Food and Nutrition Sciences
Vol. 2 No. 8 (2011) , Article ID: 7889 , 10 pages DOI:10.4236/fns.2011.28118
Anti-Helicobacter Activity of Certain Food Plant Extracts and Juices and Their Composition in Vitro
![]()
1Institute of Innovative Biomedical Technology Ltd., Riga, Latvia; 2Faculty of Biology, University of Latvia, Riga, Latvia.
Email: vizma.nikolajeva@lu.lv
Received May 4th, 2011; revised July 30th, 2011; accepted August 7th, 2011.
Keywords: Helicobacter, Antimicrobial Activity, Plant Extract, Juice
ABSTRACT
Plant material screening was performed to study anti-Helicobacter pylori activity in vitro using an agar diffusion method on Columbia blood agar. 33 substances, juices and plant extracts and 35 of their combinations were tested. Quince (Cydonia oblonga) juice demonstrated the strongest anti-H. pylori activity followed by cranberry juice. Concen-trated apple juice, plum, red currant, black chokeberry, raspberry and bilberry juice also showed significant activity. Green tea and apple pomace extract as well as sweet flag rhizome, ginger and wild bergamot extract, cherry syrup, red beet juice and whey did not exhibit anti-Helicobacter activity. Quince juice in combination with bilberry, black chokeberry, red currant juice, green tea, sweet flag rhizome or apple pomace extract as well as cranberry juice in combination with sweet flag rhizome extract demonstrated a synergistic effect on inhibition of H. pylori. The obtained results offer new perspectives for development of functional anti-Helicobacter food product(s) for dietary management of H. pylori infection. The essential components of these products could be the most active juices and extracts like quince and cranberry juice supplemented with a corresponding synergist. Further studies are required to investigate the mechanism of antibacterial action of plant products and their efficacy in vivo.
1. Introduction
Helicobacter pylori is a common bacterium and unique among pathogens. It can persist in the acidic environment of the human stomach. In vitro, urease is required for survival at pH 4.0 and below, whereas at higher acidic pH, mechanisms independent of urease are protective [1]. Expression of approximately 7% of the H. pylori genome is reproducibly altered by a shift to low pH [2]. A large number of reports have been produced on H. pylori and its pathogenetic potential. H. pylori infects at least 50% of the world’s population [3]. Usually, the infection occurs in early life [4,5] and persists throughout the course of a lifetime. The most likely mode of transmission is from person to person, by either the oral-oral route or the fecal-oral route [6]. The involvement of water and food cannot be excluded as vehicles or sources of infection [7].
The course of treatment against helicobacteriosis is usually based on classic triple therapy including proton pump inhibitors and antibacterial therapy, clarithromycin and amoxicillin. For allergic patients, amoxicillin could be replaced by metrodinazole. In cases of antibiotic resistance, the Bismuth compounds can be applied [8-10]. Currently available first-line anti-H. pylori therapies may fail in up to 30% of patients [11] leading to a significant increase of antimicrobial resistance [12-14]. New antimicrobial agents such as a combination of antibiotics with plant extracts and other natural products that possess antimicrobial activity [15,16] are, therefore, being developed to overcome this problem.
Many infections may prove amenable to safe and effective treatment with non-antibiotics [17]. Non-antibiotic therapies, including phytomedicines, probiotics, and antioxidants, have been increasingly investigated for the treatment of H. pylori [18]. The consumption of foods that inhibit the growth of bacteria may provide an alternative to current therapies [19] or complement and expedite current treatments [20]. For the present, despite investigations, phytoceuticals are not accepted as a standard treatment for H. pylori infection [21].
In general, investigations of natural anti-Helicobacter agents can be divided in two groups: 1) investigation of plants used for gastroduodenal disorder treatment in the ethnomedical traditions in different geographic regions [22]; 2) investigation of common food plants and their components. Anti-Helicobacter activity has been estimated, for example, in broccoli sprouts [23], dill, fennel, caraway and cinnamon [20] as well as in several representatives of the Asteraceae, Boraginaceae, Crassulaceae, Eriocaulaceae, Tiliaceae and Verbenaceae families [22]. In our opinion, the largest amount of information describes berries—the cranberry, bilberry, strawberry, black currant and red bilberry [24,25]. The inhibitory effect of the Japanese apricot (Prunus mume) on H. pylori-related chronic gastritis [26] is also well-known.
Taking into consideration previous findings, the aim of this study was to evaluate the anti-H. pylori effect of certain food plant extracts and juices and their composition in vitro to enable the production of new functional food product(s) with anti-Helicobacter activity.
2. Materials and Methods
2.1. Tested Substances, Juices and Extracts
33 substances, juices and plant extracts were tested (Table 1). Whey was produced by “Elpa” Ltd. (Latvia). Water extract of green tea and concentrated apple juice were obtained from “Pure Food” Ltd. (Latvia). Berries and juices were obtained from “Lases” (Latvia). Red beets were supplied by “Baldones lauki” Ltd., dried sweet flag rhizome, fresh ginger, dried wild bergamot as well as chemicals—by Kirsch Pharma GmbH (Germany). Ethanol solution of propolis was obtained from Riga Pharmaceutical Plant JSC (Latvia).
All solutions were made with distilled water. Juices were obtained from cranberries, red beets and apples by using a household juicer followed by centrifugation at 3000 rpm for 30 min. 10% extract of apple pomace was obtained after suspending 10 g of commercial apple pomace (“Lases”, Latvia) in 100 ml of 90˚C water. All materials were stored in the dark at 2˚C - 8˚C until use.
2.2. Measurement of pH
The pH values were measured using a pH meter pH/Ion 510 (Oakton Instruments, USA).
2.3. Bacteria and Culture Conditions
The strain Helicobacter pylori ATCC 43504 was used. H. pylori was cultivated on Columbia blood agar (Oxoid, UK) at 37˚C for 72 h. Plates were incubated in anaerobic conditions inside an anaerobic jar HP0011A (Oxoid, UK) containing GasPak™ Plus (Becton, Dickinson, USA).
2.4. Agar Diffusion Method
Antibacterial activity was determined by the agar diffusion method. The agar diffusion test was performed on Columbia blood agar (Oxoid, UK). Inoculum of 106 CFU of bacteria per millilitre was used. Aliquots of 85 μl of each test-sample solution were applied into 6.0 mm diameter agar wells. After incubation in anaerobic conditions at 37˚C for 72 h, the diameter of the clear zone (no growth) around the well in the bacterial lawn was mea- sured and was used to express the antimicrobial activity. The inhibition zone diameter was measured in millimeters (mm). The tests were performed in triplicate and the final results were presented as the arithmetic average.
2.5. Statistics
Statistical analysis was done by analysis of variance. P < 0.05 was considered statistically significant. Each experiment was repeated three times.
3. Results
3.1. Anti-Helicobacter Activity of Certain Substances, Juices and Extracts
The antibacterial activity against H. pylori was tested for five organic acids (Table 1) in the pH range of 1.18 to 3.11 and for three food preservatives (sorbic acid, potassium sorbate and sodium benzoate). Anti-Helicobacter properties were shown only for hydrochloric acid (0.4% solution), acetic acid (1%) and citric acid (2%) but not for ascorbic acid (0.5%) and benzoic acid (0.15%). The selected preservatives did not exhibit antibacterial activity at the tested concentrations permitted in food products.
From other tested components including fruit and berry juices, quince juice showed the greatest antibacterial activity. Its activity exceeded the activity of cranberry juice (Table 1) and it was less dependent on dilution than cranberry juice. The antibacterial activity was observed in 10% quince juice while cranberry juice retained some activity only at 20% concentration. Moreover, quince juice recovered the major part of its antibacterial activity even after five years’ storage. Equivalent activity was demonstrated by juice of wild and cultivated cranberries, Vaccinium vitis-idaea and V. macrocarpum, respectively. Experimental increase of pH of quince and cranberry juices with sodium hydrocarbonate or calcium carbonate above pH 4 completely eliminated the anti-Helicobacter activity (Table 2).
Concentrated apple juice as well as plum, red currant, black chokeberry, raspberry and bilberry juice also demonstrated significant antibacterial activity. Green tea extract and apple pomace extract as well as sweet flag rhizome, ginger and wild bergamot extract, cherry syrup, red beet juice and whey did not possess anti-Helicobacter activity (Table 1).
In general, the values of pH of the investigated solutions were in the range from 1.18 (0.4% hydrochloric acid, corresponding inhibition zone diameter 10 mm) to 8.80 (10% extract of dried sweet flag rhizome, inhibition zone diameter 0 mm). The highest pH at which antibacterial
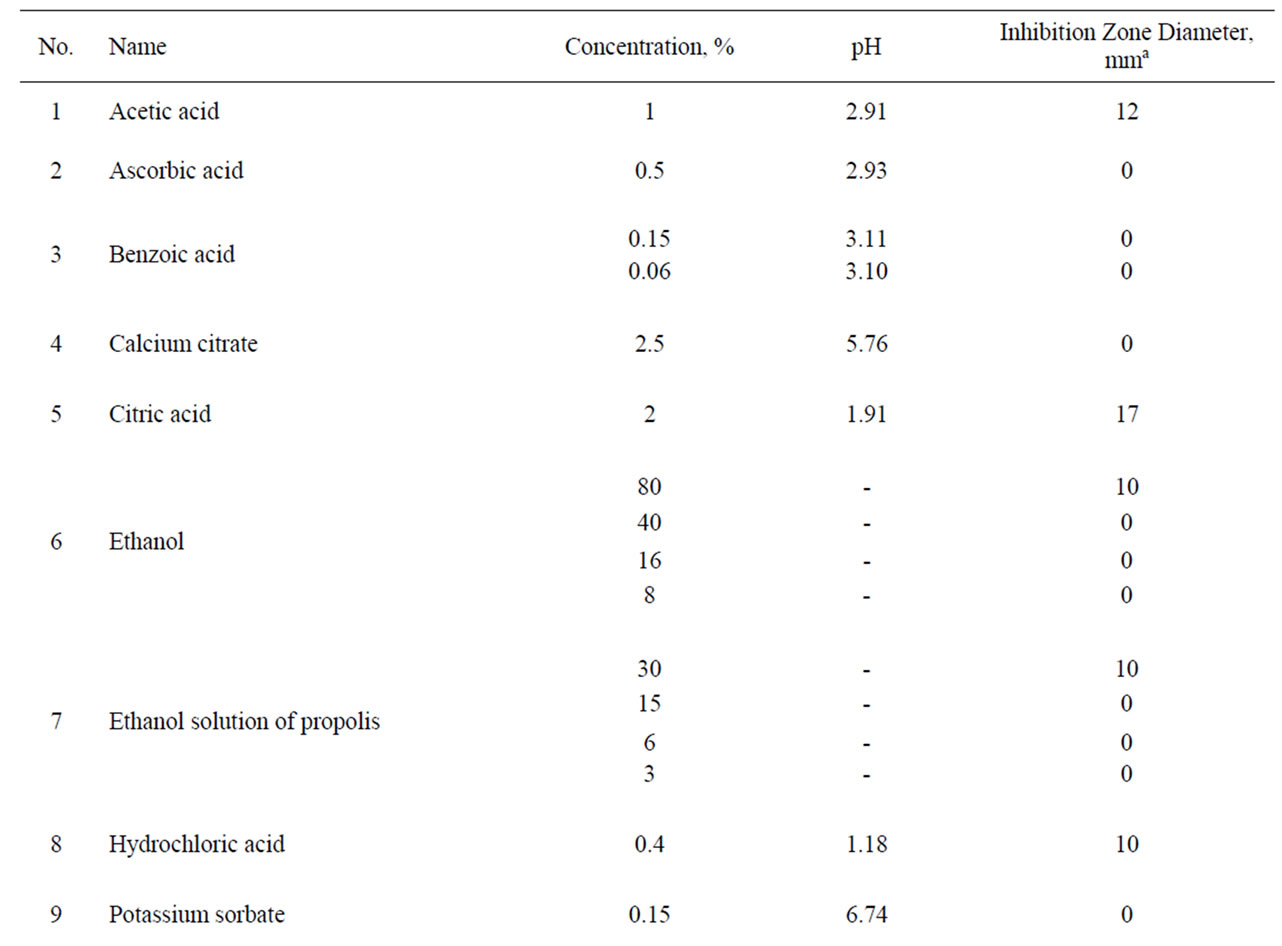
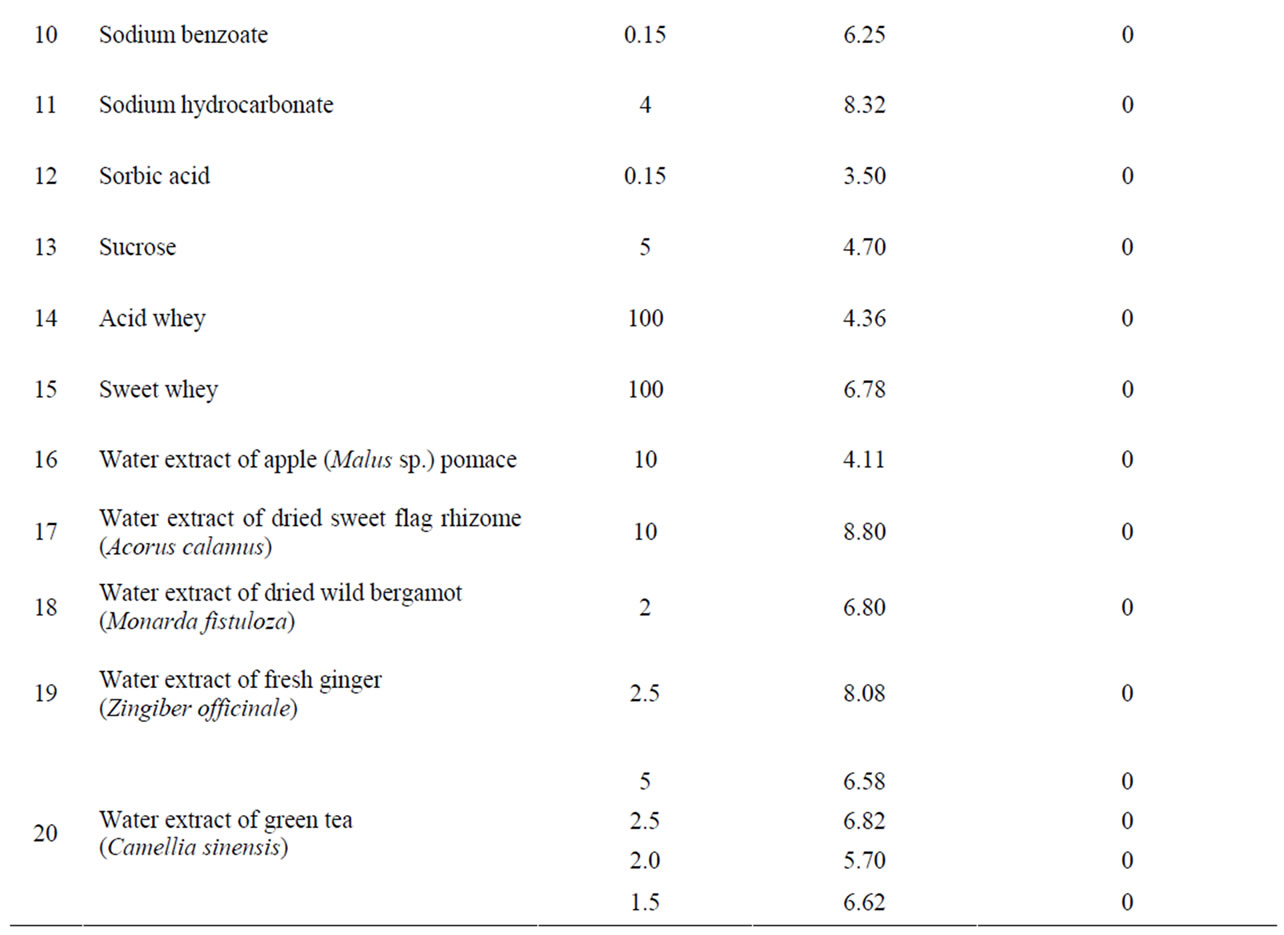
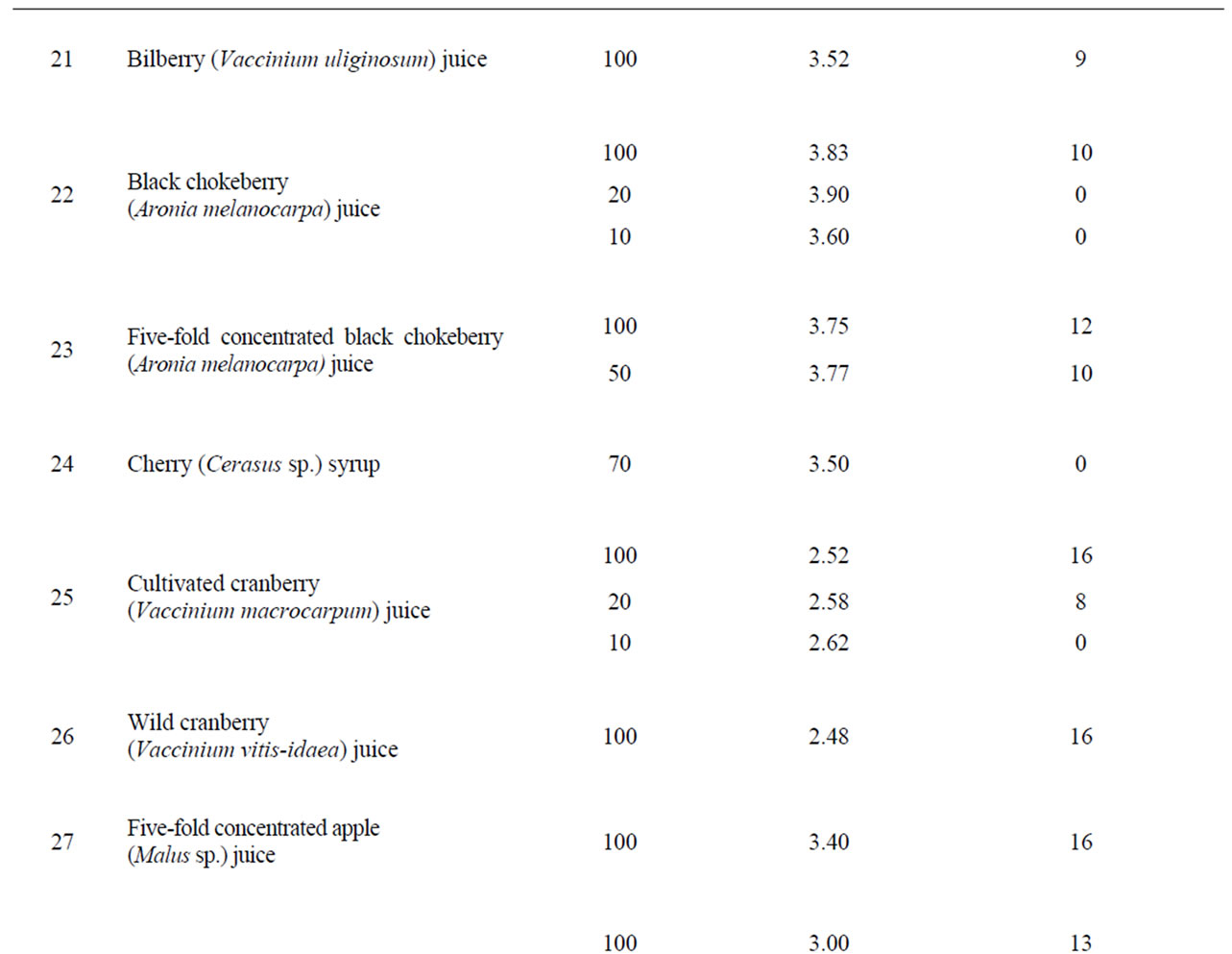
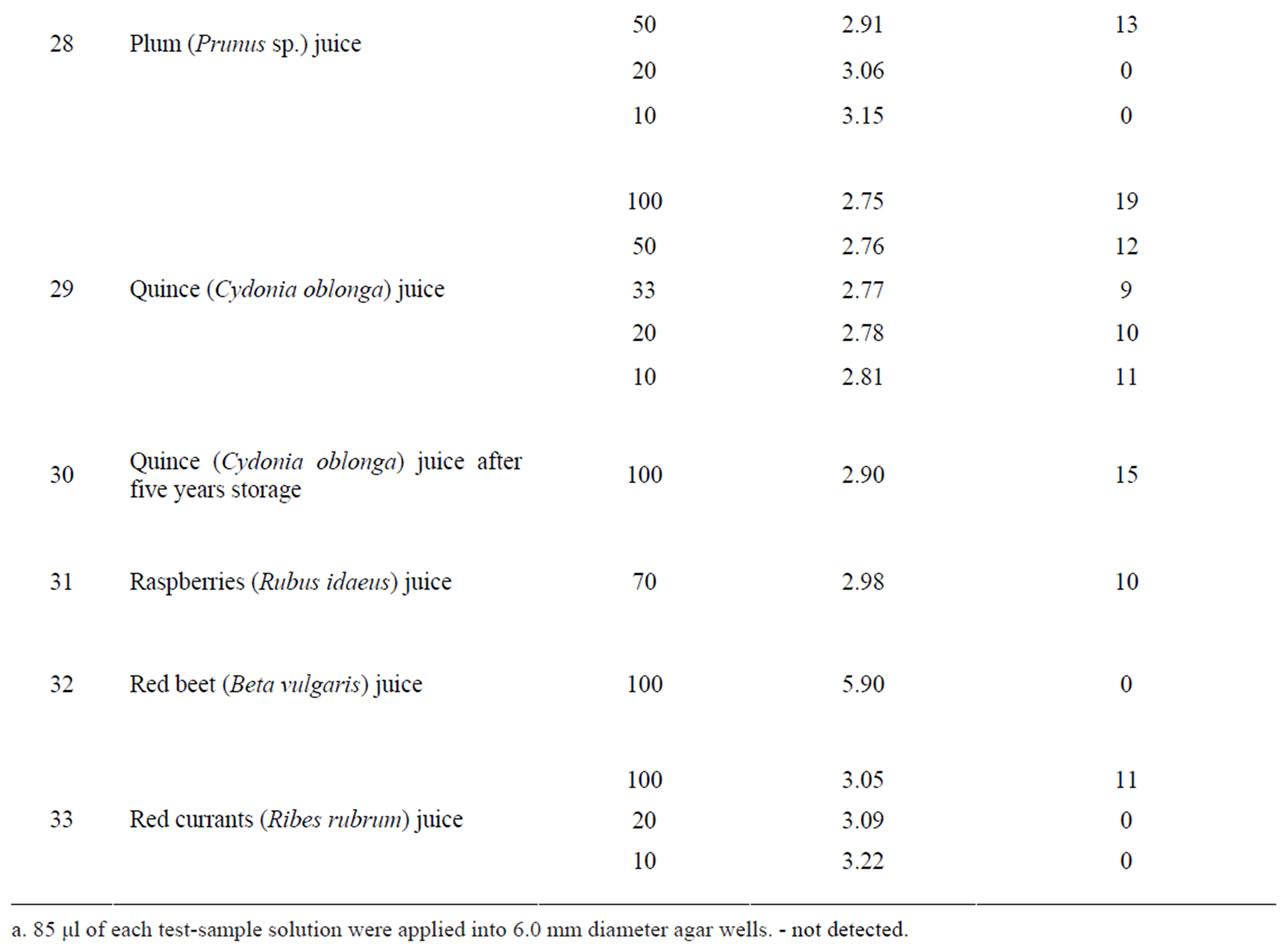
Table 1. Anti-Helicobacter activity and characteristic of certain solutions, juices and extracts.
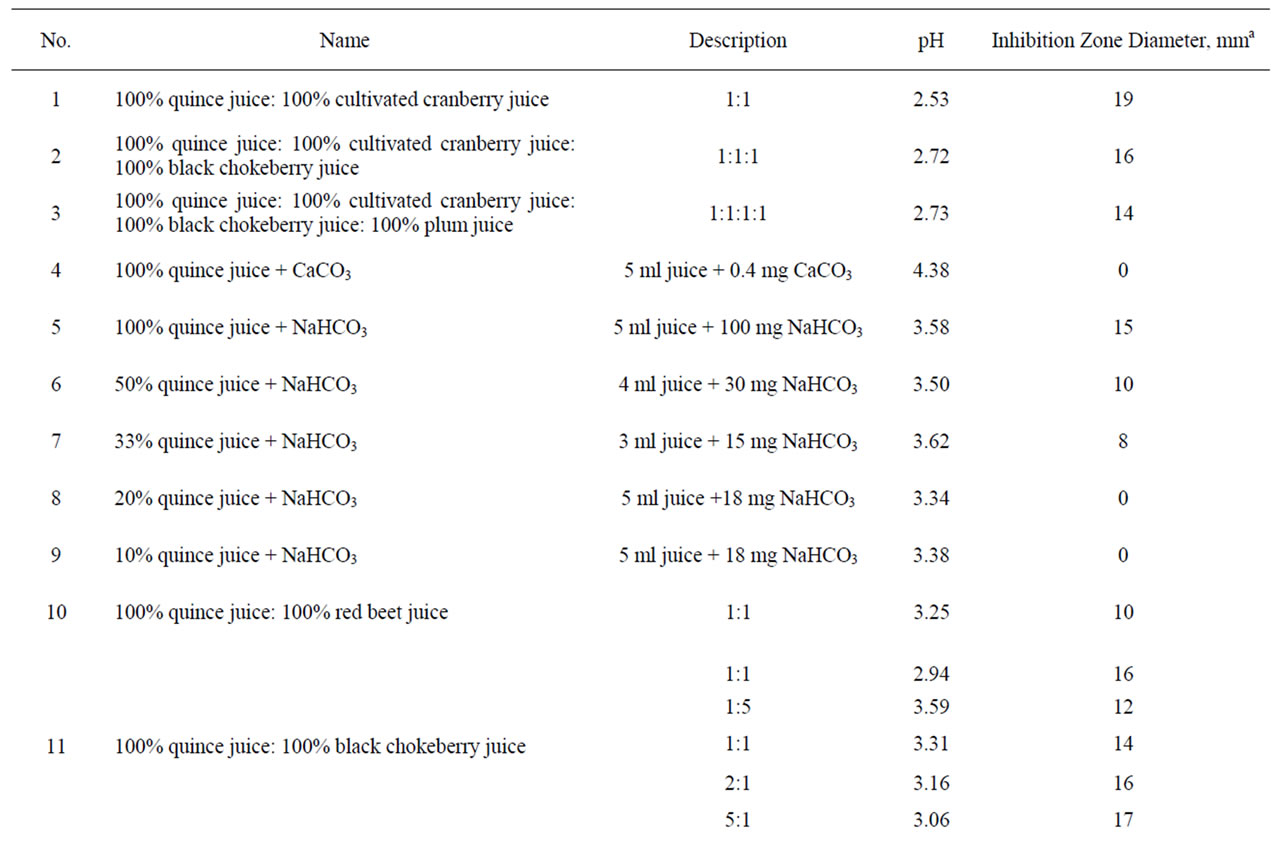
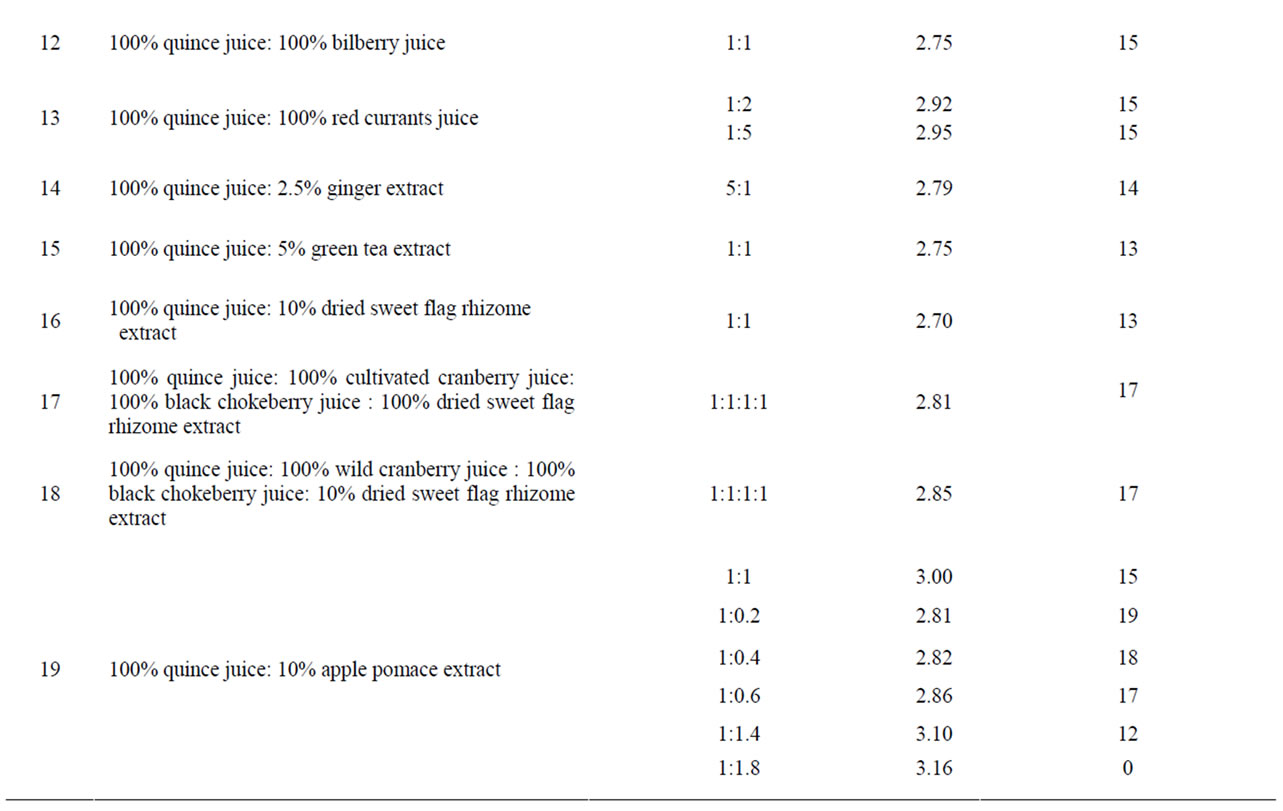
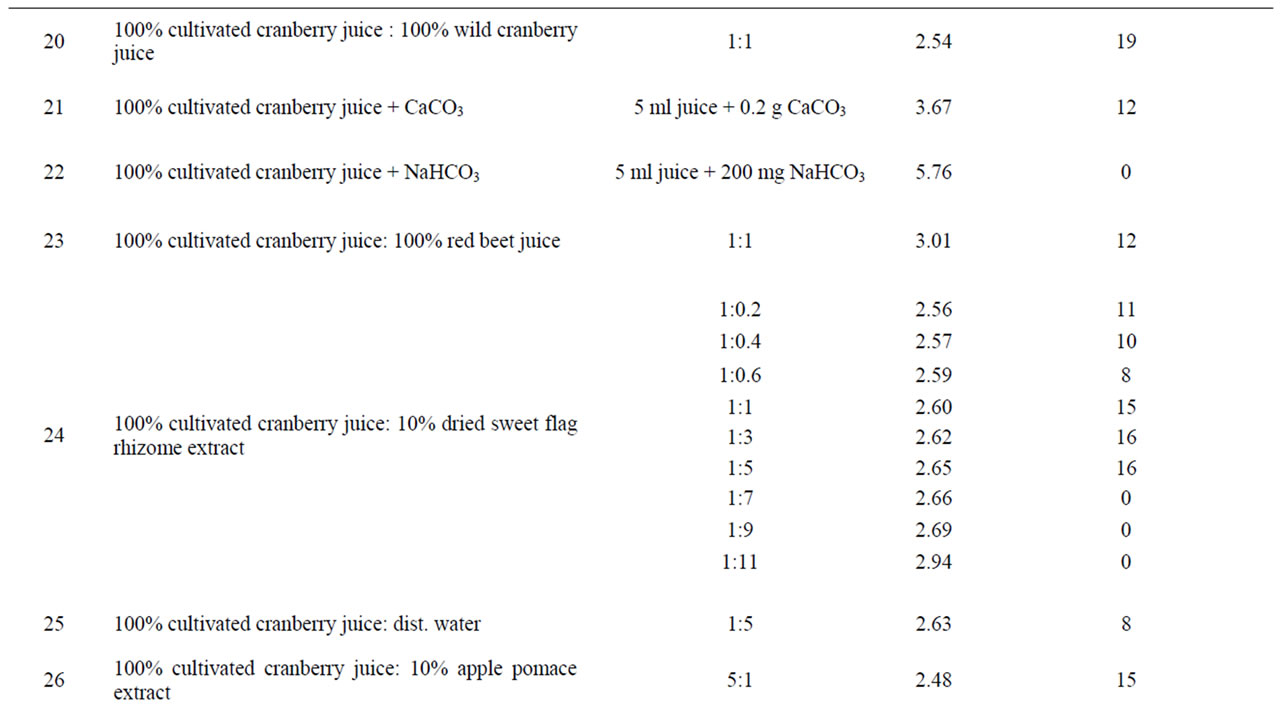
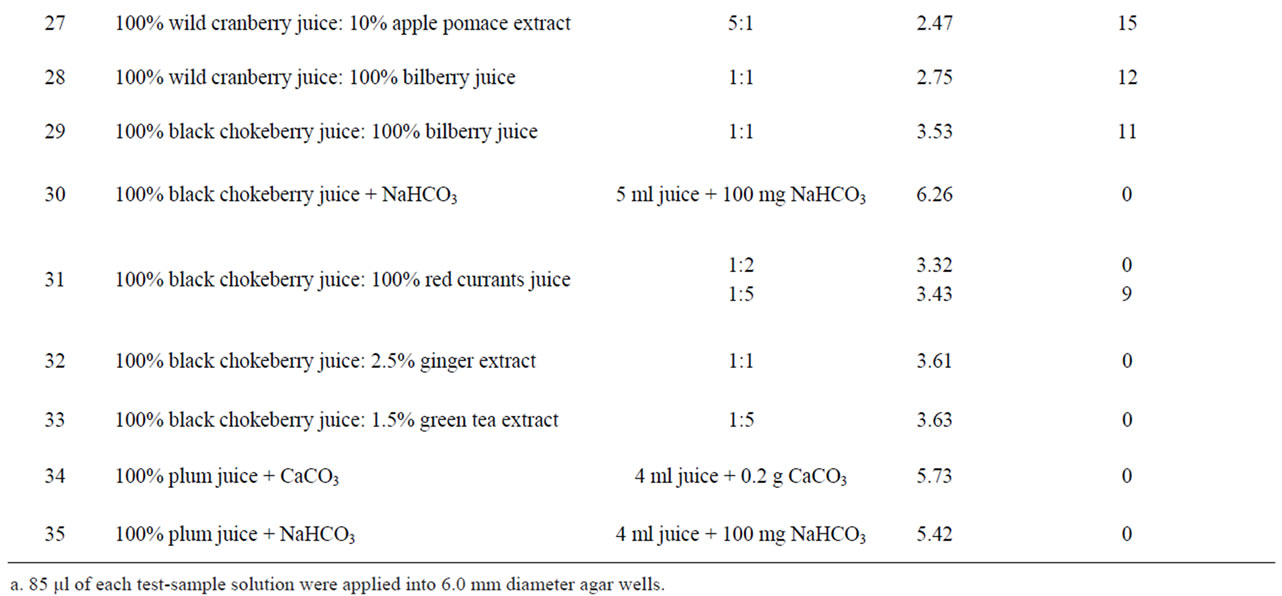
Table 2. Anti-Helicobacter activity and characteristic of different compositions.
activity was observed corresponded to 3.83 (black chokeberry juice, inhibition zone diameter 10 mm) but all substances, juices and extracts with pH ≥ 3.90 showed no observable activity.
3.2. Anti-Helicobacter Activity of Different Compositions
Quince juice in combination with bilberry, black chokeberry, red currant juice, green tea and sweet flag rhizome or apple pomace extracts showed synergistic activity (Table 2). For example, quince juice possessed antibacterial activity after dilution with apple pomace water extract (Figure 1), but the activity was not observed when this juice was diluted with water. Apple pomace extract itself exhibited no antibacterial effect.
Synergism was observed also for compositions made from cranberry juice and sweet flag rhizome extract (Figure 2). Sweet flag rhizome extract alone did not possess the anti-Helicobacter activity.
pH values of the investigated compositions fell within the range of 2.47 (wild cranberry juice and apple pomace extract in the ratio 5:1, corresponding inhibition zone
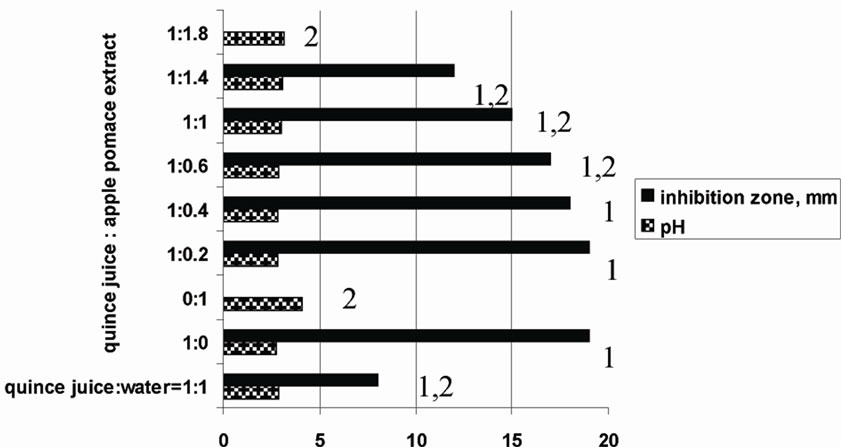
Figure 1. Dilution effect on the quince juice anti-Helicobacter activity in vitro. The inhibition zone was observed using the agar diffusion method. Aliquots of 85 μl of each test-sample solution were applied into 6.0 mm diameter agar wells. The tests were performed in triplicate and the final results were presented as the arithmetic average. 1P < 0.05 inhibition zone in comparison with apple pomace extract. 2P < 0.05 inhibition zone in comparison with quince juice.
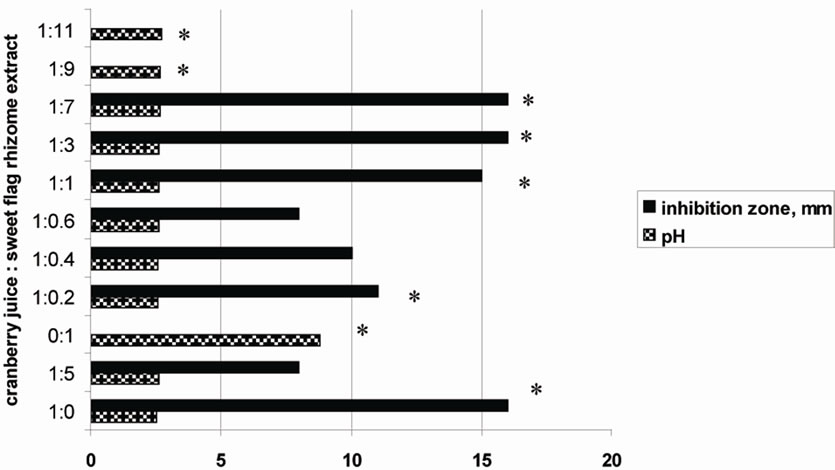
Figure 2. Synergistic effects of cranberry juice and sweet flag rhizome extract on inhibition of H. pylori. The inhibition zone was observed using the agar diffusion method. Aliquots of 85 μl of each test-sample solution were applied into 6.0 mm diameter agar wells. The tests were performed in triplicate and the final results were presented as the arithmetic average. *P < 0.05 inhibition zone in comparison with cranberry juice diluted with water in the ratio 1:5.
diameter 15 mm) to 5.76 (cranberry juice partly alkalized with NaHCO3, inhibition zone diameter 0 mm). In general, antibacterial activity was observed only for compositions in which pH did not exceed 3.67.
4. Discussion
Anti-Helicobacter activity in vitro has been estimated in many known botanical extracts—high in Myristica fragrans (seed), Zingiber officinale (ginger rhizome/root) and Rosmarinus officinalis (rosemary leaf); moderate in Achillea millefolium, Foeniculum vulgare (seed), Passiflora incarnata (herb), Origanum majorana (herb) and a (1:1) combination of Curcuma longa (root) and ginger rhizome; weak in Carum carvi (seed), Elettaria cardamomum (seed), Gentiana lutea (roots), Juniper communis (berry), Lavandula angustifolia (flowers), Melissa officinalis (leaves), Mentha piperita (leaves), Pimpinella anisum (seed), Matricaria recutita (flowers), Ginkgo biloba (leaves) [27] etc. Also, the bacteriostatic effect of dill, fennel, caraway and cinnamon extracts against Helicobacter pylori has been detected. The presumption was stated that the possible synergistic effects of different dietary combinations of these extracts may be a factor in the possible protection afforded by the traditional Iranian diet against H. pylori infection [20].
This study evaluated the anti-H. pylori potential of some food-related chemical substances (organic acids and preservatives), plant extracts and juices and ascertained the effectivity of their composition. In general, 33 individual substances, juices and extracts were tested (Table 1).
As might be expected, some acid solutions demonstrated anti-Helicobacter activity but estimated inhibition zone diameters did not correlate with the pH value. However, we can confirm that pH has significant impact on the growth of H. pylori. In our experiments, citric acid solution has a higher pH in comparison with hydrochloric acid solution, but citric acid has a larger inhibition zone diameter than hydrochloric acid (Table 1). Probably, the anion, in this case citrate, is also implicated in suppression of bacteria. At least several citrate salts (iron citrate, ammonium iron citrate and sodium ferrous citrate) possess anti-Helicobacter activity [28]. However, our experiments with 2.5% calcium citrate remained ineffective. Also, ascorbic acid showed no antibacterial activity, but this fact should be interpreted with caution because this substance is a well-known antioxidant and, therefore, could demonstrate anti-Helicobacter activity in vivo as have been shown in clinic tests [30,31].
There have been many investigations regarding antibacterial activity of different natural plant products in laboratory experiments as well as in vivo. Cranberries are considered the gold standard and they are widely used in various ways [25]. A prospective, randomized, doubleblind, placebo-controlled trial conducted in China showed that regular dietary consumption of cranberry juice can suppress H. pylori infection [32]. In our experiments, the greatest anti-Helicobacter activity was demonstrated by quince and cranberry juice, but significant activity was shown by apple, plum, red currant, black chokeberry, raspberry and bilberry juice (Table 1). Although the activity of bilberry and black chokeberry was lower than that of cranberry or quince, their pH was about 0.7 higher. It could be considered an advantage while it decreased the possibility of irritation of mucous membranes.
The antibacterial activity of cranberries and also some other berries usually is attributed to their benzoic acid content. For all that, benzoic acid (0.15%) and sodium benzoate alone did not show anti-Helicobacter activity. A high molecular mass constituent of cranberry juice has been shown to inhibit the adhesion of H. pylori to human gastric mucus in vitro [33]. In our study, the observed activity of quince juice exceeded the activity of cranberries. In the literature, we have found only one research paper [34] in which the authors noted the anti-Helicobacter action of quince (Cydonia oblonga) juice.
Several extracts that did not possess anti-Helicobacter activity alone showed significant activity in combination with others components. Such synergism was observed for sweet flag rhizome, green tea and apple pomace extract. However, other investigations have established that sweet flag (Acorus calamus) rhizome [35] as well as green tea (Camellia sinensis) [36-38] and apple peel extract [39] has antibacterial activity. Cranberry synergies with blueberry, grape seed and oregano extract [39] as well as blackcurrant oil synergy with broccoli sprouts have been reported [19]. Sometimes synergism is already used in practice. Extracts from wild blueberries, strawberries, cranberries, wild bilberries, elderberries and raspberries significantly (P < 0.05) inhibited H. pylori, compared with controls, and also increased susceptibility of H. pylori to clarithromycin, but food supplement OptiBerry, made from a blend of the abovementioned extracts, demonstrated maximal effects [24]. The antibacterial activeity of sweet flag rhizome, green tea and apple pomace extracts also could be expected in in vivo conditions.
On the basis of our experiments, the essential components of new functional anti-Helicobacter food product(s) could be the most active juices and extracts like quince, black chokeberry and cranberry juice supplemented with one or another synergist. Further studies are required to elucidate the mechanism of antibacterial action of plant juices and extracts and to investigate the efficacy in vivo.
REFERENCES
- C. C. McGowan, T. L. Cover and M. J. Blaser, “Helicobacter pylori and Gastric Acid: Biological and Therapeutic Implications,” Gastroenterology, Vol. 110, No. 3, 1996, pp. 926-938. doi:10.1053/gast.1996.v110.pm8608904
- D. S. Merrell, M. L. Goodrich, G. Otto, L. S. Tompkins and S. Falkow, “pH-Regulated Gene Expression of the Gastric Pathogen Helicobacter pylori,” Infection and Immunity, Vol. 71, No. 6, 2003, pp. 3529-3539. doi:10.1128/IAI.71.6.3529-3539.2003
- S. Perry, M. L. Sanchez, S. Yang, T. D. Haggerty, P. Hurst, G. Perez-Perez and J. Parsonnet, “Gastroenteritis and Transmission of Helicobacter pylori Infection in Households,” Emerging Infectious Diseases, Vol. 12, No. 11, 2006, pp. 1701-1708.
- H. M. Malaty, A. El-Kasabany, D. Y. Graham, C. C. Miller, S. G. Reddy, S. R. Srinivasan, Y. Yamaoka and G. S. Berenson, “Age at Acquisition of Helicobacter pylori Infection: A Follow-Up Study from Infancy to Adulthood,” The Lancet, Vol. 359, No. 9310, 2002, pp. 931-535. doi:10.1016/S0140-6736(02)08025-X
- T. Sabbi, P. de Angelis and L. Dall’Oglio, “Helicobacter pylori Infection in Children: Management and Pharmacotherapy,” Expert Opinion on Pharmacotherapy, Vol. 9, No. 4, 2008, pp. 577-585. doi:10.1517/14656566.9.4.577
- L. M. Brown, “Helicobacter pylori: Epidemiology and Routes of Transmission,” Epidemiologic Reviews, Vol. 22, No. 2, 2000, pp. 283-297.
- F. F. Vale and J. M. Vitor, “Transmission Pathway of Helicobacter pylori: Does Food Play a Role in Rural and Urban Areas?” International Journal of Food Microbiology, Vol. 138, No. 1-2, 2010, pp. 1-12. doi:10.1016/j.ijfoodmicro.2010.01.016
- L. Fischbach and E. L. Evans, “Meta-Analysis: The Effect of Antibiotic Resistance Status on the Efficacy of Triple and Quadruple First-Line Therapies for Helicbacter pylori,” Alimentary Pharmacology and Therapeutics, Vol. 26, No. 3, 2007, pp. 343-357. doi:10.1111/j.1365-2036.2007.03386.x
- D. Y. Graham and A. Shiotani, “New Concepts of Resistance in the Treatment of Helicobacter pylori Infections,” Nature Clinical Practice Gastroenterology and Hepatology, Vol. 5, No. 6, 2008, pp. 321-331. doi:10.1038/ncpgasthep1138
- B. Stenstrom, A. Mendis and B. Marshall, “Helicobacter pylori—the Latest in Diagnosis and Treatment,” Australian Family Physician, Vol. 37, No. 8, 2008, pp. 608-612.
- M. P. Della, A. Lavagna, G. Masoero, L. Lombardo, L. Crocella and A. Pera, “Effectiveness of Helicobacter pylori Eradication Treatments in a Primary Care Setting in Italy,” Alimentary Pharmacology and Therapeutics, Vol. 16, No. 7, 2002, pp. 1269-1275. doi:10.1046/j.1365-2036.2002.01244.x
- M. Heep, M. Kist, S. Strobel, D. Beck and N. Lehn, “Secondary Resistance among 554 Isolates of Helicobacter pylori after Failure of Therapy,” European Journal of Clinical Microbiology and Infectious Diseases, Vol. 19, No. 7, 2000, pp. 538-541. doi:10.1007/s100960000288
- F. Bazzoli, P. Pozzato and T. Rokkas, “Helicobacter pylori: The Challenge in Therapy,” Helicobacter, Vol. 7, Suppl. 1, 2002, pp. 43-49. doi:10.1046/j.1523-5378.7.s1.7.x
- A. Qasim and C. A. O’Morain, “Review Article: Treatment of Helicobacter pylori Infection and Factors Influencing Eradication,” Alimentary Pharmacology and Therapeutics, Vol. 16, 2002, pp. 24-30. doi:10.1046/j.1365-2036.2002.0160s1024.x
- R. N. Ndip, A. E. M. Tarkang, C. M. Echakachi, A. Malongue, J.-F. T. K. Akoachere, L. M. Ndip and H. N. Luma, “In vitro Antimicrobial Activity of Selected Honeys on Clinical Isolates of Helicobacter pylori,” African Health Sciences, Vol. 7, No. 4, 2007, pp. 228-231.
- R. N. Ndip, A. E. M. Tarkang, S. M. Mbullah, H. N. Luma, A. Malongue, L. M. Ndip, K. Nyongbela, C. Wirmum and S. M. N. Efange, “In Vitro Anti-Helicobacter pylori Activity of Extracts of Selected Medicinal Plants from North West Cameroon,” Journal of Ethnopharmacology, Vol. 114, No. 3, 2007, pp. 452-457. doi:10.1016/j.jep.2007.08.037
- C. F. Carson and T. V. Riley, “Non-Antibiotic Therapies for Infectious Diseases,” Communicable Diseases Intelligence, Vol. 27, 2003, pp. S143-146.
- M. M. Kamiji and R. B. Oliveira, “Non-Antibiotic Therapies for Helicobacter pylori Infection,” European Journal of Gastroenterology and Hepatology, Vol. 17, No. 9, 2005, pp. 973-981. doi:10.1097/00042737-200509000-00014
- J. I. Keenan, N. Salm, M. B. Hampton and A. J. Wallace, “Individual and Combined Effects of Foods on Helicobacter pylori Growth,” Phytotherapy Research, Vol. 24, No. 8, 2010, pp. 1229-1233.
- S. Sadeghian, T. R. Neyestani, M. H. Shirazi and P. Ranjbarian, “Bacteriostatic Effect of Dill, Fennel, Caraway and Cinnamon Extracts against Helicobacter pylori,” Journal of Nutritional and Environmental Medicine, Vol. 15, No. 2-3, 2005, pp. 47-55. doi:10.1080/13590840500535313
- S. Y. Lee, Y. W. Shin and K. B. Hahm, “Phytoceuticals: Mighty but Ignored Weapons against Helicobacter pylori Infection,” Journal of Digestive Diseases, Vol. 9, No. 3, 2008, pp. 129-139. doi:10.1111/j.1751-2980.2008.00334.x
- H. S. Falcao, I. R. Mariath, M. F. Diniz, L. M. Batista and J. M. Barbosa-Filho, “Plants of the American Continent with Antiulcer Activity,” Phytomedicine, Vol. 15, No. 1-2, 2008, pp. 132-146. doi:10.1016/j.phymed.2007.07.057
- A. Yanaka, J. W. Fahey, A. Fukumoto, M. Nakayama, S. Inoue, S. Zhang, M. Tauchi, H. Suzuki, I. Hyodo and M. Yamamoto, “Dietary Sulforaphane-Rich Broccoli Sprouts Reduce Colonization and Attenuate Gastritis in Helicobacter pylori—Infected Mice and Humans,” Cancer Prevention Research, Vol. 2, No. 4, 2009, pp. 353-360.
- A. Chatterjee, T. Yasmin, D. Bagchi and S. J. Stohs, “Inhibition of Helicobacter pylori in vitro by Various Berry Extracts, with Enhanced Susceptibility to Clarithromycin,” Molecular and Cellular Biochemistry, Vol. 265, No. 1-2, 2004, pp. 19-26. doi:10.1023/B:MCBI.0000044310.92444.ec
- H. Shmuely, J. Yahav, Z. Samra, G. Chodick, R. Koren, Y. Niv and I. Ofek, “Effect of Cranberry Juice on Eradication of Helicobacter pylori in Patients Treated with Antibiotics and a Proton Pump Inhibitor,” Molecular Nutrition and Food Research, Vol. 51, No. 6, 2007, pp. 746-751. doi:10.1002/mnfr.200600281
- S. Enomoto, K. Yanaoka, H. Utsunomiya, T. Niwa, K. Inada, H. Deguchi, K. Ueda, C. Mukoubayashi, I. Inoue, T. Maekita, K. Nakazawa, M. Iguchi, K. Arii, H. Tamai, N. Yoshimura, M. Fujishiro, M. Oka and M. Ichinose, “Inhibitory Effects of Japanese Apricot (Prunus mume Siebold et Zucc.; Ume) on Helicobacter pylori-Related Chronic Gastritis,” European Journal of Clinical Nutrition, Vol. 64, No. 7, 2010, pp. 714-719. doi:10.1038/ejcn.2010.70
- G. B. Mahady, S. L. Pendland, A. Stoia, F. A. Hamill, D. Fabricant, B. M. Dietz and L. R. Chadwick, “In Vitro Susceptibility of Helicobacter pylori to Botanical Extracts Used Traditionally for the Treatment of Gastrointestinal Disorders,” Phytotherapy Research, Vol. 19, No. 11, 2005, pp. 988-991. doi:10.1002/ptr.1776
- Otsuka Pharmaceutical Co., Ltd (JP), Inventors: M. Kikuchi, H. Takiza, H. Wakebe, F. Mukai, S. Shimizu, H. Okamatsu, T. Saito, S. Yamadaira, H. Kimura and K. Sasaki, “Composition, Antimicrobial Agent, Infection Preventive, and Food against Helicobacter pylori,” WIPO Patent Application WO/1998/009652, Application Number: PCT/JP/1997/ 003100. 1997.09.04.
- M. Tabak, R. Armon, G. Rosenblat, E. Stermer and I. Neeman, “Diverse Effects of Ascorbic Acid and Palmitoyl Ascorbate on Helicobacter Pylori Survival and Growth,” FEMS Microbiology Letters, Vol. 224, No. 2, pp. 247-253. doi:10.1016/S0378-1097(03)00439-7
- C. H. Chuang, B. S. Sheu, A. W. Kao, H. C. Cheng, A. H. Huang, H. B. Yang and J. J. Wu, “Adjuvant Effect of Vitamin C on Omeprazole-Amoxicillin-Clarithromycin Triple Therapy for Helicobacter pylori Eradication,” HepatoGastroenterology, Vol. 54, No. 73, 2007, pp. 320-324.
- L. Zhang, J. Ma, K. Pan, V. L. Go, J. Chen and W. C. You, “Efficacy of Cranberry Juice on Helicobacter pylori Infection: Randomised Placebo-Controlled Trial,” Helicobacter, Vol. 10, No. 2, 2005, pp. 139-145. doi:10.1111/j.1523-5378.2005.00301.x
- O. Burger, I. Ofek, M. Tabak, E. Weiss, N. Sharon and I. Neeman, “A High Molecular Mass Constituent of Cranberry Juice Inhibits Helicobacter pylori Adhesion to Human Gastric Mucus,” FEMS Immunology and Medical Microbiology, Vol. 29, No. 4, 2000, pp. 295-301. doi:10.1111/j.1574-695X.2000.tb01537.x
- S. Fattouch, P. Caboni, V. Coroneo, C. I. G. Tuberoso, A. Angioni, S. Dessi, N. Marzouki and P. Cabras, “Antimicrobial Activity of Tunisian Quince (Cydonia oblonga Miller) Pulp and Peel Polyphenolic Extracts,” Journal of Agricultural and Food Chemistry, Vol. 55, No. 3, 2007, pp. 963-969. doi:10.1021/jf062614e
- F. Aquil and I. Ahmad, “Antibacterial Properties of Traditionally Used Indian Medicinal Plants,” Methods and Findings in Experimental and Clinical Pharmacology, Vol. 29, No. 2, 2007, pp. 79-92. doi:10.1358/mf.2007.29.2.1075347
- H. C. Lee, A. M. Jenner, C. S. Low and Y. K. Lee, “Effect of Tea Phenolics and their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota,” Research in Microbiology, Vol. 157, No. 9, 2006, pp. 876-884. doi:10.1016/j.resmic.2006.07.004
- A. Rocco and G. Nardone, “Diet, H. pylori Infection and Gastric Cancer: Evidence and Controversies,” World Journal of Gastroenterology, Vol. 13, No. 21, 2007, pp. 2901- 2912.
- C. Stoicov, R. Saffari and J. Houghton, “Green Tea Inhibits Helicobacter Growth in Vivo and in Vitro,” International Journal of Antimicrobial Agents, Vol. 33, No. 5, 2009, pp. 473-478. doi:10.1016/j.ijantimicag.2008.10.032
- E. Pastene, H. Speisky, M. Troncoso, J. Alarcon and G. Figueroa, “In vitro Inhibitory Effect of Apple Peel Extract on the Growth of Helicobacter pylori and Respiratory Burst Induced on Human Neutrophils,” Journal of Agricultural and Food Chemistry, Vol. 57, No. 17, 2009, pp. 7743-7749. doi:10.1021/jf9006592
- D. A. Vattem, Y. T. Lin, R. Ghaedian and K. Shetty, “Cranberry Synergies for Dietary Management of Helicobacter pylori Infections,” Process Biochemistry, Vol. 40, No. 5, 2005, pp. 1583-1592. doi:10.1016/j.procbio.2004.06.024

