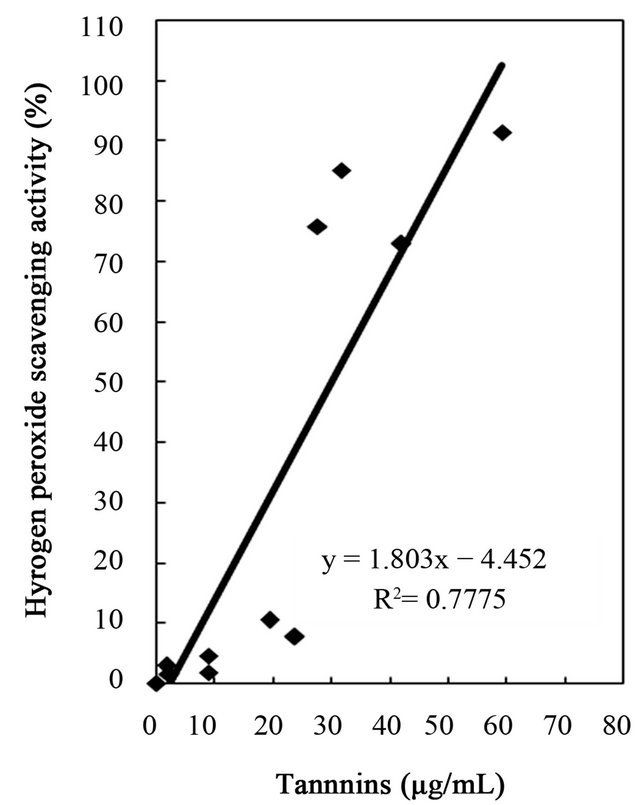
Figure 5. Correlation between hydrogen peroxide scavenging activity and total phenolics, flavonoids, and tannins of water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel.
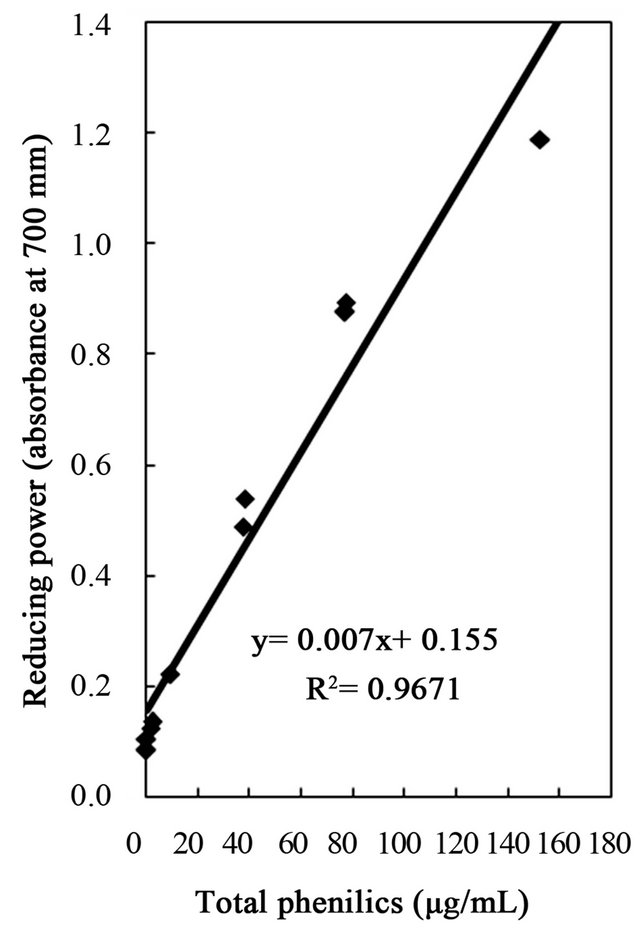
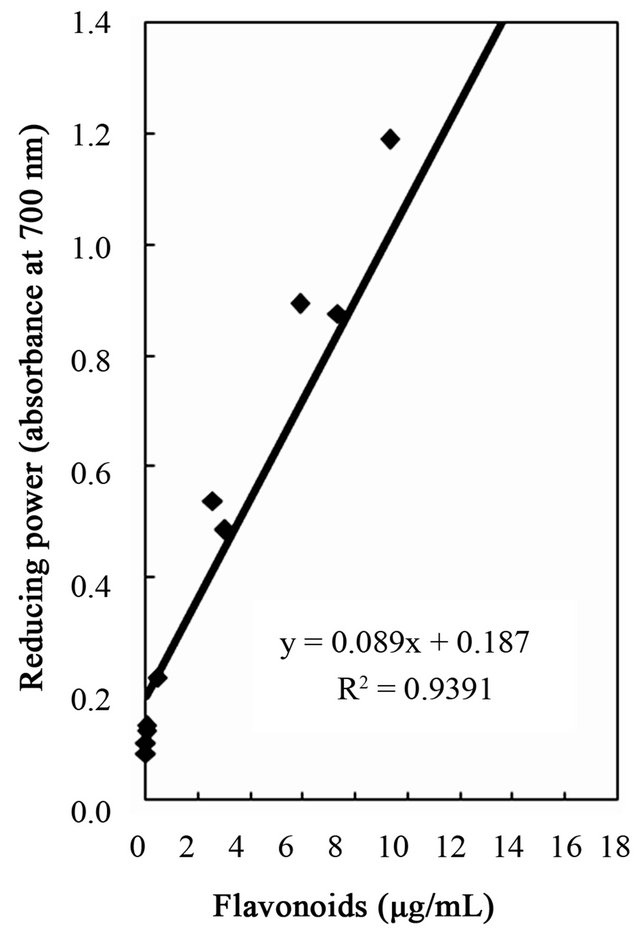
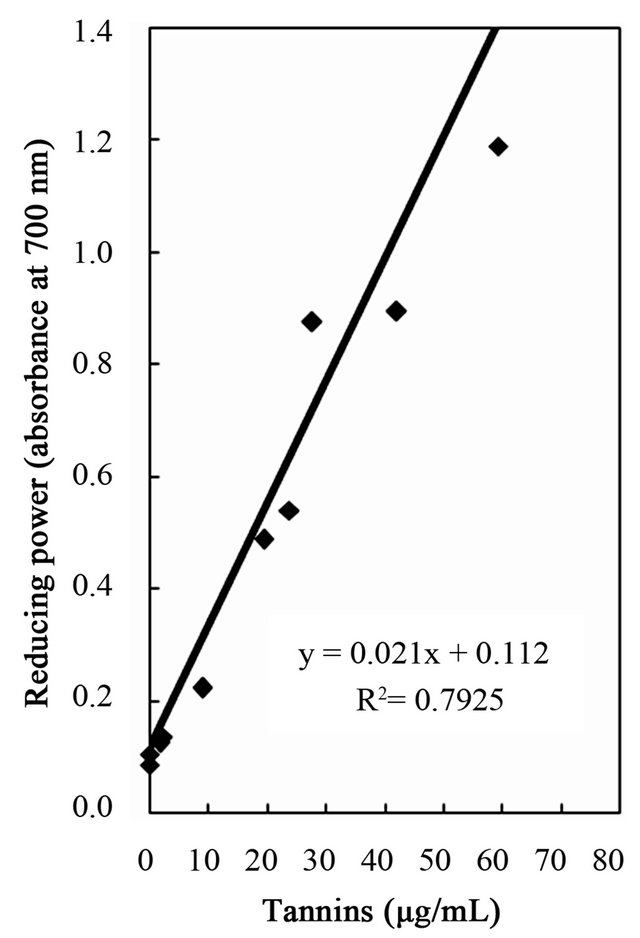
Figure 6. Correlation of reducing power to total phenolics, flavonoids, and tannins of the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel.
studies identified a positive relationship between the phenolic content and antioxidant activity in citrus peel [2,17,53]. The correlation coefficients between the flavonoids and the antioxidant activities were r2 = 0.8000 for DPPH radical scavenging, r2 = 0.8110 for hydrogen peroxide scavenging, and r2 = 0.9391 for reducing capacity (Figures 4-6), relatively lower than those between total phenolics and the antioxidant activities. These results indicates that besides flavonoids, there could be other phenolic compounds such as phenolic acids, tannic acid, proanthocyanidin among others [52], which also contribute to the antioxidant activity of dried Citrus unshiu peel. Furthermore, linear correlations between the tannin content and the antioxidant activity were also observed with correlation coefficients of r2 = 0.8803 for DPPH radical scavenging, r2 = 0.7775 for hydrogen peroxide scavenging, and r2 = 0.7925 for reducing capacity (Figures 4-6). Although relatively lower correlations were observed among tannins, except hydrogen peroxide scavenging and reducing capacity, DPPH radical scavenging showed a good correlation with the tannins. These results strongly suggest that the content of phenolic compounds in dried Citrus unshiu peel including flavonoids and tannins could be attributed to their antioxidant activity, which plays an important role as free radical terminator, ROS scavenger, or reducer via their hydrogenor electron-donating ability.
3.4. Evaluation of Antioxidant Activity Using in Vitro Biochemical Assays
3.4.1. Inhibition Activity of AAPH-Induced Oxidative Hemolysis Using Rat Red Blood Cells (RBC)
Thermal decomposition of AAPH in the reaction solution generates an initiating radical, which can attack the polyunsaturated lipids in the RBC membranes to induce lipid peroxidation. As a result, the RBC membranes are quickly damaged and eventually lead to hemolysis, because the lipid peroxidation is a free radical chain reaction and one initiating radical could induce up to twenty propagation reactions [54,55]. One the other hand, if antioxidants such as phenolic acids, flavonoids, and vitamins E, and C are present or added to the RBCs, they would react with the chain propagating peroxyl radicals to stop the peroxidation, hence inhibit hemolysis [56]. In the present study, the inhibition effects of the water and ethanol extracts obtained from dried Citrus unshiu peel against the oxidative hemolysis of rat RBC induced by AAPH, which is known as a peroxyl radical initiator, were investigated and compared at different concentrations. The inhibition activity of the water extracts increased in a concentration-dependent mode; however statistical difference between 1600 and 3200 ppm of extract concentration was not found (P < 0.05), and the inhibition activity at the two concentrations was even higher than that of positive control ferulic acid (Figure 7). On the other hand, the inhibition activity of ethanol extracts was not dependent on the concentration of extract, which suggests the inhibition activity of hemolysis might not be related to the content of phenolic compounds. Unlike the previous data from the chemical assays, there is a lack of the relationship between the content of phenolic compounds and their antioxidant activity in inhibiting peroxyl radical-induced hemolysis in biological system. This indicates that the antioxidant activity of phenolic compounds for the inhibition of peroxyl radical-induced hemolysis is not dependent on the concentration of phenolic compounds, but may also be explained in different ways such as the chemical structures, the concentration, the number, and position of hydrogendonating functional hydroxyl groups on the aromatic cycles of the phenolic molecules [57,58]. According to the study of Bocco et al. [18], methanol extracts in citrus peel are rich in flavones and glycosylated flavanones, whereas hydrolyzed extracts contain mainly phenolic acids and flavonol. Thus, the phenolic acid and flavonol among the phenolic compounds in the water extract of dried Citrus unshiu peel could selectively and effectively inhibit the perxoyl radical-induced hemolysis in cellular system.
3.4.2. Inhibition Activity of Fe2+/Ascorbate-Induced Lipid Peroxidation Using Rat Brain Tissue
The inhibition activity of nonenzymatic lipid peroxidetion induced with FeSO4-ascorbic acid on rat brain tissue homogenate was measured by the color intensity of MDA-TBA complex (Figure 8). In general, membrane phospholipids of tissue cells are the major target of oxidative damages in biological system. Lipid peroxidation, which involves the oxidative cleavage of polyunsaturated fatty acids at their double bonds, is recognized as a potential mechanism of cell injury, leading to the formation of malondialdehyde (MDA) [59]. The MDA has long served as a convenient index to determine the extent of lipid peroxidation, and Fe2+ ion is well-known as an initiator for stimulating lipid peroxidation in cellular system [60]. The inhibition activity of Fe2+/ascorbate-induced lipid peroxidation by the water and ethanol extracts of dried Citrus unshiu peel showed concentration-dependence in the tested concentration ranges, whereas that of ethanol extracts in the range of 800 to 3200 ppm was not significantly different (P < 0.05) and comparable to that of positive control a-tocopherol (Figure 8). The ethanol extracts of dried Citrus peel generally showed a higher inhibition percentage of lipid peroxidation than those of water extracts at the same level except at 3200 ppm.
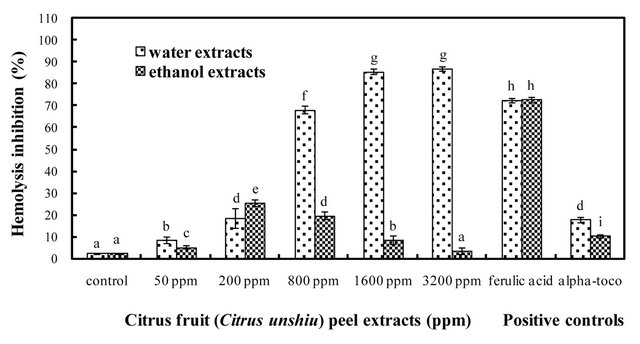
Figure 7. Inhibition activities of AAPH-induced hemolysis using rat RBC in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations; alpha-toco = a-tocopherol; Bars with different letters are significantly different (P < 0.05); n = 3.

Figure 8. Inhibition activities of Fe2+/ascorbate-induced lipid peroxidation using rat brain tissue in the water and ethanol extracts of dried citrus fruit (Citrus unshiu) peel at different concentrations; alpha-toco = a-tocopherol; Bars with different letters are significantly different (P < 0.05); n = 3.
Based on the results obtained, it is possible that the inhibition of the lipid peroxidation by Fe2+/ascorbate system could be more influenced by ethanol extract than water extract. Thus, the ethanol extract of dried Citrus unushiu peel could be an effective antioxidant to inhibit the lipid peroxidation induced by Fe2+ ion in cellular system even at low concentrations possibly due to the presence of catechins and anthocyianidines, which are not only subgroups of flavonoid family but also belong to tannins and governed by the position and number of hydroxyl groups on the B-ring of aromatic cycles in their phenolic structures [40]. According to the study reported by Seeram & Nair [61], catechins and anthocyanidins effectively inhibited the Fe(II)-induced lipid peroxidation in a liposomal model, which was reported to be more effective at inhibiting Fe(II) ion-induced peroxidation than the free radical-induced peroxidation. Thus, the ethanol extract of dried Citrus unshiu peel could be a better source of antioxidant having the chemical structure of catechins and anthocyanidins compared to the water extract.
Although further studies are needed, the results of the present study show the significant antioxidant activities of the water and ethanol extracts obtained from the dried Citrus unshiu peel by both chemical and biochemical in vitro assays. In light of these findings, the water and ethanol extracts of dried Citrus unshiu peel can be considered as potential ingredients of functional foods for use in conditions where reactive oxygen species are implicated, although preclinical and clinical studies are needed to establish the usefulness of the natural extracts of dried Citrus unshiu peel in the treatment or prevention of many human diseases.
REFERENCES
- M. Lohrasbi, M. Pourbafrani, C. Niklasson and M. J. Taherzadeh, “Process Design and Economic Analysis of a Citrus Waste Biorefinery with Biofuels and Limonene as Products,” Bioresource Technology, Vol. 101, No. 19. 2010, pp. 7382-7388. doi:10.1016/j.biortech.2010.04.078
- G. H. Xu, J. C. Chen, D. H. Liu, Y. H. Zhang, P. Jiang and X. Q. Ye, “Minerals, Phenolic Compounds, and Antioxidant Capacity of Citrus Peel Extract by Hot Water,” Journal of Food Science, Vol. 73, No. 1, 2008, pp. C11- C18. doi:10.1111/j.1750-3841.2007.00546.x
- M. B. Kim, J. S. Park and S. B. Lim, “Antioxidant Activity and Cell Toxicity of Pressurized Liquid Extracts from 20 Selected Plant Species in Jeju, Korea,” Food Chemistry, Vol. 122, No. 3, 2010, pp. 546-552. doi:10.1016/j.foodchem.2010.03.007
- U. Imeh and S. Khokhar, “Distribution of Conjugated and Free Phenols in Fruits: Antioxidant Activity and Cultivar Variations,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 22, 2002, pp. 6301-6306. doi:10.1021/jf020342j
- J. A. Ross and C. M. Kasum, “Dietary Flavonoids: Bioavailability Metabolic Effects, and Safety,” Annual Review of Nutrition, Vol. 22, 2002, pp. 19-34. doi:10.1146/annurev.nutr.22.111401.144957
- J. Silalahi, “Anticancer and Health Protective Properties of Citrus Fruit Components,” Asia Pacific Journal of Clinical Nutrition, Vol. 11, No. 1, 2002, pp. 79-84. doi:10.1046/j.1440-6047.2002.00271.x
- B. Halliwell, “Free Radicals, Antioxidants, and Human Disease: Curiosity, Cause, or Consequence?” Lancet, Vol. 344, No. 8924, 1994, pp. 721-724. doi:10.1016/S0140-6736(94)92211-X
- J. J. Pincemail, “Free Radicals and Antioxidants in Human Diseases,” In: A. E. Favier, J. Cadet, B. Kalyanaraman, M. Fontecave and J. L. Pierre, Eds., Analysis of Free Radicals in Biological Systems, Birkhauser Verlag, Berlin, 1995, pp. 83-98.
- D. Dreher and A. F. Junod, “Role of Oxygen Free Radicals in Cancer Development,” European Journal of Cancer, Vol. 32A, No. 1, 1996, pp. 30-38. doi:10.1016/0959-8049(95)00531-5
- S. Maxwell, “Anti-Oxidant Therapy: Does It Have a Role in the Treatment of Human Disease?” Expert Opinion on Investgational Drugs, Vol. 6, No. 3, 1997, pp. 211-236. doi:10.1517/13543784.6.3.211
- V. Lopez, S. Akerreta, E. Casanova, J. M. Garcia-Mina, R. Y. Cavero and M. I. Calvo, “In Vitro Antioxidant and Anti-Rhizopus Activities of Lamiaceae Herbal Extracts,” Plant Foods for Human Nutrtion, Vol. 62, No. 4, 2007, pp. 151-155. doi:10.1007/s11130-007-0056-6
- G. Satpathy, Y. K. Tyagi and R. K. Gupta, “Preliminary Evaluation of Nutraceutical and Therapeutic Potential of Raw Spondias Pinnata K., an Exotic Fruit of India,” Food Research International, Vol. 44, No. 7, 2011, pp. 2076- 2087. doi:10.1016/j.foodres.2011.01.040
- M. G. L. Hertog, E. J. M. Feskeens, C. H. Holmann, K. B. Katan and D. Kromhout, “Dietary Antioxidant Flavonoids and Risk of Coronary Heart Disease: The Zutphen Elderly Study,” Lancet, Vol. 342, No. 8878, 1993, pp. 1007-1011. doi:10.1016/0140-6736(93)92876-U
- V. Elangovan, N. Sekar and S. Govindasamy, “Chemoprotective Potential of Dietary Bioflavonoids against 20-Methylcholanthrene-Induced Tumorigenesis,” Cancer Letter, Vol. 87, No. 1, 1994, pp. 107-113. doi:10.1016/0304-3835(94)90416-2
- F. R. Marin, M. J. Frutos, J. A. Perez-Alvarez, F. Martinez-Sanchez and J. A. Del Rio, “Flavonoids as Nutraceuticals: Structure Related Antioxidant Properties and Their Role on Ascorbic Acid Preservation,” In: Atta-Ur-Rahman, Ed., Studies in Natural Products Chemistry, Elsevier Science, Amsterdam, 2002, pp. 324-389.
- D. D. Majo, M. Giammanco, M. L. Guardia, E. Tripoli, S. Giammanco and E. Finotti, “Flavanones in Citrus Fruit: Structure-Antioxidant Activity Relationships,” Food Research International, Vol. 38, No. 10, 2005, pp. 1161- 1166. doi:10.1016/j.foodres.2005.05.001
- M. A. Anagnostopoulou, P. Kefalas, V. P. Papageorgiou, A. N. Assimopoulou and D. Boskou, “Radical Scavenging Activity of Various Extracts and Fractions of Sweet Orange Peel (Citrus sinensis),” Food Chemistry, Vol. 94, No. 1, 2006, pp. 19-25. doi:10.1016/j.foodchem.2004.09.047
- A. Bocco, M. E. Cuvelier, H. Richard and C. Berset, “Antioxidant Activity and Phenolic Composition of Citrus Peel and Seed Extracts,” Journal of Agricultural and Food Chemistry, Vol. 46, No. 6, 1998, pp. 2123-2129. doi:10.1021/jf9709562
- S. Gorinstein, O. Martín-Belloso, Y. S. Park, R. Haruenkit, A. Lojek, M. Cíz, A. Gaspi, I. Libman and S. Trakhtenberg, “Comparison of Some Biochemical Characteristics of Different Citrus Fruits,” Food Chemistry, Vol. 74, No. 3, 2001, pp. 309-315. doi:10.1016/S0308-8146(01)00157-1
- J. A. Manthey and K. Grohmann, “Phenols in Citrus Peel Byproducts Concentrations of Hydroxycinnamates and Polymethoxylated Flavones in Citrus Peel Molasses,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 7, 2001, pp. 3268-3273. doi:10.1021/jf010011r
- D. K. Asami, Y. J. Hong, D. M. Barrett and A. E. Mitchell, “Comparison of the Total Phenolic and Ascorbic Acid Content of Freeze-Dried and Air-Dried Marionberry, Strawberry, and Corn Grown Using Conventional, Organic, and Sustainable Agricultural Practice,” Journal of Agricultural and Food Chemistry, Vol. 51, No. 5, 2003, pp. 1237-1241. doi:10.1021/jf020635c
- M. Y. Choi, C. Chai, J. H. Park, J. Lim, J. Lee and S. M. Kwon, “Effects of Storage Period and Heat Treatment on Phenolic Compound Composition in Dried Citrus Peels (Chenpi) and Discrimination of Chenpi with Different Storage Periods Through Targeted Metabolomic Study Using HPLC-DAD Analysis,” Journal of Paarmaceutical and Biomedical Analysis, Vol. 54, No. 4, 2011, pp. 638- 645. doi:10.1016/j.jpba.2010.09.036
- M. Ou, “Regular Chinese Medicine Handbook,” Warmth Publishing Ltd., Taiwan, 1999.
- H. J. Oh, H. M. Ahn, S. S. Kim, P. Y. Yun, K. L. Jeon, Y. H. Ko and K. Z. Riu, “Composition and Antimicrobial Activities of Essential Oils in the Peel of Citrus Fruits. Journal of Applied Biological Chemistry, Vol. 50, No. 3, 2007, pp. 148-154.
- B. B. Li, B. Smith and M. Hossain, “Extraction of Phenolics from Citrus Peels I. Solvent Extraction Method,” Separation and Purification Technology, Vol. 48, No. 2, 2006, pp. 182-188. doi:10.1016/j.seppur.2005.07.005
- K. Ghasemi, Y. Ghasemi and M. A. Ebrahimzadeh, “Antioxidant Activity, Phenol and Flavonoid Contents of 13 Citrus Species Peels and Tissues,” Pakistan Journal of Pharmaceutical Sciences, Vol. 22, No. 3, 2009, pp. 277- 281.
- J. C. Tang, J. N. Zhang, Y. T. Wu and Z. X. Li, “Effect of the Water Extract and Ethanol Extract from Traditional Chinese Medicines Angelica sinensis (Oliv.) Diels, Ligusticum chuanxiong Hort. and Rheum palmatum L. on Rat Liver Cytochrome P450 Activity,” Phytotherapy Research, Vol. 20, No. 12, 2006, pp. 1046-1051. doi:10.1002/ptr.1974
- J.-S. Kim, “Radical Scavenging Capacity and Antioxidant Activity of the E Vitamer Fraction in Rice Bran,” Journal of Food Science, Vol. 70, No. 3, 2005, pp. C208-213. doi:10.1111/j.1365-2621.2005.tb07127.x
- T. Hatano, H. Kagawa, T. Yasuhara and T. Okuda, “Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects,” Chemical and Pharmaceutical Bulletin, Vol. 36, No. 6, 1988, pp. 2090-2097. doi:10.1248/cpb.36.2090
- D. Atmani, N. Chaher, M. Berboucha, K. Ayouni, H. Lounis, H. Boudaoud, N. Debbache and D. Atmani, “Antioxidant Capacity and Phenol Content of Selected Algerian Medicinal Plants,” Food Chemistry, Vol. 112, No. 2, 2009, pp. 303-309. doi:10.1016/j.foodchem.2008.05.077
- M. Oyaizu, “Studies on Product of Browning Reaction Prepared from Glucose Amine,” Japanese Journal of Nutrition, Vol. 44, No. 6, 1986, pp. 307-315. doi:10.5264/eiyogakuzashi.44.307
- S. N. Lim, P. C. K. Cheung, V. E. C. Ooi and P. O. Ang, “Evaluation of Antioxidative Activity of Extracts from a Brown Seaweed Sargassum siliquastrum,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 13, 2002, pp. 3862-3866. doi:10.1021/jf020096b
- R. Julkunen-Tiitto, “Phenolic Contents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics,” Journal of Agricultural and Food Chemistry, Vol. 33, No. 2, 1985, pp. 213-217. doi:10.1021/jf00062a013
- Z. Marksimović, D. Malenčić and N. Kovačević, “Polyphenol Contents and Antioxidant Activity of Maydis stigma Extracts,” Bioresource Technology, Vol. 96, No. 8, 2005, pp. 873-877. doi:10.1016/j.biortech.2004.09.006
- T. B. Ng, F. Liu and Z. T. Wang, “Antioxidant Activity of Natural Products from Plants,” Life Sciences, Vol. 66, No. 8, 2000, pp. 709-723. doi:10.1016/S0024-3205(99)00642-6
- F. Liu, V. E. C. Ooi and S. T. Chang, “Free Radical Scavenging Activities of Mushroom Polysaccharide Extracts,” Life Sciences, Vol. 60, No. 10, 1997, pp. 763-771. doi:10.1016/S0024-3205(97)00004-0
- C. Sánchez-Moreno, J. A. Larrauri and F. Saura-Calixto, “Free Radical Scavenging Capacity and Inhibition of Lipid Oxidation of Wines, Grape Juices and Related Polyphenolic Constituents,” Food Research International, Vol. 32, No. 6, 1999, pp. 407-412. doi:10.1016/S0963-9969(99)00097-6
- R. P. Singh, K. N. C. Murthy and G. K. Jayaprakasha, “Studies on the Antioxidant Activity of Pomegranate (Punica granatum) Peel and Seed Extracts Using in Vitro Models,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 1, 2002, pp. 81-86. doi:10.1021/jf010865b
- E. R. Sherwin, “Oxidation and Antioxidants in Fat and Oil Processing,” Journal of the American Oil Chemists’ Society, Vol. 55, No.11, 1978, pp. 809-814.
- E. Tripoli, M. L. Guardia, S. Giammanco, D. D. Majo and M. Giammanco, “Citrus Flavonoids: Molecular Structure, Biological Activity and Nutritional Properties: A Review,” Food Chemistry, Vol. 104, No. 2, 2007, pp. 466- 479. doi:10.1016/j.foodchem.2006.11.054
- M. E. Cuverlier, H. Richard and C. Berset, “Comparison of the Antioxidative Activity of Some Acid Phenols: Structure Activity Relationship,” Bioscience Biotechnology and Biochemistry, Vol. 56, No. 2, 1992, pp. 324-325. doi:10.1271/bbb.56.324
- V. Bondet, W. Brand-Williams and C. Berset, “Kinetics and Mechanisms of Antioxidant Activity Using the DPPH× Free Radical Method,” Lebensmittel Wissenschaft und Technologie, Vol. 30, No. 6, 1997, pp. 609-615.
- H. Y. Lin and C. C. Chou, “Antioxidative Activities of Water-Soluble Disaccharide Chitosan Derivatives,” Food Research International, Vol. 37, No. 9, 2004, pp. 883- 889. doi:10.1016/j.foodres.2004.04.007
- B. Halliwell, “Reactive Oxygen Species in Living Systems; Source, Biochemistry, and Role in Human Disease,” The American Journal of medicine, Vol. 91, No. 3, 1991, pp. 14-22.
- S. M. Nabavi, M. A. Ebrahimzadeh, S. F. Navabi and M. Jafari, “Free Radical Scavenging Activity and Antioxidant Capacity of Eryngium caucasicum Trautv and Froripia subpinnata,” Pharmacologyonline, Vol. 3, 2008, pp. 19-25.
- M. A. Ebrahimzadeh, S. F. Navabi and S. M. Navabi, “Antioxidant Activities of Methanol Extract of Sambucus ebulus L. Flower,” Pakistan Journal of Biological Sciences, Vol. 12, No. 5, 2009, pp. 447-450. doi:10.3923/pjbs.2009.447.450
- M. L. Calabrò, V. Galtieri, P. Cutroneo, S. Tommasini, P. Ficarra and R. Ficarra, “Study of the Extraction Procedure by Experimental Design and Validation of a LC Method for Determination of Flavonoids in Citrus bergamia Juice,” Journal Pharmaceutical and Biomedical Analysis, Vol. 35, No. 2, 2004, pp. 349-363. doi:10.1016/S0731-7085(03)00585-5
- Z. Yi, Y. Yu, Y. Liang and B. Zeng, “In Vitro Antioxidant and Antimicrobial Activities of the Extract of Percarpium citri Reticulatae of a New Citrus Cultivar and Its Main Flavonoids,” Food Science and Technology, Vol. 41, No. 4, 2008, pp. 597-603. doi:10.1016/j.lwt.2007.04.008
- C. L. Hsu, W. L. Chen, Y. M. Weng and C. Y. Tseng, “Chemical Composition, Physical Properties, and Antioxidant Activities of Yam Flours as Affected by Different Drying Methods,” Food Chemistry, Vol. 83, No. 1, 2003, pp. 85- 92. doi:10.1016/S0308-8146(03)00053-0
- J. S. Rathee, S. A. Hassarajani and S. Chattopadhyay, “Antioxidant Activity of Nyctanthes arbor-tristis Leaf Extract,” Food Chemistry, Vol. 103, No. 4, 2007, pp. 1350- 1357. doi:10.1016/j.foodchem.2006.10.048
- M. A. Ebrahimzadeh, S. M. Navabi, A. F. Navabi, F. Bahramian and A. R. Bekhradnia, “Antioxidant and Free Radical Scavenging Activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum,” Pakistan Journal of Pharmaceutical Sciences, Vol. 23, No. 1, 2010, pp. 29-34.
- H. Liu, N. Qiu, H. Ding and R. Yao, “Polyphenols Contents and Antioxidant Capacity of 68 Chinese Herbals Suitable for Medical or Food Uses,” Food Research International, Vol. 41, No. 4, 2008, pp. 363-370. doi:10.1016/j.foodres.2007.12.012
- Y. Q. Ma, X. Q. Ye, Z. X. Fang, J. C. Chen, G. H. Xu and D. H. Liu, “Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus unshiu Marc.) Peels,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 14, 2008, pp. 5682-5690. doi:10.1021/jf072474o
- V. W. Bowry and R. Stocker, “Tocopherol-Mediated Peroxidation. The Peroxidation Effect of Vitamin E on the Radical-Initiated Oxidation of Human Low-Density Lipoprotein,” Journal of the American Chemistry Society, Vol. 115, No. 14, 1993, pp. 6029-6044. doi:10.1021/ja00067a019
- Z. Q. Liu, L. P. Ma, B. Zhou, L. Yang and Z. L. Liu, “Antioxidative Effects of Green Tea Polyphenols on Free Radical Initiated and Photosensitized Peroxidation of Human Low Density Lipoprotein,” Chemistry and Physics of Lipids, Vol. 106, No. 1, 2000, pp. 53-63. doi:10.1016/S0009-3084(00)00133-X
- F. Dai, Q. Miao, B. Zhou, L. Yang and Z. L. Liu, “Protective Effects of Flavonols and Their Glycosides against Free Radical-Induced Oxidative Hemolysis of Red Blood Cells,” Life Sciences, Vol. 78, No. 21, 2006, pp. 2488- 2493. doi:10.1016/j.lfs.2005.10.009
- C. A. Rice-Evans, N. J. Miller and G. Paganga, “Antioxidant Properties of Phenolic Compounds,” Trends in Plant Science, Vol. 2, No. 4, 1997, pp. 152-158. doi:10.1016/S1360-1385(97)01018-2
- A. Djeridane, M. Yousfi, B. Nadjemi, D. Boutassouna, P. Stocker and N. Vidal, “Antioxidant Activity of Some Algerian Medicinal Plants Extracts Containing Phenolic Compounds,” Food Chemistry, Vol. 97, No. 4, 2006, pp. 654-660. doi:10.1016/j.foodchem.2005.04.028
- C. Manna, S. D’Angelo, V. Migliardi, E. Loffredi, O. Mazzoni, P. Morrica, P. Galletti and V. Zappia, “Protective Effect of the Phenolic Fraction from Virgin Olive Oils against Oxidative Stress in Human Cells,” Journal of Agricultural Food Chemistry, Vol. 50, No. 22, 2002, pp. 6521-6526. doi:10.1021/jf020565+
- [61] B. A. Igor, I. D. Anatolli, V. B. Aleksander, A. K. Valadimer and I. P. Alla, “Chelating and Free Radical Scavenging Mechanisms of Inhibiting Action of Rutin and Quercetin in Lipid Peroxidation,” Biochemical Pharmacology, Vol. 38, No. 11, 1989, pp. 1763-1769. doi:10.1016/0006-2952(89)90410-3
- [62] N. P. Seeram and M. G. Nair, “Inhibition of Lipid Peroxidation and Structure-Activity-Related Studies of the Dietary Constitutes Anthocyanins, Anthocyanidins, and Catechins,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 19, 2002, pp. 5308-5312. doi:10.1021/jf025671q

