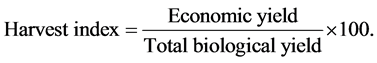American Journal of Plant Sciences
Vol.5 No.13(2014), Article
ID:46752,18
pages
DOI:10.4236/ajps.2014.513203
Influence of Soil Water Deficit and Phosphorus Application on Phosphorus Uptake and Yield of Soybean (Glycine max L.) at Dejen, North-West Ethiopia
Merkebu Getachew
Department of Plant Sciences, College of Agriculture and Natural Resources, Debre Markos University, Debre Markos, Ethiopia
Email: merkeb2020@gmail.com
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 April 2014; revised 16 May 2014; accepted 5 June 2014
ABSTRACT
A green house experiment was conducted at Dejen, Northwest Ethiopia, with the objective of quantifying the critical soil water deficit and P levels that affect yield and yield components of soybean, and determine the critical soil water deficit levels influencing P uptake in soybean. The treatment consisted of factorial combination of four available soil water (ASW) deficit levels (0%, 25%, 50% and 75%) and four levels of phosphorus (0, 10, 20, and 30 kg∙ha−1) laid out in RCBD with four replications using soy bean variety Jalale as a planting material. The experiment was conducted under green house condition at Dejen, South Ethiopia during the 2011 academic year. Air dried soil was filled in the pots and seeds were sown on May 13, 2011. Four plants were maintained on each pot after thinning till flowering but after flowering, the total number of plants per pot was reduced to three as one plant which was used for measurement of root biomass. The water deficit treatments were imposed after the plants have been fully established 2 weeks after emergence just before branching stage. The water deficiency was imposed through maintaining the soil moisture content below field capacity at the deficit levels of 25%, 50% and 75%. The 75% of ASW deficit resulted in the longest days (45) to flowering and maturity (99) compared to the 0%, 25% and 50% deficit levels. Also, the 75% of ASW deficit level resulted in shorter plants (55 cm), the lowest leaf area (82.6 cm2), the highest root to shoot ratio (0.0168) and the lowest DM accumulation (161.3 gm−2) compared to the other ASW deficit levels. Likewise, the 75% of ASW deficit level gave the lowest number of pods per plant (4.13), seeds per pod (1.69), 100 seed weight (2.54 g), seed yield (13.4 g∙m−1), above ground biomass (174.6 g∙plant−1) and harvest index (0.08) compared to the other ASW deficit treatments. The degree of sensitivity to drought increased dramatically (from 0.0423 at 25% to 0.9604 at 75%) with increase in water deficit level. Tissue analysis results indicated that the highest seed P concentration (1.285%) and uptake (432.5 g∙plant−1) were obtained at the 0 ASW deficit and 30 kg∙P∙ha−1 and the lowest were obtained at 75% ASW deficit and all rate of applied P. On the contrary, the highest straw P concentration (1.88%) and uptake (552.7 g∙plant−1) were recorded at 75% and 25% of ASW deficit levels and 30 kg∙P∙ha−1, respectively. However, the total P uptake was influenced only by ASW deficit levels in that the relatively minimum and maximum values were observed at 75% and 0% of ASW deficit levels, respectively. It can be concluded that the critical ASW deficit levels that affect yield and yield components of soybean and uptake of total P lie between 25% and 50% of available water deficit levels. The parameters started to decline significantly from the 50% of ASW deficit onwards. As it is a green house experiment, further study on more number of ASW deficit levels and soil types under different field conditions need to be done to reach at a conclusive recommendation.
Keywords:Phosphorus Uptake, Soybean, Soil Water Deficit

1. Introduction
Soybean is an important grain legume crop and it is a leading plant source of dietary protein worldwide [1] . Soybean is a nutrient rich crop. Soybean oil contains no cholesterol and has one of the lowest levels of saturated fat among vegetable oils. Soybean contains 37% - 48% Protein, 16% - 22% fats and oil; and 30% carbohydrates. This implies that the crop is a very important food, oil, fodder and industrial material [2] . The Soybean plant is harvested for their beans to be used primarily for meal and oil. Soybean is considered as a main cash crop in many lowlands and mid-altitude zones of Ethiopia [3] . It is known for its high export potential legume as vegetable and seed for a long period contributing to foreign exchange earnings of Ethiopia [2] [4] .
The suitable soybean production areas of Ethiopia have been indicated in areas with an altitude ranging between 1100 and 1850 m.a.s.l., with mean maximum temperature of less than 30˚C - 37˚C, mean minimum temperature greater than 10˚C - 12˚C, and a rainfall of 350 - 500 mm well distributed throughout the growing season [3] . There are large commercial farms concentrated in the Rift Valley areas of Ethiopia producing soybean throughout the year using rainfall and also irrigation during dry periods using the water resources around.
Soybean, as indicated by [4] , is a nodulating legume, very sensitive to environmental stresses such as low soil fertility and water deficit and grows under rain-fed conditions in the world where drought is one of the major limiting factors for plant production [5] . However, not only abiotic constraints but also biotic factors such as diseases and pests are challenging the production. Low soil fertility and drought are among the most important yield limiting environmental factors in arid and semi-arid areas. Of the various physiological problems limiting soy bean production in developing countries, drought is preceded in importance only by soil fertility problem, and approximately 60% of bean productions in the regions of the world suffer from serious drought every year. Research results had shown that water deficit has greater influence on soybean especially when it appears at reproductive and grain filling stages even though there is a genotypic difference between water uptake and use [6] .
Water is a key factor in nutrient uptake through root interception, mass flow, and diffusion, although soil types would also determine the hydraulic integrity of the soil-plant-atmosphere continuum. Substantial response to water use efficiency (WUE) and fertilization occurs in dry as well as wet conditions. Even though the response is less under water deficit, adequate nutrient availability greatly reduces drought related yield losses. The importance of adequate fertility for efficient crop water use and improvement of crop to low rainfall condition have been explained as they increase root exploration of the soil to move more portion of the root around the soil particles where nutrients reside, advances maturity, increase the mass of plant residue, decrease water requirement and increase the plant canopy so that the soil is covered more quickly to lower evaporation [7] .
Nutrients usually concentrate in the soil surface layers and their absorption is made easier because of higher microbial activity [8] . From a recent study it appears that phosphorus (P) acquisition is less in deeper-rooted plants than in shallow-rooted because, phosphate tends to reside mainly in the upper most soil layer which is most vulnerable to drying out [9] . In contrast to acquire water in dry conditions, profuse rooting in the deeper soil layers would be required. These indicate that shallow and deep rooting are rather antagonistic developments with water and P absorption [9] . This implies that the hypothesis that a shallow rooting pattern contributes to an enhanced nutrient harvest needs to be revisited when crops are grown during dry periods. A work will also be needed to assess the volumetric soil moisture threshold level at which P uptake by plants is highly influenced. Soybean is an early maturing crop growing in the semi-arid areas of the Ethiopian Rift Valley mainly following the lakes. Intermittent rainfall shortage and sometimes insufficient amount of rainfall and longer dry spell in the main cropping season are problems in the area (personal communication).
On the other hand, it is well proved in many studies that flowering and pod filling stages of the crop are highly sensitive for drought resulting in final yield reduction. However, there is no information on how much the deficit affects yield, when there is insufficient rainfall throughout the growth season. Moreover, knowledge of nutrient uptake under moisture deficit especially P, which has a specific role in nitrogen (N) fixing plant species than others due to the greatest P dependency of biological N-fixation [4] , is also nonexistent in the area. The information on soil water deficit levels affecting P uptake and yield of soy bean is also important for irrigated farms to implement deficit irrigation in order to economize water resource and reduce irrigation costs. Therefore, this study was proposed with the following objectives:
Ÿ To quantify the critical soil water deficit and P level which affect yield and yield components of soybean, and
Ÿ To determine the critical soil water deficit level influencing P uptake by soybean.
2. Materials and Methods
2.1. Experimental Materials, Design and Treatments@NolistTemp# The experiment was conducted under green house condition (where the average temp. and relative humidity were 29˚C and 40˚C, respectively) at Dejen using a pot (15 cm diameter × 11 cm depth and the soil was filled up to the top). A soybean variety, Jalale, was used for the study. The crop “soybean” is selected because it is dominantly cultivated crop in the area. Four levels of phosphorus (0, 10, 20 and 30 kg∙P∙ha−1) and 4 levels of available soil water deficit (0%, 25%, 50% and 75%) were arranged in a factorial combination using randomized complete block design (RCBD) and replicated four times. Starter fertilizer N with a recommended rate of 46 kg∙N∙ha−1 [7] was applied at equal rate to all treatments. The sources for P and N were Triple Super phosphate (TSP, 46% P2O5) and Urea (46% N) fertilizers, respectively.2.2. Determination of Available Soil Water Holding Capacity
The available soil water holding capacity (AWC) was calculated as the difference in moisture content between water held at field capacity (FC) and permanent wilting point (PWP): The matric potentials corresponding to FC (−1/3 bar) and PWP (−15 bars) were applied using the pressure plate apparatus. The equilibrium water contents at FC and PWP were then determined gravimetrically. The gravimetric water content was converted into volumetric water contents using bulk density, which was determined using the core method. The depth of AWC was calculated using the following relationship:
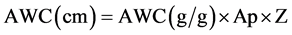 (1)
(1)
where: Ap = apparent specific gravity =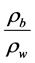 (dimensionless)Z = thickness or depth of the root zone (cm), which in this case was depth of the pot (23 cm)rb = dry bulk density of the soil (g/cm3) and rw = density of water assumed to be 1 g/cm3.
(dimensionless)Z = thickness or depth of the root zone (cm), which in this case was depth of the pot (23 cm)rb = dry bulk density of the soil (g/cm3) and rw = density of water assumed to be 1 g/cm3.
The volume of water applied per irrigation for the control (no deficit) was then calculated by multiplying determined values from Equation 1 (cm) by area of the pot (cm2). Similarly, the amounts of water to be applied per irrigation for the 25%, 50% and 75% deficit levels were calculated as:
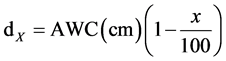 (2)
(2)
where d is the depth of irrigation water applied (cm) and x is the available water deficit levels (25%, 50% and 75%). The calculated irrigation depth was then converted into volume of water by multiplying it by the area of the pot.
The amount of water depleted was determined through daily weighing of the pots. The assumption made was that weight loss is due to loss of water from the pot through evapo-transpiration and that 1g of water occupies a volume of 1 cm3. The capacity of the pot was 2.5 m2 and the soil was filled up to the top. Each pot was irrigated with equal amount (100% ASW) for all the treatments until the water deficit treatment was imposed. After establishment, the water content in the soil was maintained at the indicated deficit levels throughout the crop growth cycle.
2.3. Experimental Procedures
The experimental soil was Eutric Fluvisol. Air dried soil was filled in the pots and seeds were sown on May 13, 2011. Four plants were maintained on each pot after thinning till flowering but after flowering, the total number of plants per pot was reduced to three as one plant was used for measurement of root biomass.
The water deficit treatments were imposed after the plants have been fully established 2 weeks after emergence just before branching stage. The water deficiency was imposed through maintaining the soil moisture content below field capacity at the deficit levels indicated above.
2.4. Agronomic Practices
Fertilizers, TSP and Urea were applied at sowing, placed on the side of the seeds at a distance of 5 cm and depth of 2 cm. During crop growth period, hand weeding and cultivation were practiced as required to avoid weed competition and improve the soil aeration. During the cropping period, anti-mite chemical (common name: Mitigun) was used to control the mite incidence encountered at the late vegetative stage.
2.5. Data Collection
2.5.1. Soil Sampling and Analysis
Soil samples were taken before sowing from a depth of 0 - 30 cm to determine particle size distribution, bulk density, total N, available P, soil pH, organic carbon, cation exchange capacity, exchangeable bases and water contents at FC and PWP. Undisturbed soil samples were taken using core sampler for the determination of water content at FC and PWP and bulk density. One composite soil sample was taken before sowing from five spots of the field selected for soil sample collection. The sampled soil was air dried, crushed and passed through a 2 mm mesh sieve following standard laboratory procedures.
Total N was determined by the micro-Kjeldahl method [10] and available P using the Olsen method [11] . Organic carbon content was determined according to the Walkley and Black method as described by [12] . Exchangeable bases were determined by the ammonium acetate method [13] . Particle size distribution was determined using the Bouyoucos hydrometer method [14] . The soil pH was measured using a glass electrode pH meter.
2.5.2. Phenological Parameters
The number of days to flowering was determined as the number of days from emergence to 75% flowering. And the number of days to maturity was taken as the number of days from emergence to 95% physiological maturity.
2.5.3. Growth Parameters
Plant height was measured starting from the basal node to the top of the main shoot at flowering and maturity. It was taken from two randomly selected plants per pot; one at flowering and the other at harvest. Leaf area was determined by tracing an individual leaf on to a square paper with a dimension of 1 cm2 and counting the number of squares fully covered by the leaf and multiplying them by the dimension of a square. In this case peripheral squares with an area greater or equal to 0.75 cm2 were considered as full squares. It was taken from two randomly selected plants per pot at flowering stage. The plant dry matter accumulation was determined as the weight of the above ground parts of the plant excluding the seed (g∙plant−1) dried at 70˚C to a constant weight. It was taken from two randomly selected plants per pot at harvest. The root to shoot ratio (RSR) was determined as the ratio of root mass to the total plant mass. The whole plant parts were oven dried at 70˚C to a constant weight and then the root and the other plant parts were weighed separately to calculate RSR. It was taken from two randomly selected plants per pot; one at flowering and the other at harvest.
2.5.4. Yield and Yield Components
Data on yield and yield components were taken from two randomly selected plants per pot at harvest except for grain yield which was taken from all plants per pot remained at harvest. Number of pods per plant was determined as the average number of pods counted per plant. And the number of seeds per pod was determined as the average number of seeds counted per pod; three pods were used per plant. Weight of hundred seeds was taken as the seed weight (g) of 100 seeds. Seed yield was the measured weight of seeds (g∙plant−1) at 10% moisture content. Above ground biomass was determined as the weight of the above ground parts of the plant including the grain (g∙plant−1) dried at 70˚C to a constant weight. And the harvest index was calculated as the ratio of seed yield to the total above ground biomass of the sampled plants.
2.5.5. Drought Sensitivity Index (DSI)
It was calculated as the ratio of seed yield difference between the control and the stressed pots to that of the control pots.
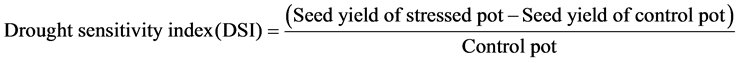
2.5.6. Plant Tissue Sampling and Analysis
After harvest, samples of seed and straw were taken from each pot within the replications. Seed and straw samples were air dried and ground separately for the analysis of seed and straw P concentrations. The measurements of P concentration of seed and straw were carried out through calcinations of both seed and straw separately at 450˚C. After calcinations, wet digestion of plants with strong H2SO4 was carried out and then P was measured using spectrophotometer at 430 nm.
The seed and straw concentrations of P were used to estimate P uptake of the plant. The P uptake values of the seed and straw were calculated by multiplying seed and straw yields per meter square by the % P concentrations of the respective tissues and total P uptake was calculated as the sum of seed and straw P uptake.
2.6. Statistical Analysis
All the agronomic data collected were subjected to analysis of variance using SAS software. Mean separations were carried out using least significant difference at 5% probability level. Log transformations were done to normalize the inter-row differences that occurred on 100 seed weight, seed yield, aboveground biomass and seed and total P uptake.
3. Results and Discussion
3.1. Selected Physical and Chemical Properties of the Experimental Soil
Results of analysis of the selected physico-chemical properties of the experimental soil are presented in TableTable 1. The particle size distribution data indicates that the soil has a loam texture with the sand separate occurring in the highest proportion. The water content retained FC and PWP was slightly higher than the average values reported in literature for a loam-textured soil [15] . The bulk density value obtained was relatively low for the textural class of loam. According to the rating of pH by [16] , the experimental soil was moderately alkaline. The soil organic matter and total N contents were medium [17] . The Olsen P content of the soil was very high [18] whereas the CEC of the soil was high [19] . As per the rating of exchangeable bases provided by [20] , the exchangeable bases ranged from low (Na+), medium (Mg2+), high (Ca2+) to very high (K+). 3.2. Crop Phenology The results of analyses of variance on selected phenological parameters of the crop are presented in Appendix Table 1 and Table 2. The mean days to flowering and maturity were highly significantly (P ≤ 0.01) affected by the available soil water deficit treatments (Table 2). Accordingly, use of 75% available soil water deficit resulted in the longest days (45) to flowering compared to the 0%, 25% and 50% deficit levels, which had the same number of days to flowering. The results obtained *Moisture content on oven dry weight basis. Table 2. Effects of available soil water deficit levels and P fertilizer rates on days to flowering and maturity of soy bean (from a two way ANOVA). Treatment means within a column followed by the same letters are not significantly different at (P = 0.05). Ns = Non-significant; SE± = Standard error of the means. here are contrary to what is normally perceived in that water deficit stress hastens flowering and maturity. However, delayed flowering in wheat due to severe water deficit stress was reported by [21] . Furthermore, the 75% available soil water deficit level resulted in less number of flowers (visual observation) compared to the control due mainly to the death and drop of flower buds. Similar to the effect on days to flowering, the 75% available soil water deficit delayed the mean days to maturity by 15, 16 and 23 days compared to the 0%, 25% and 50% available soil water deficit levels, respectively (Table 2). On the other hand, plants grown under the 50% available soil water deficit matured earlier than those grown under the 0%, 25% and 75% deficit levels whereas the 0% and 25% deficit levels resulted in statistically the same days to maturity. Similarly, [15] noted that water deficit stress may hasten or delay phenological periods depending on the time it occurs, its severity, rate of onset of the stress and type of species involved. Nevertheless, the days to flowering and maturity were not significantly (P > 0.05) affected by the different rates of P and the interaction effects of available soil water deficit by P rates. The reason why the different P rates did not affect these parameters might be related to the high content of available P of the experimental soil. Normally, plants respond to applied nutrient when the concentration of a given nutrient element in the soil is in the deficiency level. Although there was no statistical difference, the highest P rate (30 kg∙ha−1) hastened days to flowering by one day but delayed days to maturity by two days compared to 10 and 20 kg∙P∙ha −1 (Table 2). 3.3. Growth Parameters The results of analysis of variance of the selected growth parameters are presented in Appendix Table 1 and Table 3. The discussion of each of the growth parameters follows. 3.3.1. Plant Height Plant height was highly significantly responded to water deficit treatments but not to P rates and the interaction. The mean plant height values indicate that the lower water deficit levels (0% and 25%) gave taller plants than those grown under the relatively higher water deficit levels (50% and 75%). The findings of this experiment indicate that water deficit levels of up to 25% do not significantly reduce plant height. However, the highest water deficit level decreased mean plant height by about 44% compared to the no deficit or control treatment (Table 3). The findings of this study are consistent with the findings of other researchers. As indicated by [22] , drought stress affects tissue expansion both through reduced cell expansion and division. [23] found that plant height and leaf number of common bean were significantly affected by water deficit (50% FC). [23] also indicated that with Table 3. Effects of available soil water deficit levels and P fertilizer rates on mean growth parameters of soy bean (from a two way ANOVA). Treatment means within a column followed by the same letters are not significantly different at (P = 0.05). PH = Plant height, LA = Leaf area, RSR = Root to shoot ratio and DMA = Dry matter accumulation. Ns = Non-significant; SE± = Standard error of the means. holding irrigation at any growth stage significantly reduced plant height of common bean and, from the different growth stages, water stress at the vegetative stage caused the highest reduction in plant height. Similarly, [24] revealed that moisture stress during vegetative growth stage was so severe that plants remained without sufficient stature to support high yields. 3.3.2. Leaf Area The mean leaf area was highly affected by the moisture deficit stress (Appendix Table 1). Greater LA was recorded at 0% followed by 25% available soil water deficit level with small significant difference between them. And the lowest LA was observed at 75% followed by the 50% available soil water deficit (Table 3). Leaf area decreased steeply from 25% ASW deficit onwards. The reason for the decline of LA in the relatively lower levels of ASW or high water deficit level might be due to stunted growth resulting from the water deficit stress. However, the effect of P application and also its interaction with moisture deficit were not significant (P > 0.05). Specific leaf area determines the physiological cost of producing total leaf area. It relates dry matter production to leaf area expansion and consequently to radiation interception, photosynthesis and transpiration [25] . According to [25] , dry matter yield and leaf area decreased in response to water stress in soy bean and sorghum. [26] also got all plants under −15 bars water potential to be dead as a result of reduced water potential due to a highly retarded plant growth such as plant height, leaf area and leaf number. 3.3.3. Root to Shoot Ratio (RSR) The mean root to shoot ratio was highly significantly affected by the different available soil water deficit levels; but not by the P rates and the interaction (Appendix Table 1). Accordingly, the highest mean root mass ratio was obtained from the 75% followed by the 50% ASW deficit (Table 3). On the other hand, the lowest mean root mass ratio was recorded from plants grown under the 0 and 25% ASW deficit. The results obtained in this experiment indicate that water deficit stress increases the root biomass compared to the shoot and thus results in high root mass ratio. Related work by [27] also indicated that water stress increases root to shoot ratio. The reason for the high root to shoot ratio of plants under low available soil water level might be due to a relative increase of root in depth and lateral growth in search of water as compared to the growth in their aboveground parts of these plants [5] . Nevertheless, the different P rates and its interaction with the available soil water deficit did not significantly affect the root mass ratio (P > 0.05). This is contrary to the findings of other studies [28] , which indicated that application of P alleviated the adverse effects of water deficit stress by increasing root dry weight, length and surface area. The high available soil P might have offset this contribution of P for plants grown under water deficit conditions. 3.4. Dry Matter Accumulation The results of analysis of variance indicate that moisture stress significantly (P ≤ 0.01) affected dry matter accumulation (Appendix Table 1). The control and 25% deficit level resulted in the highest mean dry matter accumulation followed by the 50% and 75% deficit levels (Table 3). Following this, the 50% and 75% deficit levels decreased the dry matter accumulation by 48% and 68.5%, respectively, compared to the control. Nevertheless, this parameter did not respond significantly (P > 0.05) to the different P rates and the interaction. Greater accumulation of dry matter is one of the important inputs to assure total translocation of photosynthate materials to the seed. As reported by several researchers, if water is a deficit factor, there will be a retarded growth in the vegetative and reproductive parts of plants. [29] indicated that shoot biomass accumulation is considered as an important trait to attain high seed yield in seed legumes. On the other hand, water deficit can decrease plant height and total biomass [29] [30] also observed that water deficit retarded plant growth which in turn resulted in production of low biomass and, hence, low grain yield. 3.5. Yield and Yield Components The results of analysis of variance of yield and yield components and the mean values of the parameters are indicated in Appendix Table 2 and Table 4, respectively. 3.5.1. Pods per Plant A significant difference (P ≤ 0.01) was observed among water deficit treatments on mean pod number per plant Table 4. Effect of available soil water deficit levels and P fertilizer rates on yield and yield components of soybean (from a two way ANOVA). Treatment means within a column followed by the same letters are not significantly different at (P = 0.05). P/P = Pods per plant, S/P = Seeds per pod, SW = 100 seed weight, GY = Grain yield, AGBM = Aboveground biomass, HI = Harvest index and ns = Non-significant; SE± = Standard error of the means. due to the available soil water deficit, whereas the difference was insignificant (P > 0.05) for P rates and the interaction of water deficit and P rates (Appendix Table 2 and Table 4). As can be observed from the Table, the 0% and 25% deficit levels gave the highest number of pods per plant compared to the 50% and 75% deficit levels. Compared to the 0% deficit level, the 75% deficit level reduced the mean pod number by 65.24%. Similarly, number of pods per plant decreased consistently with increase in water deficit level. The findings of this study indicate that up to 25% available soil water deficit level, the plant processes that affect the formation of pods may not be affected. The reduction in pod number per plant in soy bean grown under water deficit conditions could be attributed to abortion and abscission of flowers [20] , early ovary expansion [31] and retarded vegetative growth, which may contribute to a decrease in number of pod holding branches. The results of this study are in agreement with the findings of other researchers, who also observed a decrease in pod number per plant. [32] with held water during the flowering and pod-filling stages and found a significant decrease in pod number per plant. [32] also reported reduction in pod number per plant due to water deficit stress. 3.5.2. Seeds per Pod Similar to the mean pod number per plant, the mean number of seeds per pod was significantly affected (P ≤ 0.01) by the water deficit level (Appendix Table 2 and Table 4). Consequently, the 0% and 25% deficit levels produced the highest mean number of seeds per pod. The 75% deficit level gave the lowest number of seeds per pod that was reduced by 48.3% compared to the 0% deficit (control). This might be due to the negative effect of water deficit stress on meiosis and pollen fertility as observed in many cereals [33] . As indicated by [34] , during meiosis cells are extremely sensitive to moisture content, and drought at this stage will cause pollen sterility. Unlike the results obtained from the water deficit treatments, application of different rates of P did not significantly affected (P > 0.05) number of seeds per pod. Similarly, interaction effect was non-significant indicating that applied fertilizer did not contribute any better to withstand the negative impacts of water deficit stress and increase seed number per pod. This might be attributed to the fact that the experimental soil already contained high amount of available P and the applied P was only luxury consumption. 3.5.3. Hundred Seed Weight Hundred seed weight responded to water deficit treatments significantly (P ≤ 0.01) (Appendix Table 2 and Table 4). The 0% and 25% available water deficit levels produced seeds that were heavier than those grown under 50% and 75% deficit levels. Also, the 50% available soil water deficit produced seeds that were heavier than those grown under the 75% deficit level. The 75% deficit level, compared to the control, reduced hundred seed weight by 89.99% indicating that the water deficit at this level was so severe that the crop produced extremely light seeds. Furthermore, seeds produced under 75% deficit levels were very small in size and shriveled in their morphology. This might have contributed to the relatively small 100 seed weight recorded under the 75% deficit level. As reported by many researchers [4] [5] [35] , pod filling stage is sensitive to water deficit and this might occur due to source limitation making the sink to accumulate more dry matter. Therefore, the influence of water stress on 100 seed weight was probably due to the effect of drought on photosynthesis and other related physiological and metabolic processes. Cellular water deficit mostly result in a concentration of solutes, loss of turgor, denaturation of proteins, disruption of membrane integrity, decrease in cell expansion and failure of photosynthetic process [28] ; these might interfere with the formation of dry matter in the source tissue and its further accumulation in the sink. [34] also reported reduced grain weight of faba bean cultivars due to water deficit stress. Similar to what has been observed on most crop parameters, the different P fertilizer rates and their interaction with the water deficit levels did not significantly affect (P > 0.05) the mean 100 seed weight. 3.5.4. Seed Yield Seed yield responded highly significantly (P ≤ 0.01) to the different water deficit levels (Appendix Table 2 and Table 4) but not to the different P rates and its interaction with water deficit levels. The highest mean seed yield was obtained from the 0% and 25% deficit levels while the lowest was obtained from the 75% ASW deficit. The 50% and 75% deficit levels reduced the mean seed yield by 56.0% and 96.0%, respectively, as compared to the no deficit treatment (control). It is well known that the ultimate effect of drought in legumes and cereals is reduction of grain yield. In the present study, the stress at the 75% deficit had affected seed yield so severely to the degree that the amount was extremely low, far from normal size and weight and also shriveled in morphology. The stress was severe not only for the 75% but also for the 50% ASW deficit treatment that the decline in seed yield was much greater than at the control treatment. The findings of this study are in agreement with the findings of most researchers, who had proved the fact that water deficit can decrease seed yield to the different levels depending on the extent of the stress and on growth stage at which the stress occurred. [36] obtained a 20% yield reduction with stress during early and late vegetative growth and 50% reduction when stressed during early pod filling stage. The same effect of water stress on growth stage was also reported by [35] from the stress applied before flowering and sustained to maturity; the yield reduction was approximately 40% and 80% for the drought tolerant and susceptible bean lines, respectively. [37] also reported a yield reduction of 40% - 89% in water stressed bean genotypes. Additionally, [37] on the effect of water stress during seed filling and leaf senescence in soybean, indicated that water stress during seed filling could reduce yield by accelerating leaf senescence and shortening the seed filling period. [38] studied the effects of water limitation on growth and grain filling of faba bean cultivars and found out that water deficit stress considerably reduced grain yield due to large reductions in growth, grain filling duration, grain weight and grains per plant. [38] noted that the length of grain filling period is an important determinant of yield of all grain crops. Continuous water deficit stress from the beginning of grain filling stage until maturity shortened grain filling duration in soybean plants (up to 7 days earlier) resulting in smaller grains and lower yield [39] . As indicated by many researchers, water stress decreases the final leaf area, net photosynthesis, light use efficiency [40] [41] [42] , pod retention and filling by reducing the availability of assimilates and distorting hormonal balance [43] [44] and final yield reduction will be the consequence. 3.5.5. Above Ground Biomass The results of analysis of variance on aboveground biomass revealed that the effect of the different water deficit treatments was significant (P ≤ 0.01), while the effect of the different P rates and the water deficit level by P rates interaction were non-significant (P > 0.05) (Appendix Table 2 and Table 4). The results depicted in Table 4 indicate that the 75% water deficit level gave the lowest mean aboveground biomass yield compared to the other treatments whereas the control and 25% deficit levels produced the highest aboveground biomass. Moreover, the decrease in above ground biomass increased consistently with an increase in water deficit from 25% to 75% deficit level. The highest water deficit level imposed (75%) decreased the above ground biomass by 79.4% compared with the control. However, the control and 25% ASW deficit gave statistically the same aboveground biomass (Table 4) indicating that imposing a water deficit up to 25% does not affect aboveground biomass yield significantly. This can be adopted in areas where there is scarcity of water for irrigation. However, allowing deficit that exceeds the 50% and 75% of the available soil water can affect aboveground biomass and many other yield and yield components considerably. Over all plant growth is a process of biomass accumulation and is a consequence of the interaction of photosynthesis, respiration, long-distance transport, water relations and mineral nutrition processes. Water deficit stress affects these processes considerably. Many other researchers have also showed that water deficit decreased biomass production of different crops. 3.5.6. Harvest Index This was calculated as Harvest index (HI) was highly significant among the available soil water deficit levels but not among the P rates and their interaction with soil water deficit (Appendix Table 2 and Table 4). The mean HI was the highest in the control and then declined sharply as the deficit level increased to 75% (Table 4). Following this, the smallest HI, as compared to all the other treatments, was observed in the highest water deficit level imposed. The 75% and 50% deficit levels reduced the HI of the control by 80% and 12.5%, respectively. The 0% deficit and 25% deficit levels, on the other hand, resulted in the same HI. The decrease in HI when the available soil water level was low could be due to the reason that stressed plants had poor capacity to support seed filling from the plant reserves because of retarded growth and development as a result of the water deficit. Furthermore, the extremely low HI of the 75% deficit level could be attributed to the extremely low mean seed yield obtained from this treatment. As noted by [45] , HI is determined by the potential sink size, transient photosynthesis during grain formation and by the mobilization of stored plant reserves into the growing grain, all of which are affected by water deficit stress. [46] reported a decrease in HI due to water deficit stress imposed during the reproductive stage. [46] also observed greater flower and pod abscission in drought stress at flowering and pod filling stages decreasing final yield and HI. 3.6. Drought Sensitivity Index Drought sensitivity index measures the degree of sensitivity of plants for the given level of available soil water deficit. As indicated in Appendix Table 2 and Table 5, the degree of sensitivity to drought increased dramatically with increase in water deficit level albeit plants grown under the 25% deficit level showed low sensitivity compared to the 50% and 75% deficit levels. It seems that the water regime between no deficit and 25% deficit was sufficient range in which plants did not show any significant change in their sensitivity to drought. However, those plants grown under water deficit levels of 50% and 75% showed significantly higher degree of sensitivity to the stress applied indicating that the stress intensity was severe at the mentioned water levels. [47] indicated that water potential of stressed soy bean plants decreased rapidly from the control levels of −7 to −9 bars to −18 bars within 7 days after beginning of the stress. On the other hand, [47] reported that leaf water potential of soybean during stress fell as low as −23 bars. In the present study drought sensitivity index, similar to the other parameters, did not respond to the different rates of applied P and their interaction with the ASW deficit levels. 3.7. Plant Tissue Phosphorus Concentration The concentration of P in the tissues of grain and straw were significantly affected (P ≤ 0.01) by the interaction effect of available soil water deficit levels by P rates. While the ANOVA output of both are grown in Appendix Table 2, the seed and straw P concentrations as influenced by the interaction effect of ASW deficit and P fertilizer levels are presented in Table 6 and Table 7, respectively. Table 5. Effect of available soil water deficit levels and P fertilizer rates on drought sensitivity index of soybean (from a two way ANOVA). Treatment means within a column followed by the same letters are not significantly different at (P = 0.05). Ns = Non-significant; SE± = Standard error of the means. Table 6. Seed P conc. (%) of soybean as influenced by the interaction effect of available soil water deficit levels by P fertilizer (from a two way ANOVA). Interaction as well as main effect’ means followed by the same letters are not significantly different at (P =0.05), SE± = Standard error of the means. Table 7. Straw P conc. (%) of soy bean as influenced by the interaction effect of available soil water deficit with P fertilizer (from a two way ANOVA). Interaction as well as main effect’ means followed by the same letters is not significantly different at (P = 0.05); SE± = Standard error of the means. 3.7.1. Seed P Concentration The result of analysis of variance showed that the highest value of grain P concentration was obtained from the interaction of 0 deficit levels by 30 kg∙P∙ha−1 followed by the no water deficit by 20 and 10 kg∙P∙ha−1 (Table 6). On the other hand, the lowest seed P concentration was obtained from the interaction of 75% ASW deficit by all the P rates. Averaged over the different P rates, the control resulted in the highest mean seed P concentration compared to the other water deficit levels and the 75% deficit gave the lowest seed P concentration. Similarly, when averaged over the water deficit levels, the 30 kg∙P∙ha−1 gave the highest mean seed P concentration and the lowest was obtained from the 0 kg∙P∙ha−1. The increase in the seed P at the higher available soil water level (0% deficit) and greater P rate were clearly shown in the result. This increase in seed P concentration could be due to sufficient amount of water for greater mass movement of applied P and consequent translocation of the absorbed P to the seed. On the contrary, the decrease in the amount of P at the lowest level of available soil water might be due to lack of water for mass movement of applied P towards the plant root and its limited translocation to the seed. Studies conducted on sorghum and beans also indicated that phosphorus concentration in plant tissues of both crops increased with increasing P level whether plants were stressed or not [34] . In the experiment conducted by [39] on soybean grown under dry and P deficient soil, there had been also observed limited P accumulation and translocation to the seed. 3.7.2. Straw P Concentration The results depicted in Table 7 indicate that the highest mean straw P concentration was obtained from the interaction of 75% water deficit level by 30 followed by 75% and 20 kg∙P∙ha−1 and 75% by 10 kg∙P∙ha−1 while the lowest was obtained from the interaction of 0% ASW deficit 30 kg∙P−1. Averaged over the different P rates, the 75% ASW deficit gave the highest mean straw P concentration whereas the lowest was obtained from the 0% ASW deficit. Pooled over the water deficit levels, the 30 kg∙P∙ha−1 resulted in the highest straw P concentration. The higher straw P concentration obtained at the relatively lowest level of ASW deficit might be related to inadequate water which was not enough to translocate the absorbed P in the straw at the previous growth stages before pod filling so that, much of P remained accumulated in the straw. This could be the reason why the grain P concentration at the highest deficit level was the lowest. And the reverse might be true for accumulation of lowest amount of P at the relatively highest ASW level (0% deficit). 3.8. Plant Tissue Phosphorus Uptake 3.8.1. Seed P Uptake The interaction effect of available soil water deficit by P fertilizer significantly (P ≤ 0.01) affected the mean seed P uptake (Appendix Table 2 and Table 8). The highest seed P uptake was obtained from the interaction effect of 0% ASW deficit by 30 kg∙P∙ha−1 application whereas the lowest was obtained from the 75% ASW deficit by all rates of applied P fertilizer. The observed least seed P uptakes were 98.6% less than the highest. Except the highest P uptake observed at the given levels all the rest combinations of 0% and 25% ASW deficit by all rates of P gave statistically not significant amounts. However, there observed the interaction effects were more pronounced on the use of higher levels of ASW and applied P than at the lower. The amounts of seed P uptake were relatively greater due to the use of greater amounts of water and P fertilizer combinations than the lower. The enhancement of the uptake at the mentioned levels could be due to greater translocation of absorbed phosphate ions to the seeds from what has been taken by the plant as a result of the relatively high amount of applied water and P fertilizer and the reverse could be true for the poor uptake at the highest available water deficit and low P. Other experiments also support this result in that P supply was curtailed by high moisture stress [48]. Similarly, soy bean grown under dry and P deficient soil showed limited P accumulation and translocation to the seed. 3.8.2. Straw P Uptake The results obtained on straw P uptake also had significantly higher variations because of the interaction effect of ASW deficit by applied P (Appendix Table 2 and Table 9). The highest uptake was observed on the interaction of 25% ASW deficit by 30 kg∙P∙ha−1 and the least at 75% ASW deficit by 0 kg∙P∙ha−1 indifferent from seed P uptake. The recorded highest straw P uptake was 62.37% greater than the least. This result was supported by [48] that water deficit has a direct long term influence on the uptake and accumulation of P by crops. [48] also Table 8. Seed P uptake of soy bean (g∙plant−1) as influenced by the interaction effect of available soil water deficit with P fertilizer (from a two way ANOVA). Interaction as well as main effects’ means followed by the same letters is not significantly different at (P = 0.05); SE± = Standard error of the means. Table 9. Straw P uptake (g∙m−2) of soy bean as influenced by the interaction effect of available soil water deficit by P fertilizer (from a two way ANOVA). Interaction as well as main effect’ means followed by the same letters is not significantly different at (P = 0.05); SE± = Standard error of the means. observed reduced shoot transportation of P by intact tomato plants exposed to low water potential. The influence of water deficit and level of applied P on straw P uptake was great as it was on grain P uptake that except few interaction effects majority of lower water deficit and higher P level combinations gave relatively higher straw P uptake. The relatively lower uptake observed in some higher use of ASW and P fertilizer might be due to enhanced translocation of the straw P accumulation to the seed, this could be especially because of the presence of greater amount of available soil water. 3.8.3. Total P Uptake Total P uptake, calculated as the sum of seed and straw P uptake, was significantly affected by the water deficit treatment only (Appendix Table 2). Accordingly, the mean total P uptake decreased with increase in water deficit level (Table 10). Therefore, the highest total P uptake was recorded at the 0% and 25% ASW deficit levels while the lowest was obtained from the highest (75%) water deficit level (from a two way ANOVA). The imposed water deficit levels might have created deficiency at their lower regimes as it was observed in the uptake to be lower for all higher and lower rates of P. This implicate that the water deficit effect was more important on total P uptake whatever the rate of applied phosphorus was. Phosphorus uptake by plants is often reduced during drought, as the concentration of P ion is greater on the upper surface of the soil in antagonistic with the root growth and development of affected plants. Table 10. Total P uptake (g∙m−2) of soy bean as influenced by available soil water deficit and P fertilizer. Treatment means within a column followed by the same letters are not significantly different at (P = 0.05), Ns = Non-significant; SE± = Standard error of the means. 4. Summary and Conclusions Knowledge of nutrient uptake and yield relative to drought is very important especially for shallow rooted crops like soybean and soil P which is mostly concentrated on the surface unlike soil water level found deeper during water deficit stress. Soybean is known for its high export potential as vegetable and seed crop for a long period contributing to foreign exchange earnings of Ethiopia. So, this experiment was conducted with the aim of quantifying the critical soil water deficit and P levels that affect yield, and also determining the critical soil water deficit levels influencing P uptake. Factorial combination of four available soil water deficit levels (0%, 25%, 50% and 75%) and four levels of phosphorus (0, 10, 20, 30 kg∙P∙ha−1) were laid out in RCBD with four replications. The experiment was conducted under greenhouse condition using variety, Jalale, as a planting material on Eutric Fluvisol with a loamy texture. Highly significant differences were observed only because of the applied ASW deficit in most of the selected parameters contrary to the effect of applied P, which were not significant except on few due to the inherently very high soil P level found in the soil. From the findings of this experiment, it can be concluded that the critical ASW deficit levels that affect yield and yield components of soybean and uptake of total P lie between 25% and 50% of available water deficit levels. The results revealed that the 25% of ASW had the same effect as the control on most of the crop parameters measured and the total P uptake. The parameters started to decline significantly from the 50% of ASW deficit onwards. In conclusion, since, this is a green house experiment, further study on more number of ASW deficit levels and soil types under different field conditions needs to be done to reach at a comprehensive recommendation. Normally, plants respond to applied nutrient when the concentration of a given nutrient element in the soil is in a deficiency level and there observed no significant differences among the applied P due to the inherently high P concentration of the experimental soil. Acknowledgements This work was supported by Debre Markos University Research and Community Service office. Hence, I would like to acknowledge the University for all the necessary and available facilities, equipments and services delivered. Special thanks are forwarded for the Plant Science section of Debre Markos University and regional soil laboratory for permitting me to accommodate the green house for conducting the experiment and the Soil Section staff for their cooperation to use their laboratory with its equipments. Lastly, I remain sincere, grateful and indebted to all of my friends who visited and assisted me during the course of the study. References