American Journal of Plant Sciences
Vol. 4 No. 12A (2013) , Article ID: 41274 , 5 pages DOI:10.4236/ajps.2013.412A1010
Forage Legume Seeds Submitted to in Vitro and in Situ Fermentation Techniques
![]()
1Department of Animal Science and Production, Federal University of Espírito Santo, Vitoria, Brazil; 2Laboratory of Plant Science, State University of Norte Fluminense Darcy Ribeiro, Rio de Janeiro, Brazil; 3Department of Animal Science and Production, Federal Rural University of Rio de Janeiro, Rio de Janeiro, Brazil; 4Department of Animal Science and Production, Federal University of Jequitinhonha and Mucuri Valleys, Diamantina, Brazil; 5Department of Animal Science and Production, Federal University of Pampa, Uruguaiana, Brazil.
Email: *brunodeminicis@gmail.com, henrique@uenf.br, joaocarlosbq@gmail.com, araujosac@yahoo.com.br, deisecastagnara@yahoo.com.br
Copyright © 2013 Bruno B. Deminicis et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received October 4th, 2013; revised November 6th, 2013; accepted December 18th, 2013
Keywords: Germination; Ruminal Liquid; Fabaceae Seeds
ABSTRACT
The present study was carried out to evaluate the survival rate of four tropical legume seeds submitted to different periods of ruminal incubation simulated by in vitro and in situ fermentation. The species studied were butterfly pea, stylosanthes, archer and perennial soybean. Four Holstein dairy cows fistulated in rumen were used in the in situ stage. A completely randomized design was used, in a 4 × 6 factorial arrangement (four species, six treatment periods) in the in vitro stage, with four repetitions. A completely randomized block design, arranged in a 4 × 6 factorial scheme (four species × six incubation periods), was used in the in situ stage, with four repetitions. The seeds (50 units of only one species at each repetition) were placed in erlenmeyer flask (250 mL) and were put in a 10 × 15 cm nylon bag in the in vitro stage. The times of incubation were: 6, 12, 24, 48, 96 and 144 hours and the time zero was estimated in the laboratory. The results demonstrate that, when submitted to ruminal incubation, due to their hard and impermeable teguments, legume seeds presented high resistance potential, and, thus, greater chances of germinating after passage through the gastrointestinal tract of bovines, when defecated in pastures. However, stylosanthes are not recommended for insertion in bovine feeding for this purpose, because they do not resist ruminal digestion.
1. Introduction
The use of legumes is an alternative to decrease erosion and restore and maintain soil fertility of Brazilian pastures. The benefits of inclusion of legumes in pastures are unquestionable. Unfortunately, however, such procedure is rarely adopted in livestock production system. Moreover, in Brazil, there are just a few studies aiming at developing technology for legume seed dispersion by introducing seeds in the diet of cattle, goats, sheep and/or horses [1-3]. On the other hand, many studies using the collection of manure from cattle grazing reported that many plant species are able to survive passage through the digestive tract of ruminants [4,5]. Until now, however, Ecology and Animal Science handle the dispersal of legume seeds differently. But it is a relevant issue for both fields and is related to the dynamics of plants in Brazilian pastures.
The advantages of using the in vitro technique to determine the survival of seeds after ruminal digestion are the speed, physical-chemical uniformity of the fermentation microenvironment and the fact that it is not necessary to maintain fistulated animals. Most in vitro methods, however, may fail because they do not use correctly the inoculum, buffers or the equipment that ensures the conditions of pH, anaerobiosis, number of micro-organisms and nutrients that are essential for their performance [6]. The main disadvantage of the in vitro method is that it does not reproduce the in vivo environment perfectly. However, this disadvantage can be reversed when the objective of the assay is to determine the survival/germination of seeds after ruminal digestion, since the in vitro conditions may be controlled so as to prevent physicalchemical fluctuations in the environment.
Thus, this study aimed to analyze the effects of ruminal fermentation in seeds of four tropical forage legumes, using the in vitro and in situ fermentation technique on the survival/germination of seeds.
2. Methods
The experiments were conducted at the Laboratory of Animal Science and Animal Nutrition and at the Laboratory of Plant Science of the UENF, in Campos dos Goytacazes—RJ. The following species were used: Clitorea ternatea, Stylosanthes spp. cv. Campo Grande, Neonotonia wightii and Macrotyloma axillare.
The in vitro assay was arranged in a completely randomized design, with 4 × 6 factorial scheme (four species × six fermentation times) and four replications. The seeds of the four species (50 seeds of only one species per replicate) were placed together with rumen fluid obtained via ruminal fistula plus a buffer to simulate digestion in the reticulum—rumen and omasum at a temperature of 39˚C and pH of 6.9. The rumen fluid was collected before morning feeding via rumen cannula, using a vacuum pump and a Kitassato, with capacity of 2000 mL, always kept in waterbath at 39˚C. The inocula were prepared according to [7], modified according to [8]. The seeds were placed in 250 mL erlenmeyeres containing 10 ml of ruminal fluid and 40 mL of buffer solution, at a temperature of 39˚C. The fermentation times were: 6, 12, 24, 48, 96 and 144 hours, and the time zero was performed in the laboratory. CO2 was inoculated in about 30 seconds before and after the addition of ruminal fluid. At the end of the fermentation periods, the jars were drained and the seeds were washed in the same jar, five to six times, with distilled water. Then, the seeds were submitted to germination test. The seeds were placed in a BOD germinator (12 h of light) at 25˚C, where they remained for 10 days, using four replicates of 50 seeds per replication. The seeds were placed for germination in rolled germitest paper moistened with distilled water at the proportion of 2.5 times the paper weight. The percentages of germination of each species were determined at each fermentation time.
The in situ assay used crossbred cattle (Holstein × Zebu), live weight of 500 kg, with rumen cannula, housed in individual pens. The diet consisted of chopped elephant grass, mineral salt and water ad libitum. The total daily amount of food was provided at two different times, 8 am and 6 pm. The amount offered was adjusted according to the leavings observed every day. The leavings were controlled so that they would be 10% of the amount offered on the previous day, so as to ensure voluntary intake of animals. The assay was arranged in a randomized block design, with four species and four replications. The equivalent to 50 seeds of only one type was placed in each 10 × 15 cm nylon bag per replication. The incubation times were: 6, 12, 24, 48, 96 and 144 hours, and the time zero was performed in the laboratory. At incubation, the bags were tied with nylon thread and attached to a 540-g stainless steel cylindrical rod, which was secured by an iron chain with length of 50 cm, in the cannula, during the incubation period. After “in situ” incubation, the bags were washed with distilled water, and the seeds were submitted to the germination test. The seeds were placed in BOD germinator (12 h of light) at 25˚C, where they remained for 10 days. The percentages of germination of each species were determined at each incubation time. After testing the germination of seeds, a high percentage of hard seeds was observed, except for stylosanthes. Thus, scarification of the hard seeds was carried out with water sandpaper number 100, until grooves were observed in the integument of the seeds to break dormancy. The germination test was performed after scarification. The seeds were placed in BOD germinator at 25˚C, with 12 hours of light.
The results for germination were given in percentage, and were subjected to the analysis of variance, using the Tukey test at 5% significance for the comparison of the averages. Then, a regression analysis was performed. All values were transformed into arcsen 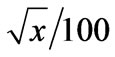 for the analysis of variance.
for the analysis of variance.
3. Results and Discussion
Regression Non-linear regression analysis allowed the achievement of the equations that describe the behavior of germination percentage (GP%) and hardness percentage (%HP) of the seeds submitted to different periods of in vitro fermentation with rumen fluid and artificial saliva (Figures 1 and 2) and the percentage of germination (GP%) and percentage of hardness (%HP) of the seeds subjected to different ruminal in situ incubation period (Figures 3 and 4).
As for the percentage of germination, the seeds presented little increase as fermentation period increased, until 48 hours. After this period, the value decreased slightly. However, the seeds of stylosanthes behaved differently from the others and rapidly reduced the number of germinated seeds, precisely because of the number of dead seeds observed in the germination test. This behavior can be explained by the fact that the integument of stylosanthes is not very resistant, since recommendation for breaking dormancy requires immersion in concentrated sulfuric acid for a maximum of 10 minutes [9]. [10] studied the process of Phyllanthus emblica seed dispersal by ruminants in tropical forests and observed deers (Axis deer) regurgitating intact seeds of Phyllanthus emblica after retaining them in their rumen for 7 - 27 hours, and
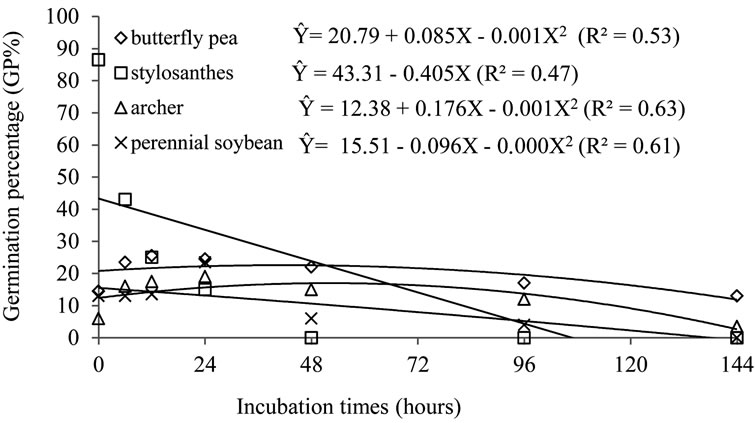
Figure 1. Regression for germination percentage of hard seeds of butterfly pea, stylosanthes, perennial soybean and archer, due to periods of in vitro fermentation.
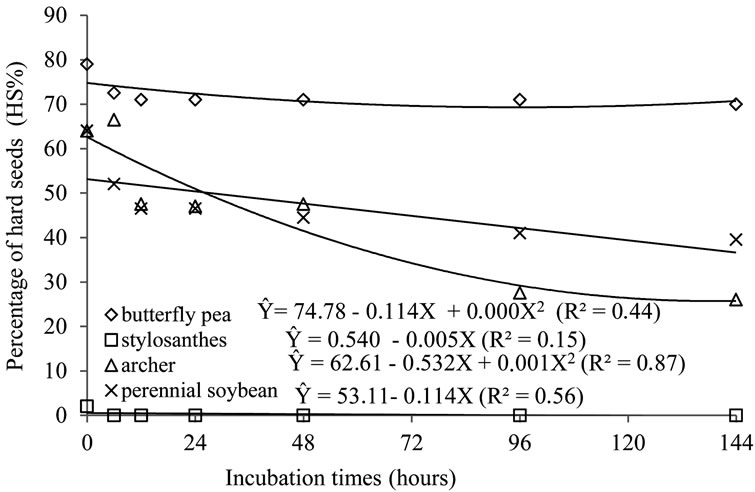
Figure 2. Regression for hardness percentage seeds of butterfly pea, stylosanthes, perennial soybean and archer, due to periods of in vitro fermentation.
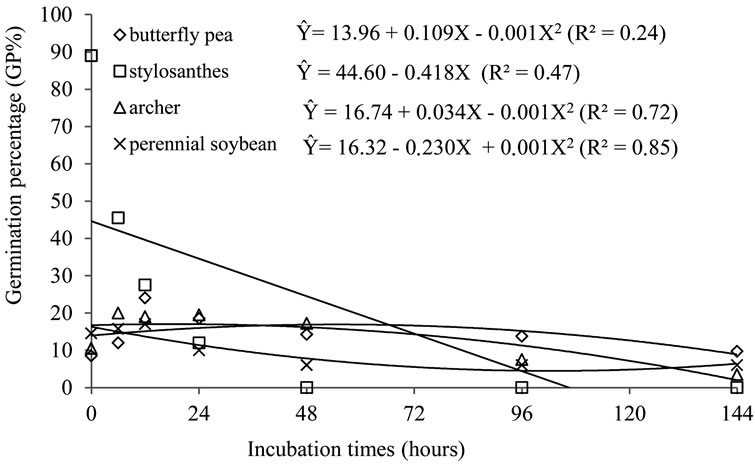
Figure 3. Regression for the germination percentage of seeds of butterfly pea, stylosanthes, perennial soybean and archer, due to ruminal incubation periods (in situ).
that a significant fraction (22%) of the seeds germinate, although the percentage of original germination of the seeeds consumed is 72%, which suggests that size and hardness of seeds ensure their regurgitation, instead of being defecated.
The response of hard seeds was similar for all species. In other words, the number of hard seeds decreased as time of ruminal incubation increased, although the slopes
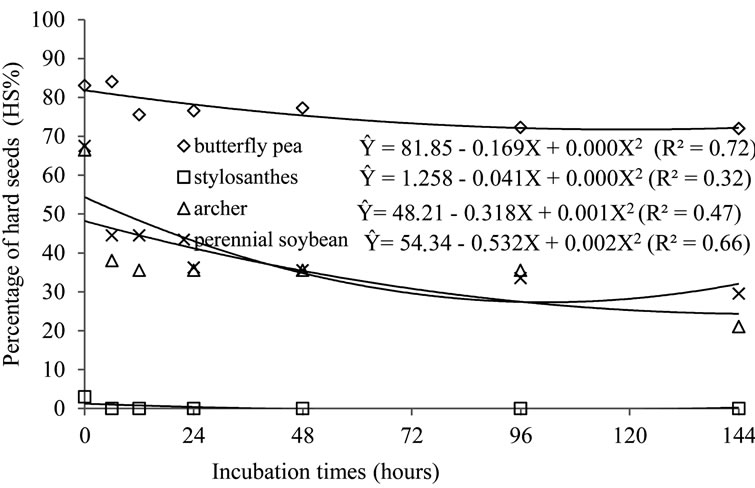
Figure 4. Regression for the hardness percentage seeds of butterfly pea, stylosanthes, perennial soybean and archer, due to ruminal incubation periods (in situ).
of the curves were different, i.e. more or less accentuated, in relation to one another. However, the seeds of the species archer, butterfly pea and perennial soybean presented high resistance and survival rates, exactly due to the hardness of their teguments.
In a study on the germination of scarified and nonscarified Enterolobium cyclocarpum seeds subjected to in vitro fermentation technique using rumen fluid (bovine) and cecal inoculum (horse), [11]. observed that, after 24 hours of exposure to in vitro fermentation, 90% of the scarified seeds died, while all non-scarified seeds germinated in the germination test. This behavior suggests that scarification of the seeds promoted by microbiota, temperature and immersion in rumen fluid has little effect on the permeability of the tegument of seeds of butterfly pea, perennial soybean and archer, while the seeds of stylosanthes suffer lethal damage when passing through rumen, regardless of length of stay [12]. Investigated the germination of seeds incubated in the rumen of sheep and goats and observed high germination rate when the tegumenting was eroded, which occurs when seeds are incubated in the rumen (32% within 48 hours of ruminal incubation, while the control reached 12%). [13] studied the survival of seeds of 25 species of plants introduced to the feeding of deer (Dama dama L) and found that, out of the 25 species of plants whose seeds had been ingested by the animals, 24 survived and germinated in their faeces (0.5% to 42% of the ingested seeds) and 50% of all the seeds were recovered within the first 25 h, at a 13 to 38-h range.
Legume seeds have physical latency due to the presence of a hard and impermeable tegument. Thus, they need to be scarified to germinate [14]. In Salta, a province of the Chaco region, in Argentina, [15] examined the germination of Caesalpinia paraguariensis after passage through the digestive tract of cattle, and observed that seed germination is low and germination percentage does not increase, because the viable seeds are dormant due to their strong impermeable tegument. For [16], species with impermeable teguments present higher percentages of germination after passage through the digestive tract of cattle, although no significant difference can be noticed in germination, survival or speed rates. The hypothesis that latency is broken when seeds pass through the digestive tract of animals is widely accepted, but some results are conflicting [14,17-22]. It can be observed in Figures 5 and 6, which show no dormancy breaking in most seeds subjected to either in vitro or in situ fermentation.
However, these results suggest that most seeds survive and may germinate. It is clear that seeds introduced into the diet of these animals that survive rumen degradation and remain dormant may germinate in the feces of cattle in the field.
4. Conclusions
Ruminal digestion, simulated by in vitro and in situ fermentation techniques, showed that seeds with resistant teguments have high potential to be introduced into the diet of cattle, for ruminal digestion poses low risk, mainly to the seeds of butterfly pea (cunhã), perennial soybeans and archer.
In this study, stylosanthes should not be inserted in the
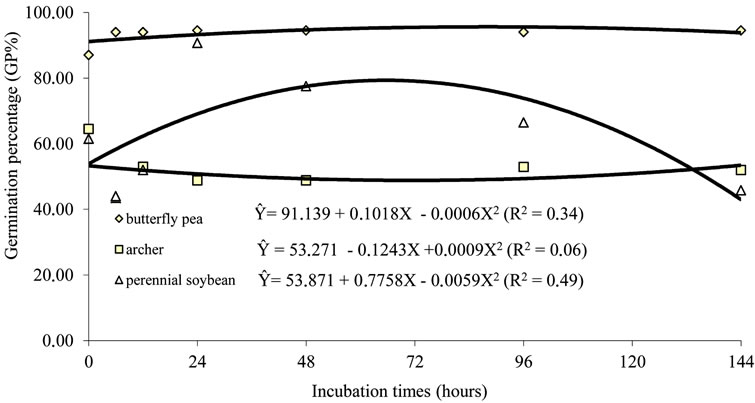
Figure 5. Regression for the percentage of germination of “hard” seeds of butterfly pea, perennial soybean and archer, due to periods of in vitro fermentation and after scarification.
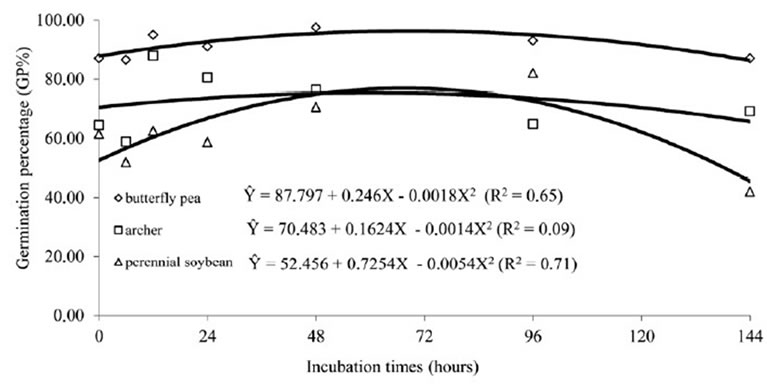
Figure 6. Regression for the percentage of germination of “hard” seeds of butterfly pea, perennial soybean and archer, due to periods of incubation in situ.
feeding of cattle aiming to be introduced in the pastures precisely because it is sensitive to the ruminal digestion stage. Further studies should be conducted for better knowledge about these results and for assessing the possible introduction of legumes through bovines into pastures.
5. Acknowledgements
FAPERJ—Carlos Chagas Filho Foundation for Research Support of Rio de Janeiro State, CNPq—Coordination of Improvement of Higher Education Personnel, CAPES—National Council for Scientific and Technological Development, UFRRJ—Federal Rural University of Rio de janeiro, UFC—Federal University of Ceará, EMBRAPA—Brazilian Agricultural Research Corporation, PESAGRO-RIO—Agricultural Research Corporation of Rio de Janeiro State, UNIOESTE—University of West of Paraná State.
REFERENCES
- J. F. Valentim and J. C. Carneiro, “Dormancy Breaking and Planting of Kudzu in Farming Systems and Agroforestry,” Embrapa-CPAF-Acre, Rio Branco, Technical Instructions No. 17, 1998.
- B. B. Deminicis, J. C. C. Almeida, S. A. C. Araújo, M. C. Blume, H. D. Vieira and L. B. Dobbss, “Overcoming of Dormancy in Seeds of Eight Tropical Forage Legumes,” Archivos de Zootecnia, Córdoba, Vol. 56, No. 215, 2007, pp. 347-350.
- T. O. Silva, J. C. C. Almeida, N. S. Rocha, Z. S. Costa, G. P. Lima, P. H. Grassi, T. C. Ferreira, R. P. Araújo and J. B. R. Abreu, “Dispersal and Germination of Tropical Forage Legumes in the Faeces of Cattle,” International Congress of Animal Science, Londrina, 2007, p. 201.
- S. Stender, P. Poschlod, E. Vauk-Hentzelt and T. Dernedde, “Die Ausbreitung Durch Galloway-Rinder,” Verhandlungen der Gesellschaft für Ökologie, Vol. 27, 1997, pp. 173-180.
- R. J. Pakeman, G. Digneffe and J. L. Small, “Ecological Correlates of Endozoochory by Herbivores,” Functional Ecology, Vol. 16, No. 3, 2002, pp. 296-304. http://dx.doi.org/10.1046/j.1365-2435.2002.00625.x
- P. J. Van Soest, “Nutritional Ecology of the Ruminant,” 2nd Edition, Cornell University Press, Ithaca, 1994.
- J. M. A. Tilley and R. A. Terry, “A Two-Stage Technique for the in Vitro Digestion of Forage Crops,” Journal of the British Grassland Society, Vol. 18, No. 2, 1963, pp. 104-111.
- G. T. Santos, U. Cecato and L. P. Rigolon, “Chemical Composition and in Situ Fermentation of Leucaena (Leucaena leucocephala) and Desmodium (Desmodium ovalifolium) Undergoing Conservation in the Form of Hay and Silage,” Annual Meeting of the Brazilian Society of Animal Science, Fortaleza, July 1996, pp. 347-349.
- N. F. Seiffert, “Methods of Seed Scarification Tropical Forages,” EMBRAPA Gado de Corte, Campo Grande, 6 Pages. (Technical Communication, 13), 1982.
- S. Prasad, J. Krishnaswamy, R. Chellam and S. P. Goyal, “Ruminant-Mediated Seed Dispersal of an Economically Valuable Tree in Indian Dry Forest,” Biotropica, Vol. 38, No. 5, 2006, pp. 679-682. http://dx.doi.org/10.1111/j.1744-7429.2006.00182.x
- D. H. Janzen, M. W. Demment and J. B. Robertson, “How Fast and Why Do Germinating Guanacaste Seeds (Enterolobium cyclocarpum) Die inside Cows and Horses?” Biotropica, Vol. 17, No. 4, 1985, pp. 322-325.
- A. Robles and J. Castro, “Effect of Thermal Shock and Ruminal Incubation on Seed Germination in Helianthemum apenninum (L.) Mill. (Cistaceae),” Acta Botánica Malacitana, No. 27, 2002, pp. 41-47.
- A. M. Mouissie, C. E. J. V. D. Veen, G. F. Veen and R. V. Diggelen, “Ecological Correlates of Seeds Survival after Ingestion by Fallow Deer,” Functional Ecology, Vol. 19, No. 2, 2005, pp. 284-290. http://dx.doi.org/10.1111/j.0269-8463.2005.00955.x
- I. Izhaki and D. G. Ne’eman, “Hares (Lepus spp.) as Seed Dispersers of Retama raetam (Fabaceae) in a Sandy Landscape,” Journal of Arid Environments, Vol. 37, No. 2, 1997, pp. 343-354. http://dx.doi.org/10.1006/jare.1997.0273
- B. P. Ortega, M. L G. Viana and S. M. Larenas, “Seed Germination of Caesalpinia paraguariensis (Fabaceae): Agents of Livestock Scarifiers and Effect,” Journal of Tropical Biology, Vol. 49, No. 1, 2001, pp. 301-304.
- B. Peco, L. Lopez-Merino and M. Alvir, “Survival and Germination of Mediterranean Grassland Species after Simulated Sheep Ingestion: Ecological Correlates with Seed Traits,” Actaoecologica, Vol. 30, 2006, pp. 269-275.
- D. H. Janzen, “Enterolobium cyclocarpum Seed Passage Rate and Survival in Horses, Costa Rican Pleistocene Seed Dispersal Agents,” Journal of Ecology, Vol. 62, No. 3, 1981, pp. 593-601.
- R. M. Peinetti, M. A. Pereyra, A. Kin and A. Sosa, “Effect of Cattle Ingestion on Viabilty and Germination Rate of Caldén (Prosopis caldenia) Seeds,” Journal of Range Management, Vol. 46, No. 6, 1993, pp. 483-486. http://dx.doi.org/10.2307/4002856
- M. Campos and M. Ojeda, “Dispersal and Germination of Prosopis flexuosa (Fabaceae) Seeds by Desert Mammals in Argentina,” Journal of Arid Environments, Vol. 35, No. 4, 1997, pp. 707-714. http://dx.doi.org/10.1006/jare.1996.0196
- B. P. Ortega, “Emergency and Survival of Seedlings of Prosopis ferox Cardones National Park. (Salta, Argentina),” Licentiate Thesis, Universidad Nacional de Salta, Salta, 1999.
- M. A. Manhães, L. C. S. Assis and E R. M. Castro, “Frugivory and Seed Dispersal of Miconia urophylla (Melastomataceae) by Birds in a Fragment of Secondary Atlantic Forest in Juiz de Fora, Minas Gerais State, Brazil,” Brazilian Journal of Ornithology, Vol. 11, No. 2, 2003, pp. 173-180.
- E. R. Chang, E. L. Zozaya, D. P. J. Kuijper and J. P. Bakker, “Seed Dispersal by Small Herbivores and Tidal Water: Are They Important Filters in the Assembly of Salt-Marsh Communities?” Functional Ecology, Vol. 19, No. 4, 2005, pp. 665-673. http://dx.doi.org/10.1111/j.1365-2435.2005.01011.x
NOTES
*Corresponding author.

