American Journal of Plant Sciences
Vol.3 No.10(2012), Article ID:24127,4 pages DOI:10.4236/ajps.2012.310171
In Vitro Antioxygenic Activity of Ridge Gourd (Luffa acutangula) Pulp, Peel and Their Extracts on Peroxidation Models
![]()
Department of Cereals and Pulses Technology Defence Food Research Laboratory, Siddarthanagar, India.
Email: *gksh60@gmail.com
Received July 23rd, 2012; revised August 20th, 2012; accepted September 1st, 2012
Keywords: Ridge Gourd; Antioxygenic Activity; Solvent Extraction; Phenolics; Flavonoids
ABSTRACT
Ridge gourd pulp and peel powders as well as their various solvent fractions were evaluated for antioxygenic activity using different methods. Ridge gourd pulp and peel powders at 2% level and their ethanol/water soluble extracts exhibited strong antioxygenic activity in sunflower oil stored at 37˚C. The water soluble extract devoid of any antioxygenic activity in sunflower oil. Ridge gourd pulp and peel powders as well as their extracts were evaluated for their antioxygenic activity using linoleic acid peroxidation, β-carotene-linoleic acid bleaching and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) methods. Ethanol/water extracts from ridge gourd pulp and peel showed highest antioxygenic activity followed by water extracts, while the petroleum ether extract showed moderate antioxygenic activity. Ridge gourd peel powder and its extracts showed slightly higher antioxygenic activity than ridge gourd pulp powder and its extracts. This may be attributed to the presence of higher amounts of phenolics and flavonoids which have been reported as potential antioxidants.
1. Introduction
One of the major causes of food quality deterioration is lipid peroxidation, a free radical mediated process responsible for both the development of objectionable odour and flavour in foods [1,2]. Tocopherol, butylated hydroxy anisole (BHA), butylated hydroxy toluene (BHT) and tertiary butyl hydro quinone (TBHQ) are important synthetic antioxidants that protect polyunsaturated fatty acids from oxidative degradation. However, due to their unstable, highly volatile nature and toxicological side effects [3,4], there has frequently been some questions raised about their efficiency since their first introduction into the food industry [5,6]. TBHQ has been banned in Japan and certain European countries while BHA and BHT are reported to be carcinogenic [7]. Hence, research into safer and more effective natural antioxidants is under way and several natural sources are being examined [8-12] that act as a stable and efficient antioxidant to prevent lipid deterioration of foods [13]. Fruits, vegetables, herbs and oil seeds have been long recognized as a good source of phenolic compounds such as phenolic acids, flavonoids, isoflavonoids, flavones, anthocyanins, catechins etc rather than having micronutrients [14-17], act as antioxidant compounds impart greater benefits to human being.
Ridge gourd is a popular vegetable consumed in Asian, African and Arabic countries and a common vegetable in daily Indian diet. Data on the antioxygenic activity of ridge gourd is unavailable. Therefore, the present study was undertaken to extract antioxidant compounds separately from pulp and peel of ridge gourd using various solvents and the relative antioxygenic activities of these extracts evaluated using different assays.
2. Materials and Methods
Refined sunflower oil (Sunpure brand) without added synthetic antioxidant was supplied by sunflower oil producers (M/s MK Agrotech, Srirangapattanam, Karnataka, India). Good quality ridge gourd was procured from local market. Linoleic acid, catechin, ammonium thiocyanate, tween 20, tween 40, 1,1-diphenyl-2-picryl-hydrazyl (DPPH) and Tertiary Butyl Hydro Quinone (TBHQ) were procured from Sigma chemicals, Mumbai, India. Ferrous chloride, sodium dihydrogen phosphate, disodium hydrogen phosphate, chloroform, methanol, petroleum ether (40˚C - 60˚C) and gallic acid were procured from E-Merck, Mumbai, India, while β-carotene was procured from Fluka chemicals, (Fluka chemie, GMbH, Buchs, Switzerland). Ethyl alcohol was distilled before use, while all other chemicals were of analytical reagent grade and used as such.
2.1. Preparation of Ridge Gourd Pulp and Peel Powders
Good quality ridge gourd was washed in a running tap water to remove the adhering dust and durt. Ridge gourd was trimmed and hand peeled and peel portion was collected separately. Remaining portion (ridge gourd pulp) was diced in to small pieces (1 - 2 mm thickness) manually. Both peel and pulp were dried at 70˚C separately in a fluidized bed drier (Model 30D, Chemec Engineering, Mumbai, India) to a moisture level of 7% - 8%. Dehydrated ridge gourd pulp and peel were ground to a fine powder in an ultra centrifugal mill (Model Restch R1, Haan, Germany) using 1 mm sieve and used for experimental studies.
2.2. Solvent Extraction Procedure
Ridge gourd pulp and peel powders (20 gm) were taken individually in glass columns (2.5 × 30 cm) and sequentially eluted with 1000 ml each of petroleum ether (40˚C - 60˚C), ethanol/water (80:20 v/v) and distilled water. Each fraction was separately evaporated using a rotary vacuum evaporator (Model Superfit PBU6 Continental instruments, Mumbai, India) and preserved at 5˚C prior to use. A flow chart for the preparation of various fractions from ridge gourd is given in Figure 1.
Powders (0.2 g) of ridge gourd pulp and peel as well as their solvent extracts (0.1 g) were extracted with 25 ml of 70% methanol (70 ml methanol + 30 ml distilled water) for 1 h under sonication and the extracts were filtered. The residues were re-extracted for 1 h and both the filtrates were combined and made up to 50 ml with 70% methanol. The total phenolics and flavonoids were estimated according to the method of Gerard and Roberts [18].
2.3. Test for Antioxygenic Activity in Sunflower Oil
Ridge gourd pulp and peel as well as their various solvent fractions were evaluated for antioxygenic activity in refined sunflower oil. Samples (100 g) of sunflower oil both with and without 0.5, 1.0 and 2.0 g powder each of ridge gourd pulp and peel and an equivalent amount (2 g of powder) of various ridge gourd fractions were incubated in 250 ml glass beaker at 37˚C ± 1˚C. Initially and after regular intervals of 30 days, 20 g samples were removed and analysed for peroxide value (PV) as well as thiobarbituric acid (TBA) values by the methods reported earlier [19]. Antioxygenic activity was calculated as the ratio between the peroxide or thiobarbituric acid value of control and the peroxide or thiobarbituric acid value of the samples.
2.4. Determination of Antioxygenic Activity Using β-Carotene-Linoleic Acid System
Antioxygenic activities of ridge gourd pulp, peel and their extracts were measured by monitoring the coupled oxidation of β-carotene and linoleic acid with the method reported by Peterson et al. [20]. β-carotene (2 mg) was dissolved in 20 ml chloroform and 3 ml of this solution was added to 40 mg of linoleic acid and 400 mg of tween 40. The chloroform was removed at 40˚C under a stream of nitrogen gas. Oxygenated deionised water (100 ml) was added and the solution mixed well. Aliquots (3 ml) of β-carotene-linoleic acid emulsions were mixed with 100 μl of the methanolic extracts (diluted with methanol to obtain equivalent of 1 mg of starting material/100 μl). Oxidation of the emulsion was monitored spectrophotometrically (Shimadzu UV 1601 UV-Vis Spectrophotometer) by measuring absorbance at 470 nm at 15 min intervals upto 120 min at 50˚C against a blank consisting of 100 μl of water and emulsion without β-carotene. Con-
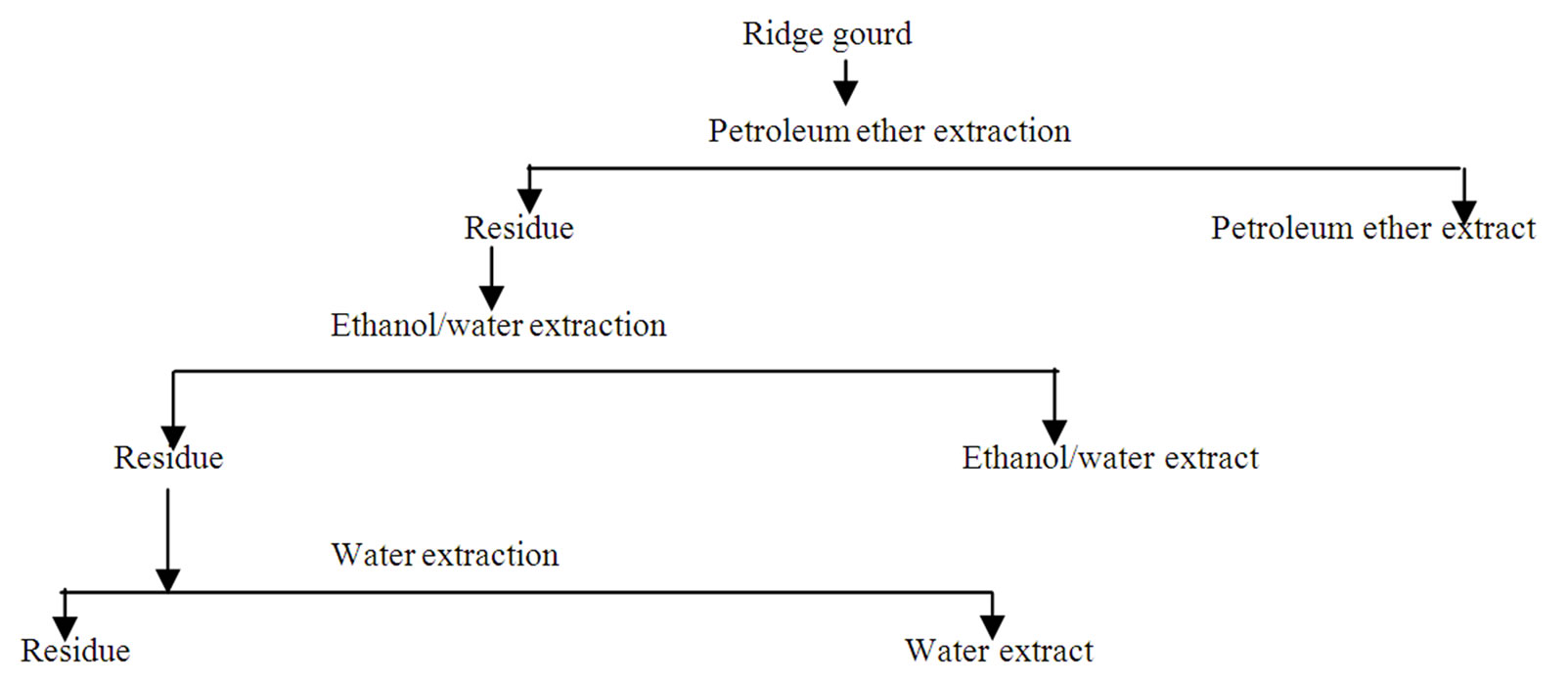
Figure 1. Scheme for the solvent extraction from ridge gourd.
trol contained 100 μl of methanol in place of the extract. Antioxygenic activity was expressed as percentage inhibition, relative to the control, in terms of bleaching of β-carotene after incubation for 120 min using the formula
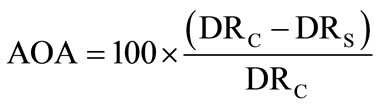
where AOA is the antioxygenic activity, DRC is the degradation rate of control and DRS is degradation rate of sample.
2.5. Determination of Antioxygenic Activity Using Linoleic Acid Peroxidation Method
The antioxygenic activities of ridge gourd pulp and peel as well as their extracts were determined using thiocyanate method [21]. The linoleic acid emulsion was prepared by homogenizing 0.28 g of linoleic acid and 0.28 g of tween 20 as an emulsifier and 50 ml of phosphate buffer (0.2 M, pH 7.0). The test samples were prepared in methanol-water mixture (80:20 v/v) and 1 ml of each of them was mixed with 2.5 ml of linoleic acid emulsion, 2 ml of phosphate buffer and incubated at 37˚C for 120 h. The mixture prepared as above without test sample served as a control. Aliquots (0.1 ml) were drawn from the incubated mixtures at intervals of 24 h and mixed with 4.7 ml of 75% ethanol, 0.1 ml of 30% ammonium thiocyanate and 0.1 ml of 0.2 mM ferrous chloride in 3.5% HCl and allowed to stand at room temperature for 3 min. After 3 min, the colour developed was measured at 500 nm. A control was performed using linoleic acid without the test sample. Synthetic antioxidant TBHQ was used as a control for comparison. The degree of linoleic acid peroxidation was calculated at 96 h using the formula:

2.6. Determination of Antioxygenic Activity Using DPPH Radical-Scavenging Method
The antioxygenic activities of ridge gourd pulp and peel as well as their extracts and TBHQ was measured in terms of hydrogen donating or radical-scavenging ability using DPPH method [22]. Different amounts of vegetable extracts (100, 300, 500 and 1000 μl) equivalent to 1, 3, 5 and 10 mg/ml of vegetable powders and their extracts were placed in separate test tubes. The volume was adjusted to 1 ml with methanol, 5 ml aliquots of 0.2 mM methanolic solution of DPPH were added to each tube and shaken vigorously. The tubes were allowed to stand at room temperature (30˚C ± 0.5˚C) for 30 min. The control was prepared as above without any extract and methanol was used for the base line correction. The absorbance of the samples was measured at 517 nm. The radicalscavenging activity was expressed as percentage of inhibition and calculated using the formula:

2.7. Statistical Analysis
All the reported values are mean of three replicates and were subjected to two way analysis of variance (ANOVA). Significant differences (p ≤ 0.01) between the means were tested by Duncan’s multiple range test using statistical software (Statistica, Ver. 7.1 Series 1205) at p ≤ 0.01 significance levels. The correlation coefficients were also calculated and tested for significance at p < 0.05 levels.
3. Results and Discussion
3.1. Total Phenolics and Flavonoid Contents of Ridge Gourd Pulp, Peel and Their Extracts
The total phenolics and flavonoid contents of ridge gourd pulp, peel and their solvent extracts are shown in Table 1. Ridge gourd peel contained slightly higher amounts of phenolics (6830 ppm) and total flavonoids (1025 ppm) as compared to ridge gourd pulp which contained 6250 and 875 ppm of phenolics and flavonoids respectively. Among the different extracts of ridge gourd pulp and peel, ethanol/water extract showed higher amounts of phenolics and flavonoids followed by water and petroleum ether extracts.
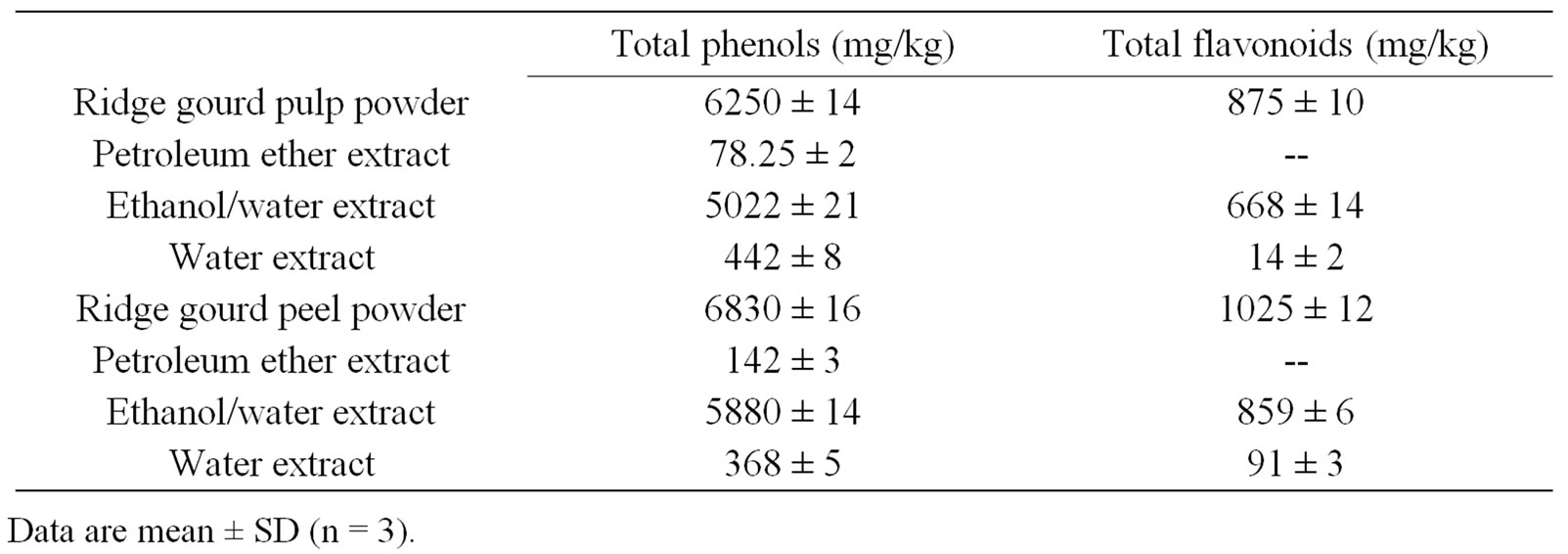
Table 1. Total phenol and flavonoid contents of ridge gourd pulp , peel and their extracts.
3.2. Antioxygenic Activity of Ridge Gourd Pulp, Peel and Their Extracts in Sunflower Oil
Effect of ridge gourd pulp and peel powders at 0.5% to 2% levels on the rate of autooxidation in refined sunflower at 37˚C ± 1˚C is shown in Table 2" target="_self"> Table 2. Addition of these powders even at 0.5% level significantly (p ≤ 0.01) retarded the rate of lipid peroxidation in sunflower oil. The catalytic action of these vegetable powders was dependant on concentration and antioxygenic activity increased with rise in concentration of these vegetable powders from 0.5% to 2%.
As both ridge gourd pulp and peel at 2% level showed highest antioxygenic activity in refined sunflower oil stored at 37˚C and therefore, their effect in refined sunflower oil were also evaluated at elevated temperature (80˚C ± 1˚C) normally encountered for the preparation of various traditional Indian foods. It is interesting to observe that, at higher temperature, ridge gourd peel powder showed higher antioxygenic activity as compared to TBHQ, clearly indicating the degradation of TBHQ and beneficial effect of ridge gourd peel and pulp in retarding the rate of autooxidation in refined sunflower oil at higher elevated temperature (Table 3).
Table 4 shows the effect of ridge gourd pulp, peel (2 g/100g) and their various extracts equivalent to 2 g/100g of vegetable powder obtained by sequential extraction with petroleum ether, ethanol/water (80:20 v/v) and water on peroxide and malonaldehyde formation in sunflower oil in comparison to TBHQ. It may be observed that both ridge gourd pulp and peel powders at 2% level and their ethanol/water soluble extracts showed strong antioxygenic activity, whereas their petroleum ether extracts exhibited only moderate activity (Table 5). Ridge gourd pulp, peel and their extracts significantly (p ≤ 0.01) reduced peroxide and malonaldehyde formation in sunflower oil during storage at 37˚C. The control gave higher peroxide (PV) and thiobarbituric acid (TBA) values than the samples containing ridge gourd pulp and peel or their ethanol/water soluble extracts (Table 4). The antioxygenic activities of ridge gourd pulp and peel powders were found comparatively higher than that of its ethanol/water soluble fraction (Table 5). The water soluble fractions of ridge gourd pulp and peel were practically devoid of any antioxygenic activity. The higher antioxygenic activity of ethanol/water soluble fractions may be due to the solubility of phenolics and flavonoid compounds as well as sugars in the ethanolic medium which are known to have antioxygenic activity in lipids.
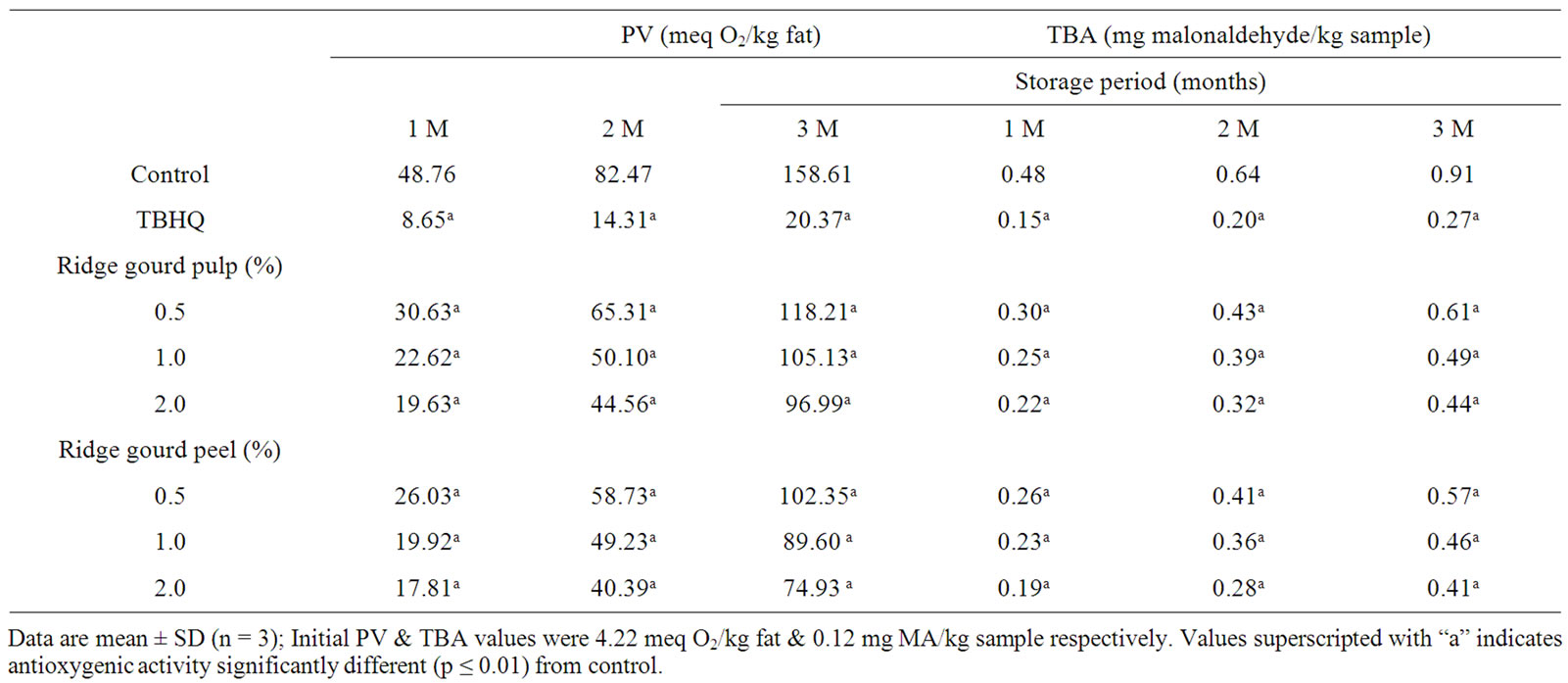
Table 2. Changes in peroxide value (PV) and thiobarbituric acid (TBA) value of ridge gourd pulp and peel powders in sunflower oil during storage at 37˚C.
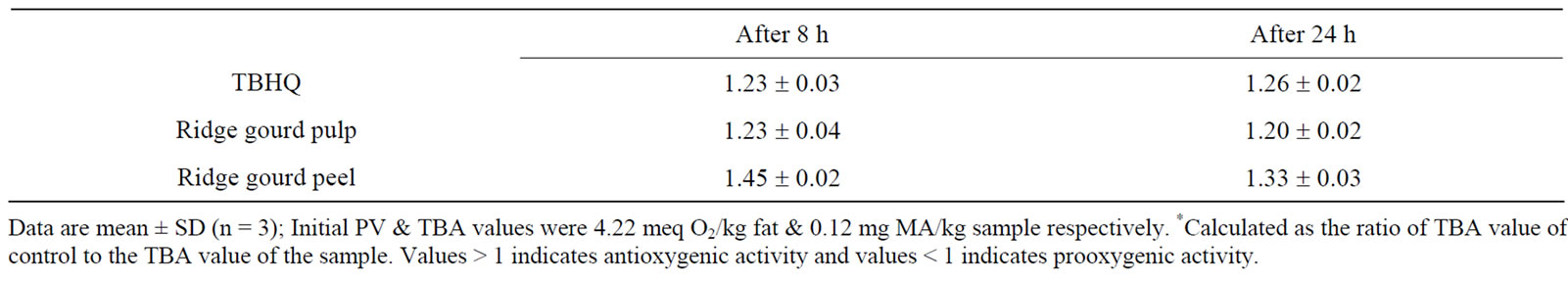
Table 3. Antioxygenic activity* of ridge gourd pulp and peel powders during storage in sunflower oil at 80˚C.
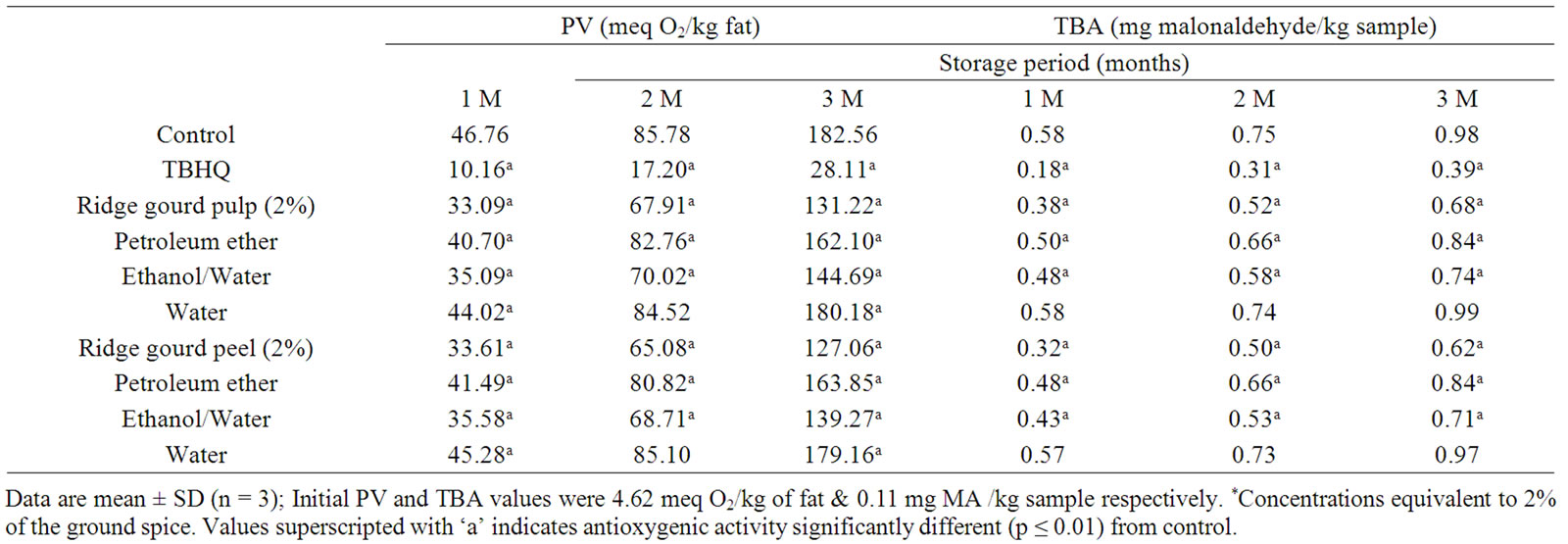
Table 4. Changes in peroxide value (PV) and thiobarbituric acid (TBA) value of ridge gourd pulp, peel and their various solvent extracts* in sunflower oil during storage at 37˚C.
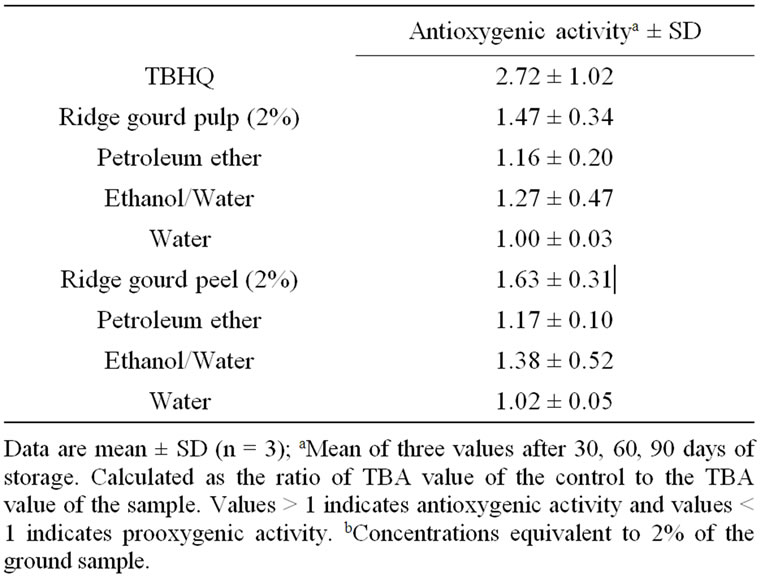
Table 5. Antioxygenic activity of ridge gourd pulp, peel and their various solvent extractsb during storage in sunflower oil at 37˚C.
3.3. Antioxygenic Activity of Ridge Gourd Pulp, Peel and Their Extracts Using β-Carotene-Linoleic Acid Bleaching Method
The antioxygenic activities of ridge gourd pulp, peel powders as well as their extracts and TBHQ as measured by the bleaching of β-carotene are shown in Figure 2" target="_self"> Figure 2. It can be seen that ridge gourd peel showed slightly higher antioxygenic activity than that of ridge gourd pulp. Relatively, ethanol/water extracts from both pulp and peel showed the highest antioxygenic activity followed by water extracts, while the petroleum ether extracts showed the least activity. However, the higher antioxygenic activity of ridge gourd peel and its ethanol/water extracts than its pulp and ethanol/water extracts may be due to the presence of higher amounts of both phenolics and flavonoids (Table 1). Flavonoids and phenolics have been reported as potent antioxidants in β-carotene-linoleic acid bleaching systems [2,23,24].
3.4. Antioxygenic Activity of Ridge Gourd Pulp, Peel and Their Extracts in Linoleic Acid Peroxidation System
The antioxygenic activities of ridge gourd pulp, peel powders as well as their extracts were measured using the thiocyanate method. Figure 3 indicates the percentage inhibition of linoleic acid after the addition of pulp and peel powders as well as their extracts and TBHQ. The ridge gourd pulp, peel and their petroleum ether, ethanol/water, water extracts and TBHQ gave a lower increase in peroxidation levels over the 96 h of testing. After 96 h, the inhibition percent of ridge gourd pulp, peel, their petroleum ether, ethanol/water and water extracts were 51.08%, 53.4%, 31.25%, 35.94%, 47.60%, 49.77% and 38.55%, 40.87% respectively. However, these values were found to be lower than that of the TBHQ (84.05%). Relatively ridge gourd peel powders and their extracts exhibited slightly higher antioxygenic activity than their ridge gourd pulp counter parts.
3.5. Scavenging Effect on 1,1-Diphenyl-2-Picryl Hydrazyl (DPPH) Radical
The profile of scavenging activities of ridge gourd pulp, peel powders as well as their extracts were determined and compared with those of TBHQ (Figures 4 and 5). The order of antioxygenic activity (1 mg/ml of ridge gourd pulp, peel and their extracts) ridge gourd peel (ethanol/ water extract) > ridge gourd peel > ridge gourd pulp > ridge gourd pulp (ethanol/water extract) > ridge gourd peel (water extract) > ridge gourd peel (petroleum ether extract > ridge gourd pulp (water extract) > ridge gourd pulp (petroleum ether extract). Even at 10 mg/ml concentration, both ridge gourd pulp and peel powders and their extracts exhibited lower scavenging activity to that of TBHQ. However, the scavenging activity of both ridge
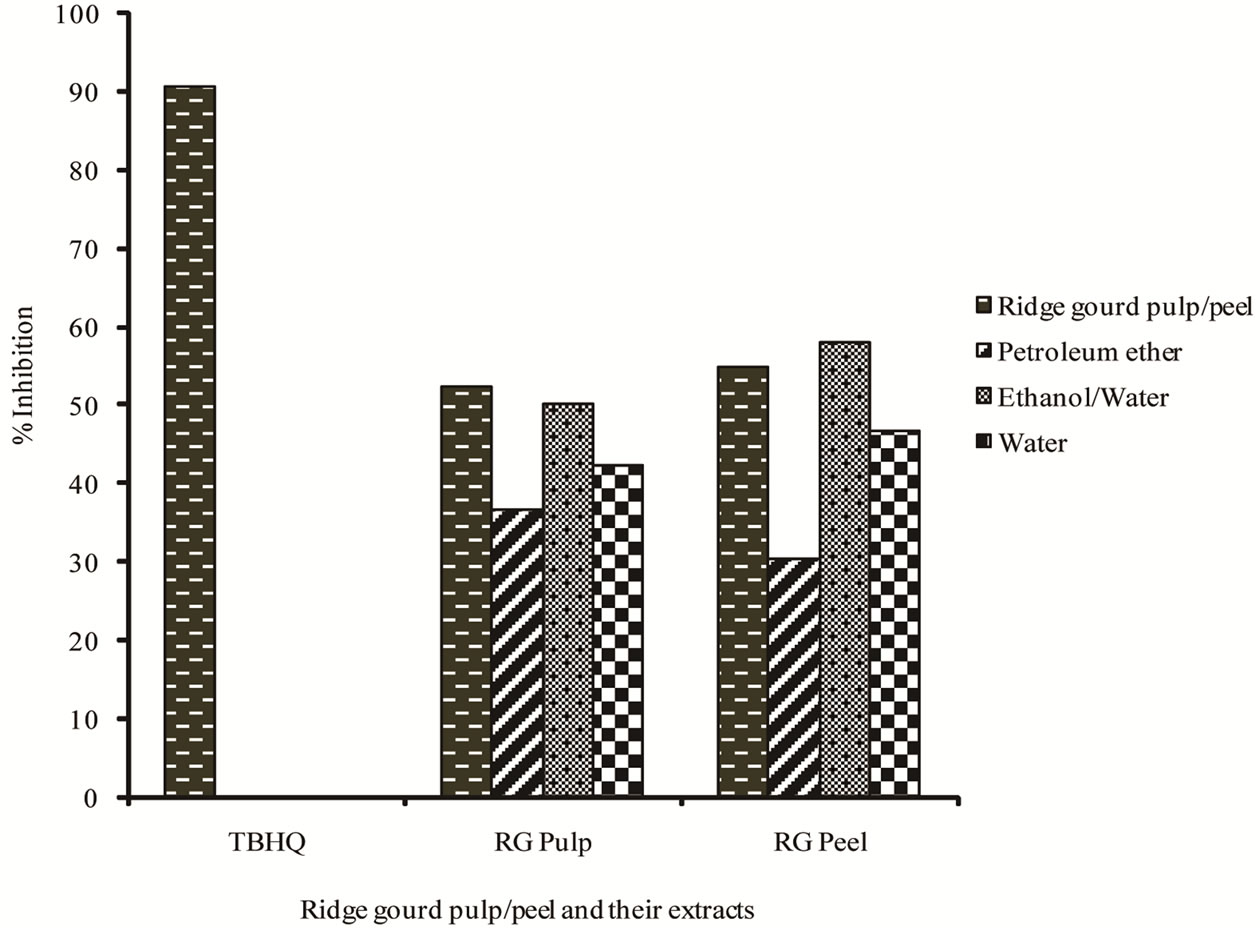
Figure 2. Antioxygenic activity of ridge gourd pulp/peel and its various solvent extracts using b-carotene bleaching method.
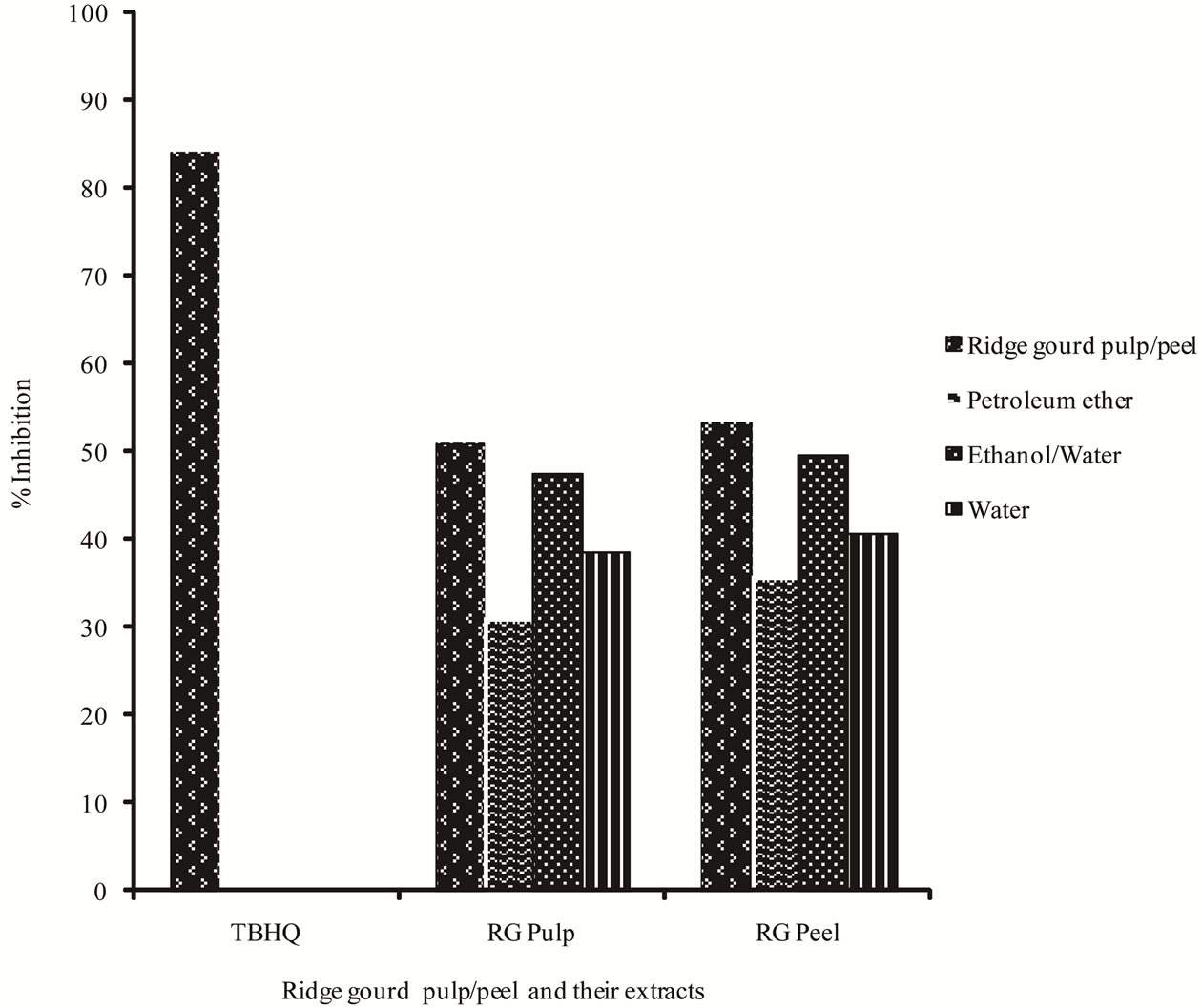
Figure 3. Antioxygenic activity of ridge gourd pulp/peel and its various solvent extracts using ammonium thiocyanate method.
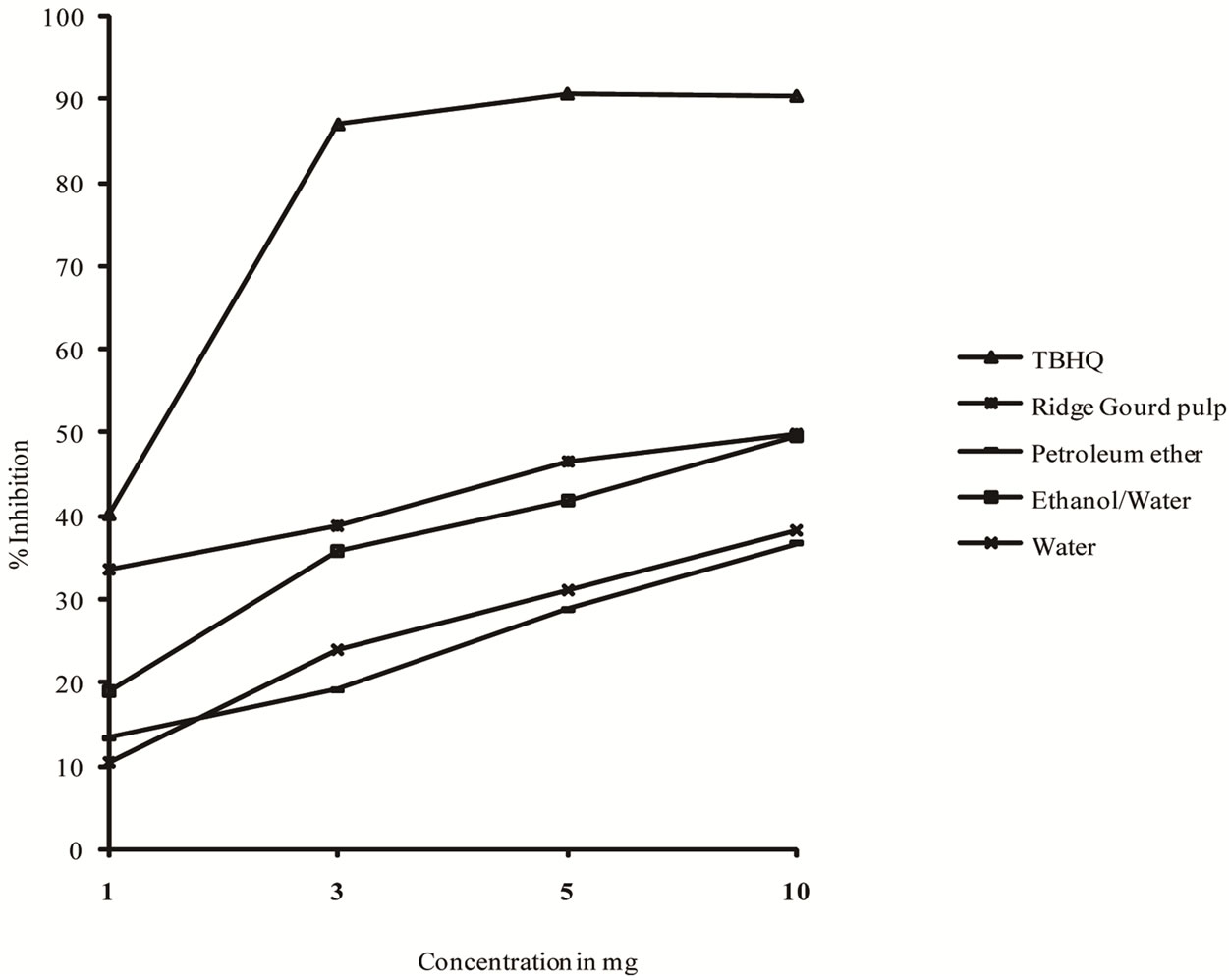
Figure 4. Antioxygenic activity of ridge gourd pulp and its various solvent extracts using DPPH radical scavenging method.
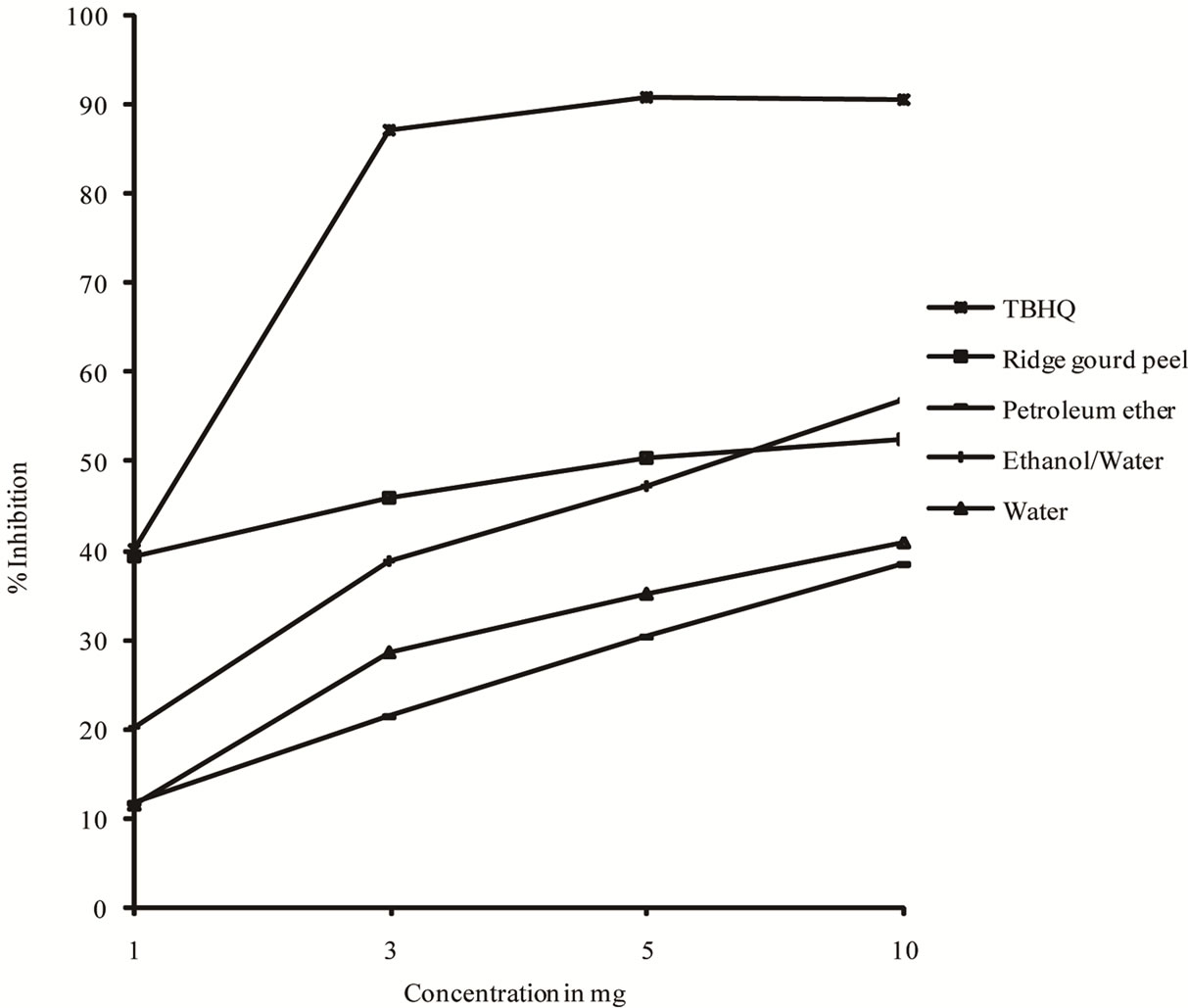
Figure 5. Antioxygenic activity of ridge gourd peel and its various solvent extracts using DPPH radical scavenging method.
gourd pulp and peel powders and their extracts increased with increasing concentrations.
3.6. Correlation Analysis
Total phenolics and total flavonoids from ridge gourd showed a positive correlation (r ≥ 0.80) with antioxygenic activity as determined by β-carotene bleaching, ammonium thiocyanate and DPPH radical scavenging methods. The correlation was found significant (p < 0.05) between total phenolics, β-carotene bleaching method and DPPH radical scavenging methods in case of ridge gourd pulp, while in case of ridge gourd peel, the correlation between total phenolics and ammonium thiocyanate method was significant (p < 0.05). The correlation between total flavonoids and antioxygenic activities determined by ammonium thiocyanate, β-carotene bleaching and DPPH radical scavenging methods were found insignificant (p > 0.05) in case of ridge gourd pulp except for DPPH radical scavenging methods and total flavonoid of ridge gourd peel was significantly (p < 0.05) correlated with DPPH radical scavenging only.
4. Conclusion
The results of the present work indicate the presence of compounds possessing antioxygenic activity in pulp and peel of ridge gourd. Ridge gourd peel had higher antioxygenic activity as compared to ridge gourd pulp which may be ascribed to their different phenolic and flavonoid compositions. Ethanol/water extract from both pulp and peel exhibited higher antioxygenic activities than did other solvent extracts, when evaluated for antioxygenic activity by any of the methods. Because this is the first report on the description of antioxygenic activity from ridge gourd, further studies are needed on the isolation and characterization of individual phenolic and flavonoid compounds to elucidate their antioxidant mechanism. However, ridge gourd pulp and peel, and particularly their ethanol/water extracts have great potential as natural antioxidants.
REFERENCES
- N. V. Yanishlieva, A. K. Eldin, E. M. Marinova and A. G. Toneva, “Kinetics of Antioxidant Action of α and ∆ Tocopherols in Soybean and Sunflower Triacylglycerols,” European Journal of Lipid Science and Technology, Vol. 104, No. 5, 2002, pp. 262-270. doi:10.1002/1438-9312(200205)104:5<262::AID-EJLT262>3.0.CO;2-B
- A. Padmashree, N. Roopa, A. D. Semwal, G. K. Sharma, G. Agathian and A. S. Bawa, “Star Anise (Illicium verum) and Black Caraway (Carum nigrum) as Natural Antioxidants,” Food Chemistry, Vol. 104, No. 1, 2007, pp. 59-66. doi:10.1016/j.foodchem.2006.10.074
- A. Dapkevicius, R. Venskutonis, T. A. Vanbeek and P. H. Linssen, “Antioxidant Activity of Extracts Obtained by Different Isolation Procedures from Some Aromatic Herbs Grown in Lithuania,” Journal of Science of Food and Agriculture, Vol. 77, No. 1, 1998, pp. 140-146. doi:10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K
- P. P. Rajaram, S. N. Mansingraj, U. J. Umesh, V. D. Vishal and P. G. Sanjay, “Antiaflatoxigenic and Antioxidant Activity of an Essential Oil from Ageratum conyzoides L.,” Journal of Science of Food and Agriculture, Vol. 90, No. 4, 2010, pp. 608-614.
- D. L. Mahdavi and D. K. Salukha, “Toxicological Aspects of Food Antioxidants,” In: D. L. Mahdavi, S. S. R. Deshpande and D. K. Salukha, Eds., Food Antioxidants, Dekker, Newyork, 1995, pp. 267-292.
- S. M. Barlaw, “Toxicological Aspects of Antioxidants Used as Food Additives,” In: B. J. F. Hudson, Ed., Food Antioxidants, Elsevier, London, 1990, pp. 253-307
- A. A. M. Adel, A. S. Mohamed, S. Iryna and M. Awad, “Antioxidant Properties of Various Solvent Extracts of Potato Peel, Sugar Beet Pulp and Sesame Cake,” Journal of Science of Food and Agriculture, Vol. 90, No. 2, 2010, pp. 218-226. doi:10.1002/jsfa.3796
- R. M. Kalyan, A. Craig and E. Nuran, “Biologically Important Thiols in Aqueous Extracts of Spices and Evaluation of Their in Vitro Antioxidant Properties,” Food Chemistry, Vol. 118, No. 3, 2010, pp. 589-593. doi:10.1016/j.foodchem.2009.05.025
- B. Nada, D. Sanja, J. Milka, V. Vlatka, I. Jasna, P. Slobodan and Z. Irena, “Supercritical Carbon Dioxide Extraction of Antioxidant Fractions from Selected Lamiaceae Herbs and Their Antioxidant Capacity,” Innovative Food Science and Emerging Technology, Vol. 11, No. 1, 2010, pp. 98-107. doi:10.1016/j.ifset.2009.08.013
- A. Padmashree, G. K. Sharma, A. D. Semwal and A. S. Bawa, “Studies on the Antioxygenic Activity of Bitter Gourd (Momordica charantia) and Its Fractions Using Various in Vitro Models,” Journal of Science of Food and Agriculture, Vol. 91, No. 4, 2011, pp. 776-782. doi:10.1002/jsfa.4251
- L. Mei, Y. Bo, Z. Maomao and C. Jie, “Antioxidant Capacity and Major Phenolic Compounds of Spices Commonly Consumed in China,” Food Research International, Vol. 44, No. 2, 2011, pp. 530-536. doi:10.1016/j.foodres.2010.10.055
- L. Wei, Z. Mouming, Y. Bao, R. Jiaoyan, S. Guanglin and R. Guohua, “Antioxidant and Antiproliferative Capacities of Phenolics Purified from Phyllanthus emblica L. Fruits,” Food Chemistry, Vol. 126, No. 1, 2011, pp. 277- 282.
- J. Pokorny, N. Yanishlieva and M. Yordon, “Antioxidants in Food; Practical Applications,” Abington Hall, Abington, 2011, p. 30.
- F. Shahidi, “Natural Antioxidants: An Overview,” In: Shahidi Ed., Natural Antioxidants, Chemistry, Health Effects and Applications, AOCS Press, Champaign, 1997, pp. 1-11.
- L. U. Thompson, P. Robb, M. Serraino and F. Cheung, “Mammalian Lignan Production from Various Foods,” Nutrition and Cancer, Vol. 16, No. 1, 1991, pp. 43-52. doi:10.1080/01635589109514139
- H. Wang, G. Cao and K. L. Rrior, “Total Antioxidant Capacity of Fruits,” Journal of Agricultural and Food Chemistry, Vol. 44, No. 3, 1996, pp. 701-705. doi:10.1021/jf950579y
- W. Zheng and S. Y. Wang, “Antioxidant Activity and Phenolic Compounds in Selected Herbs,” Journal of Agricultural and Food Chemistry, Vol. 49, No. 11, 2001, pp. 5165-5170. doi:10.1021/jf010697n
- K. A. Gerard and J. S. Roberts, “Microwave Heating of Apple Mash to Improve Juice Yield Quality,” Lebensmittel-Wissenschaft Technol, Vol. 37, No. 5, 2004, pp. 551- 557. doi:10.1016/j.lwt.2003.12.006
- A. D. Semwal, G. K. Sharma and S. S. Arya, “Pro or Antioxygenic Activity of Tejpat (Cinnamomum tamala) and Red Chilli (Capsicum anum) in Sunflower Oil,” Journal of Science of Food and Agriculture, Vol. 79, No. 12, 1999, pp. 1733-1736. doi:10.1002/(SICI)1097-0010(199909)79:12<1733::AID-JSFA431>3.0.CO;2-4
- D. M. Peterson, C. L. Emmons and A. H. Hibbs, “Phenolic Antioxidants and Antioxidant Activity in Pearling Fractions of Oat Groats,” Journal of Cereal Science, Vol. 33, No. 1, 2001, pp. 97-103. doi:10.1006/jcrs.2000.0347
- G. K. Jayaprakasha, R. P. Singh and K. K. Sakariah, “Antioxidant Activity of Grape Seed (Vitis vinifera) Extracts on Peroxidation Model in Vitro,” Food Chemistry, Vol. 73, No. 3, 2001, pp. 285-290. doi:10.1016/S0308-8146(00)00298-3
- S. Gorinstein, R. Haruenkit, S. P. Yong, S. T. Jung, Z. Zachweija and Z. Jastrzehski, “Bioactive Compounds and Antioxidant Potential in Fresh and Dried Jaffa Sweeties: A New Kind of Citrus Fruit,” Journal of Science of Food and Agriculture, Vol. 84, No. 12, 2004, pp. 1459- 1463. doi:10.1002/jsfa.1800
- G. K. Jayaprakasha, P. S. Negi, B. S. Jena and J. Mohan Rao, “Antioxidant and Antimutagenic Activities of Cinnamomum zeylanicum Fruit Extracts,” Journal of Food Composition and Analysis, Vol. 20, No. 3-4, 2007, pp. 330-336. doi:10.1016/j.jfca.2006.07.006
- P. Siddaraju and K. Becker, “Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera) Leaves,” Journal of Agricultural and Food Chemistry, Vol. 50, No. 8, 2003, pp. 2144-2155. doi:10.1021/jf020444+
NOTES
*Corresponding author.

