American Journal of Plant Sciences
Vol.08 No.06(2017), Article ID:76358,10 pages
10.4236/ajps.2017.86088
Studying on Tyrosinase Inhibition Activity of Some Vietnamese Folk Plants Aims to Use in Skin-Whitening Cosmetics
Nguyen Thi My Hanh1, Nguyen Kim Phi Phung2, Quach Ngo Diem Phuong1
1Department of Plant Biotech and Biotransformation, Faculty of Biology-Biotechnology, University of Science, Vietnam National University-Ho Chi Minh City, Ho Chi Minh City, Vietnam
2Department of Organic Chemistry, Faculty of Chemistry, University of Science, Vietnam National University-Ho Chi Minh City, Ho Chi Minh City, Vietnam

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 4, 2017; Accepted: May 20, 2017; Published: May 23, 2017
ABSTRACT
White mulberry Morus alba and jackfruit Artocarpus heterophyllus are two of seven chosen Vietnamese folk plants which showed the highest tyrosinase inhibition activity. Besides, both of them showed antioxidant and antibacterial activities. Especially, the Sulforhodamine B (SRB) assay test result of the root core extract of M. alba figured out its non-toxicity on foreskin fibroblasts.
Keywords:
Morus alba, Tyrosinase Inhibition Activity, Antibacterial, Antioxidant

1. Introduction
Tyrosinase plays a key role in melanin biosynthesis―the skin color determining factor. The melanin pigment is a polymer family produced inside the melanosomes and synthesized from the amino acid L-tyrosine that is converted by the enzyme tyrosinase to dopaquinone. The composition of the mixed melanins in many different ways forms many colour variations of the skin and hair [1] .
Plant extracts that have a good inhibitory effect on melanin formation may be a good choice for the cosmetic purposes of whitening skin. In addition, they have relatively fewer side effects [2] .
The purpose of this study is finding some Vietnamese folk plants which can possess skin whitening capability and other necessary properties for cosmetics. Seven chosen plants in this report have been thought having the special capability in Vietnamese traditional medicine.
2. Experimental
2.1. General
Tyrosinase activity assay was carried out with Sigma T3824-25KU mushroom tyrosinase enzyme and measured by Perkin Elmer 2030 Elisa reader.
Fibroblast from human foreskin was supplied from the Department of Genetics, Faculty of Biology, Vietnam National University-HCMC.
E. coli, Salmonella spp, Samonella typhimurium, Pseudomonas aeruginosa, Bacillus subtilis, Bacillus cereus, Streptococus spp, Staphylococus aureus were deposited from Department of Microbiology, Faculty of Biology-Biotechnology, University of Science, Vietnam National University-HCMC.
All data were analysed by Microsoft Excel 2007, SAS 9.1 for window. The figures are presented as Mean ±SD. The statistical significance at P (p-value) < 0.05.
2.2. Plant Materials
All of plant materials were authenticated by Department of Ecology and Evolutionary Biology, Faculty of Biology, University of Science, Vietnam National University-HCMC (Table 1).
2.3. Preparation of Different Extracts
All parts of plant material were washed, dried at 45˚C, ground into small pieces. For selection experiments, each plant material (1 g) was soaked in 10mL ethanol 80% at 60˚C in 60 mins. The mixture was cooled to room temperature and centrifuged at 6000rpm in 10 mins at the same condition. The supernatant was filtered by lab filter papers and the filtrate was used for the analysis. In other experiments, materials were soaked in ethanol 80% and filtered, repeated 4 times. The filtrates were evaporated under reduced pressure to dryness to afford crude extracts [3] . This crude extract was then successively eluted with n-hexane, chloroform and water to afford 3 different fraction extracts.
2.4. Tyrosinase Activity Assay
Each plant extract was assayed for tyrosinase inhibition by measuring its effect
Table 1. Seven chosen plants with parts, time and place of collection.
on tyrosinase activity using a 96-well reader. The reaction was carried out at room temperature in 30 µL potassium phosphate buffer (50 Mm, pH 6.8) containing 343 U/mL mushroom tyrosinase and 110 µL L-tyrosine 2 mM (the reaction mixture was preincubated for 15 mins before adding L-tyrosine). Then the reaction mixture was incubated for other 15 mins before measuring OD490 nm. The reaction mixture without enzyme was served as blank. The reaction mixture with the corresponding solvents (without plant material) was used as control. Acid kojic is the positive control. The percentage inhibition of tyrosinase was calculated as follows:
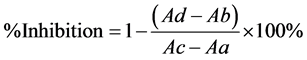
Aa: OD490 nm of reaction mixture without extract and enzyme
Ab: OD490 nm of reaction mixture without enzyme
Ac: OD490 nm of reaction mixture without extract
Ad: OD490 nm of full reaction mixture
2.5. Phytochemical Evaluation
The ethanolic extracts were subjected to the chemical tests for the identification of various active constituents: phenolic, alkaloid, flavonoid, terpenoid-steroid and glycoside.
2.6. Reducing Power Activity
The reducing power of extract was determined by the method of Yen and Duh (1993) [4] . 1ml of each extract was added phosphate buffer (200 mM, pH 6.6, 2.5 mL) and 1% potassium ferricyanide (2.5 mL). The mixture was incubated for 20 mins at 50˚C. After incubation, 10% trichloroacetic acid (2.5 mL) was added to the mixture, followed by centrifugation at 6000 rpm for 10 mins. The upper layer (1 mL) was mixed with distilled water (2 mL) and 0.1% ferric chloride (0.5 mL) and the absorbance of the resultant solution was measured at 700 nm. Vitamine E was used as positive control.
2.7. Antibacterial Activity Test
Cultures of E.coli, Salmonella spp, Samonella typhimurium, Pseudomonas aeruginosa, Bacillus subtilis, Bacillus cereus, Streptococus spp, Staphylococus aureus were inoculated separately on the surface of Luria-Bertani agar plates by surface spreading using a glass plate spreader to obtain a uniform inoculum. Antibacterial activity of the plant extracts was tested using the agar well diffusion method, whereby, wells of 6 mm diameter and 5 mm depth were made on the solid agar. The aqueous and the ethanolic extracts at the concentration of 60 mg/mL (about 50 µL) were dropped onto corresponding wells. Water containing 1% DMSO and ethanol 80% were used as negative controls. All the tests were run in triplicates. The set up was incubated for 24 hours at 37˚C. After that, the zones of inhibition were measured using a ruler.
2.8. Toxicity Test
To each well of the 96 well microtitre plate, 0.1 mL of the diluted cell suspension (approximately 10,000 cells) was added. After 24 hours, when a partial monolayer was formed, the supernatant was flicked off, washed once and 100 µL of different test concentrations were added to the cells in microtitre plates. The plates were then incubated at 37˚C for 72 hours in 5% CO2 incubator. After 72 hours, 50% trichloroacetic acid (50 µL) was gently added to each well. The plates were then incubated at 4˚C for one hour further. The plates were flicked and washed five times with tap water to remove traces of medium, sample and serum, and then were air dried. The air dried plates were stained with 0.2% SRB (100 µL) and kept for 30 minutes at room temperature. The unbound dye was removed by rapidly washing five times with 1% acetic acid. The plates were then air-dried. 10 mM Tris base (100 µL) was then added to each well to dissolve the dye. The plates were shaken vigorously for 5 minutes. The absorbance was measured using microplate reader at a wavelength of 492 nm and 620 nm. The selected extracts in previous experiment were diluted in 0.5% DMSO at the concentration of 80, 110, 140, 170, 200 ppm for this test. Camptothecin (2.5 ppm) is the positive control, water containing 0.5% DMSO is the negative control. The percentage growth inhibition was calculated using following formula:
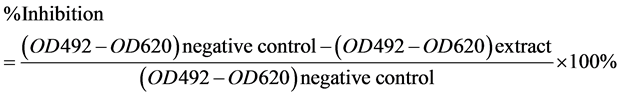
3. Results
3.1. Anti-Tyrosinase Activity
The preferred parts of seven plants were chosen to test anti-tyrosinase activity, and the result was presented in Table 2. The result in Table 2 showed that mulberry and jackfruit leaves had the highest %I. It meant that these plants are potential for whitening cosmetics. Therefore, they were successively chosen for testing anti-tyrosinase activity to select the most potential part of each plant.
Their different parts, leaves, stem, root core and root bark, were tested and the result was presented in Table 3. Following Table 3, mulberry seemed to be better than jackfruit. In almost parts of plant, the %I values of the mulberry are often higher. Besides, the root core of mulberry also exhibited strong inhibitory activity against tyrosinase in the comparison with the corresponding one of stem, leave and root bark.
Fractionation of the extract from the mulberry root core was carried out. The extract was partitioned with n-hexane, chloroform, absolute ethanol and water in succession. Four different fractions of mulberry root core extract were tested anti-tyrosinase activity and the result was presented in Table 4.
IC50 values of ethanolic and aqueous fractions were 3.91 ppm and 4.27 ppm, respectively. The inhibitory activities of these two fractions were found stronger than that of kojic acid 1.38 and 1.26 times, respectively. This result reinforced
Table 2. %I of preferred parts of seven plants.
a, b, c, means different signs reflecting significant difference at the level of p < 0.05.
Table 3. %I of four parts of mulberry and jackfruit.
a, b, c, means different signs reflecting significant difference at the level of p < 0.05.
Table 4. IC50 of four fractions of the mulberry root core.
a, b, c, means different signs reflecting significant difference at the level of p < 0.05.
the potential value of mulberry for cosmetics. The result in Table 5 also indicated that active constituents were frequently polar and soluble in water and ethanol.
3.2. Phytochemical Evaluation
The raw mulberry and jackfruit root core extract were dissolved separately in ethanol 80% to achieve the concentration of 3 mg/mL. Then they were subjected to the chemical tests for the identification of various active constituents. The result was presented in Table 5. The result in Table 5 showed that mulberry and jackfruit root cores contained phenolics, alkaloids, flavonoids, steroids and glycosides. There were remarks that the precipitate content in tannin identification test and the foam height in saponin identification test of jackfruit were higher and more stable than those of mulberry.
Table 5. Results of phytochemical evaluation of the aqueous ethanolic extracts of mulberry and jackfruit root core.
“+”: positive; “−”: negative.
3.3. Reducing Power Activity
For evaluating the in vitro antioxidant activity, the mulberry and jackfruit root core extracts were dissolved separately in ethanol and water to achieve the concentration of 2 mg/mL. Then they were subjected to the chemical tests (protocol mentioned above) for the identification of various active constituents. The result was presented in Table 6. The OD700 value of the mulberry extract in water was 3.87 times higher than that of the jackfruit extract in water. In general, the mulberry extract in both water and ethanol showed stronger antioxidant activity than those of jackfruit extract. Besides, active components seemed to be present in this extract, or other words, the strong polarized constituents in extracts maybe take in charge this antioxidant activity. Actually, some other studies indicated that the strong polarized substrates such as phenol played an important role in the antioxidant activity due to their hydroxyl’s free radical scavenger capability.
3.4. Antibacterial Activity Test
The extracts were dissolved separately in ethanol and water to achieve the concentration of 60 mg/mL. Each solution (50 µL) was loaded into each well for this test. B. cereus and P. aeruginosa were strongly inhibited by mulberry root core extract in ethanol (15.17 and 15.00 mM, respectively), whereas the mulberry root core extract in water inhibited well against Staphylococcus aureus (11.50 mM).
Table 6. OD700 of extracts of mulberry and jackfruit root core.
a, b, c, means different signs reflecting significant difference at the level of p < 0.05.
E.coli and Salmonella typhimurium were almost the most tenacious species. Shortly, mulberry exhibited antibacterial activity strong and wide range than that of jackfruit and the ethanolic extract or aqueous extract showed the same result (Table 7, Figure 1).
In all of the previous experiments, mulberry root core extract showed its excellent characteristics for skin-whitening cosmetics such as anti-tyrosinase, antioxidant and antibacterial activities. That is the reason why it was chosen for the toxicity test on human foreskin fibroblast using SRB assay (Table 8). This method not only allows a large number of samples to be tested within a few days, but also requires only simple equipment and inexpensive reagents. The SRB assay is therefore an efficient and highly cost-effective method for screening [5] . Mulberry root core extract in water inhibited tyrosinase with the concentration not exceeding 100 ppm. Therefore, in this assay, it was dissolved back in water containing 0.5% DMSO at the concentrations of 80, 110, 140, 170, 200 ppm to test the toxicity on cells. The result with % of death cells at 200 ppm was 3.48% meant that this extract was not toxic on human foreskin fibroblast. In conclusion, at the best concentration for tyrosinase inhibition (<100 ppm), mulberry root core extract was not toxic for normal human skin cells.
4. Discussion
4.1. Anti-Tyrosinase Activity
In this study, we demonstrated that mulberry inhibited tyrosinase better than other selected plants. This strengthened the scientific basis of natural beautifying?remedy which used mulberry plant parts. Actually, in many Asian countries, mulberry leaves were used to make tea or its branches were for the semi-solid extract. These remedies were believed to afford a pinkish white skin for the user. Nowadays, a lot of cosmetic producers put mulberry extracts into their products such as: soap bar and shower cream of Silk by Oriflame, Rose & Mulberry by Prôvence.
Of all parts, we also found that the root core of the plant had the best depigmenting capability. This result was suitable for structural characteristics of
Table 7. The results of antibacterial activity of mulberry and jackfruit root core extracts (The diameter of inhibition zone was calculated in mm).
a, b, c, means different signs reflecting significant difference at the level of p < 0.05.
Table 8. Percentage of death cells in SRB assay of mulberry root core extract on human foreskin fibroblast.
Figure 1. Chart of antibacterial activity of the extracts (DT-N = A extract in water, MIT-N = B extract in water, DT-E = A extract in ethanol, MIT-N = B extract in ethanol, N = water control, E = ethanol control).
magnoliophyta: diversity of secondary metabolites that are accumulated in root, especially the root core. Following to the transportation of the plant vascular, most of the compounds synthesized by plants will tend to accumulate in the roots under the force of gravity. The published studies have shown that valuable substances are often more concentrated in roots. For example, the tropane alkaloids are found in the roots of the Solanaceae families. Hyoscyamine and scopolamine, are derived from phenylalanine and ornithine or arginine that are synthesized almost exclusively only in the roots. Isoflavonoid rotenone is found in the roots of some woody legumes (Derris, Lonchocarpus). Alkaloid camptothecin, from the roots of Chinese medicinal plant Camptotheca acuminata, is one of the plant-derived anticancer drugs.... Besides, the root core is where many nutrient compounds and secondary compounds accumulate more than root cortex. This result can be explained by the structural properties of the plant roots, the protective root sheaths, and the chemical composition of the cuticle and suberin which is essentially lipid dissolve and retain the less polar compound groups while the root core with the phloem tissue has capable of storing various types of compounds. Therefore, the ability to obtain compounds that inhibit tyrosinase activity at the root core is significantly higher than that of the cortex. Moreover, ethanol and water fraction of mulberry root core extract exhibited stronger depigmenting efficacy than kojic acid, the positive control. The inhibition of tyrosinase might be due to the hydrogen bonding formation of the hydroxyl groups of the phenolic compounds of the extracts with the active site of the enzyme [6] .
4.2. Phytochemical Evaluation
Many phytochemical compounds can be isolated from mulberry as well as jackfruit. In some previous studies, mulberry is reported to contain constituents such as tannins, phytosterols, saponins, triterpenes, flavonoids, benzofuran derivatives, morusimic acid, anthocyanins, anthroquinones, glycosides and oleanolic acid [7] . But tannins and saponins content of jackfruit were maybe higher than those of mulberry. Both of them are important sources of compounds which are useful in treating many diseases.
4.3. Reducing Power Activity, Antibacterial Activity Test and Toxicity Test
Results of above Yen & Duh assay indicated that antioxidants’ content of mulberry was much than jackfruit in both their ethanol and water extracts. Some previous studies suggested that extracts with high antioxidant activity showed high phenolic content. Phenolic compounds are known as powerful chain breaking antioxidants and act as free radical terminators, may contribute directly to antioxidative action. These compounds are very important constituents of plants and their radical scavenging ability is due to the presence of hydroxyl groups.
In the other hand, mulberry seemed to have a wide antibacterial zone, from entero-bacteriaceae to human microbiota. With this characteristic, mulberry extract will keep finish products more stable, shelf-life long lasting and disinfectant themselves as well as make customers’ skin clean and sanitized. For any cosmetic product, ability of anti-S.aureus, P.aeruginosa, Streptococcus spp. (the remarkable bacteria in cosmetics) is an advantage.
Finally, at concentration of mulberry root core extract which inhibited maximum tyrosinase activity (< 100 ppm), it was found non toxic for human foreskin fibroblast. In general, the ethanolic or aqueous mulberry extracts are almost safe for cosmetic applications.
5. Conclusion
From this study, it was observed that mulberry, especially its root core, possessed the highest skin whitening capability in comparison to other chosen Vietnamese folk plants. In this case, water is properly the best solvent for extracting due to its non-toxicity and cheapness. Mulberry root core also has many other properties which are very useful in cosmetics industry like antioxidant, antibacterial… Besides, jackfruit can be a potential candidate for the topical applications as skin whitening agents. We need to do more researches to find out natural sources for making best and safest cosmetic products.
Acknowledgements
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number C2016-18-16.
Cite this paper
Hanh, N.T.M., Phung, N.K.P. and Phuong, Q.N.D. (2017) Studying on Tyrosinase Inhibition Activity of Some Vietnamese Folk Plants Aims to Use in Skin-Whitening Cosmetics. American Journal of Plant Sciences, 8, 1319-1328. https://doi.org/10.4236/ajps.2017.86088
References
- 1. Smit, N., Vicanova, J. and Pavel, S. (2009) The Hunt for Natural Skin Whitening Agents. International Journal of Molecular Sciences, 10, 5326-5349.
https://doi.org/10.3390/ijms10125326 - 2. Özer, Ö., Mutlu, B. and Kivcak, B. (2007) Antityrosinase Activity of Some Plant Extracts and Formulations Containing Ellagic Acid. Pharmaceutical Biology, 45, 519-524.
https://doi.org/10.1080/13880200701446746 - 3. Rohini, S., Shalini, M., Narayanaswamy, N. and Balakrishnan, K. P. (2012) Application of Natural Products in Cosmetics: A Study of Ixoracoccinea Extracts for Their Antityrosinase and Antioxidant Activities. International Journal of Research in Cosmetic Science, 2, 1-7.
- 4. Yen, G.C. and Duh, P.D. (1993) Antioxidative Properties of Methanolic Extracts from Peanut Hulls. Journal of the American Oil Chemists’ Society, 70, 383-386
https://doi.org/10.1007/BF02552711 - 5. Vichai, V. and Kirtikara, K. (2006) Sulforhodamine B Colorimetric Assay for Cytotoxicity Screening. Nature Protocols, 1, 1112-1116.
https://doi.org/10.1038/nprot.2006.179 - 6. Baek, H.S., Rho, H.S., Yoo, J.W., Ahn, S.M., Lee, J.Y., Jeonga-Lee, J.L., Kim, M.K., Kim, D.K. and Chang, I.S. (2008) The Inhibitory Effect of New Hydroxamic Acid Derivatives on Melanogenesis. ChemInform, 29, 43-46.
https://doi.org/10.1002/chin.200823080 - 7. Devi, B., Sharma, N., Kumar, D. and Jeet, K. (2013) Morus alba Linn: A Phytopharmacological Review. International Journal of Pharmacy and Pharmaceutical Sciences, 5, 14-18.



