American Journal of Plant Sciences
Vol.3 No.1(2012), Article ID:16646,8 pages DOI:10.4236/ajps.2012.31010
The Ultrastructure Characteristics of Secretory Cavities Associated with the Secretory Products of Ginkgo biloba
![]()
1College of Forest Resources and Environment, Nanjing Forestry University, Nanjing, China; 2College of Forestry, Shanxi Agricultural University, Taigu, China.
Email: frpeng@njfu.edu.cn
Received October 10th, 2011; revised November 9th, 2011; accepted December 7th, 2011
Keywords: Ginkgo biloba; Secretory Cavity; Ultrastructure; Secretory Products
ABSTRACT
The origin, development and ultrastructure of secretory cavities in leaf of Ginkgo biloba and its relation to the secretory products were studied by optical and electron microscope. The results indicated that the formation pattern of the secretory cavities was schizo-lysigenous in nature. First, some original central secretory cells in the center of secretory cavities expanded, dissolved then moved aside schizogeneously forming an intercellular space. Later the central and peripheral secretory cells continued to dissolve making the intercellular space larger. This process continued until the central secretory cells degenerated, autolyzed and separated off into cavities. It has been assumed that the secretory products in the secretory cavities of Gingko biloba were synthesized mainly in the plastids and the endoplasmic reticulum (ER). However it was our observation that the Mitochondrion, Golgi Body and Cytoplasm were also involved in the synthesis and translocation of the secretions. After synthesized in ER and plastids, the secretory products approached the plasmalemma and fused their membranes with the latter in the form of samll vesicles, and then was eliminated to the spaces between the plasmalemma and the wall. At last the secretory products percolated through the wall that faded into an even looser mesh of fibrillar material toward the cavity.
1. Introduction
The Ginkgo tree is the only living representative of the order Ginkgoales. The importance of Ginkgo biloba is thus two fold not only because of its importance as a link to our botanical ancestry but also due to its pharmacological properties, in particular chemicals such as flavonoids and ginkgolides. These chemicals have numerous medicinal, and therefore commercial, properties and are used to assist organ dilatation, blood circulation and the prevention of blood vessel hardening/aging. Although usage of the effective components of Ginkgo biloba is widespread research into the synthesis, accumulation mechanism and changes within these metabolites is limited. Previous research has identified the different secretory cavities (canals) in the stems, leaves, bud scale, and embryo of Ginkgo biloba, and the close correlation between the active substances (i.e. those with pharmacological properties) and the growth development of secretory tissues within the different secretory cavities [1]. However there have been divergent views on the mode of the originand development of the secretory cavities. Early research differed in this respect with some viewing theformation of the secretory cavities in Ginkgo biloba was lysigneous while others puttingforward the theory that it was schizo-lysigenous [2,3]. More recently experiments conducted by electron microscope add to this inconsistent field of thought with the most recent study by Alain (1990) claiming the formation was lysigneous in nature [1]. Given this background this paper attempts to clarify the relationship between the ultrastructure of the secretory cavities of leaves in Ginkgo biloba and the secretory products produced using histochemical methodology and microstructural and ultrastructuralobservation. Its purpose is to provide theoretical foundation for the exploitation of pharmacological resources of Ginkgo biloba.
2. Materials and Methods
2.1. Experiment Material
Two or three-year-old G. biloba seedlings grown in the nursery garden of Nanjing Forest University, Nanjing, Jiangsu Province, China. Stem tips, tender stems, bud scales, roots, tender leaves and mature leaves were collected at 8 separate intervals during the growing periods of April to November (2009) and April to May (2010). In order to ensure consistent results the samples were observed using a variety of observation techniques.
2.2. Paraffin Section Method
The fresh samples were cut into small blocks of 2 - 5 mm3 and fixed by FAA or Nawaxing Ⅲ. The samples were then treated with the traditional paraffin section method. The sections of 6 - 10 µm thickness were stained with Fanhong-gulv and Teifan-hematoxylin and mounted by neutral gel. The sections were photographed and observed using an Olympus microscope.
2.3. Semi-Section Method
The samples were cut into the small blocks of 1 mm3 and fixed with 4% glutaraldehyde mixed with PH 7.0 phosphoric acid buffer for 3 - 6 hours at room temperature. The samples were rinsed with a phosphonic acid buffer then treated again with 1% osmic acid for a period of 2 - 3 hours. The samples were then rinsed again and dehydrated with a series of alcohol mixtures. Finally the samples were treated with epoxy propane and embedded in Epon812 and sliced with a LKB-V section-cutter. The semi-sections of 2 µm thicknesses that weren’t stained were treated with Teifan-hematoxylin, transparentized with xylene and embedded in neutral gel.
2.4. Ultra-Thin Section Method
The ultra-thin samples were stained with acetum uranyl and citrate plumbum, photographed and observed with Rili-600 transmission electron microscope.
3. Results
3.1. Secretory Cavities Development Phase
The observed sections of the leaves, stems and buds of Ginkgo biloba during the development phase indicated that the formation mode of the secretory cavities was schizo-lysigenous. The development of the secretory cavities of the leaves was taken as a control example to illustrate the general development of secretory cavities (Figure 1(a)). The leaf sections were continuously observed during the different development stages. It was found that the emergence of secretory lumens in leaves is earlier than other parts of the plant. The original secretory cavity cells could be observed in immature phyllomes. According to the structural features of secretory cavities in different development phases, the development of secretory cavities was divided into the following 5 phases.
3.1.1. Secretory Cavities Original Cell Stage
The secretory cavities of Ginkgo biloba originated from a clump of densely packed rounded or oval cells. These cells differed from the peripheral cells. The cells had the features of isodiametric and polyhedral shape, dense cytoplasm, deep stains and big nuclei. Whereas the peripheral cells are circular in shape with a higher quantity of vacuolized cells (Figure 1(b)).
3.1.2. Intercellular Space Formation Phase
The original cell clumps rapidly vacuolized and the intercellular layers of central cells expanded, dissolved and finally formed the intercellular space. The intercellular space then expanded outwards along the cell wall to form an irregular lumen made up of 4 - 6 secretory cells. At this time the secretory cells were still intact (Figure 1(c)).
3.1.3. Secretory Cavities Enlargement Phase
Around the intercellular space the peripheral cells moved rapidly outwards to enlarge the secretory cavity. Simultaneously the outer sheath cells extended in tangential directions to create more space for the secretory cells moving outwards (Figure 1(d)).
3.1.4. Secretory Cavities Dissolving Phase
Once the intercellular space reached a critical point the partially developed secretory cells moved inward and individual cells began to dissolve and slough off to form secretory cavities. The secretory cells at this stage were irregular and the nuclei (which had begun to disintegrate) could still be observed in the partial secretory cells (Figure 1(e)).
3.1.5. Secretory Cavities Maturity Phase
In this last stage of differentiation the secretory cells ceased dissolving and moving in tangential directions. The secretory cavities thus reached their largest size in terms of diameter. The mature secretory cavities were round in shape and surrounded by 4 - 6 flat secretory cells (extended in tangential directions). The peripheral sheath cells constituted about 2 - 5 layers and were similar in appearance to the secretory cells (Figure 1(f)).
3.2. Development of Secretory Cavities Ultrastructure
3.2.1. Secretory Cavities Original Cell Stage
The ultrastructure of the secretory cavities in the leaves contained several original secretory cavity cells (with large nuclei and dense cytoplasm) in the phyllome with mesophylle, which had not differentiated completely. Subsequently the number of the original cells increased by the method of anticlinal division (helixical in arrangement). Later the peripheral cells split periclinally to form 2 - 3 layers of sheath cells elongating in tangential direction with a few small vacuoles. At this stage the to
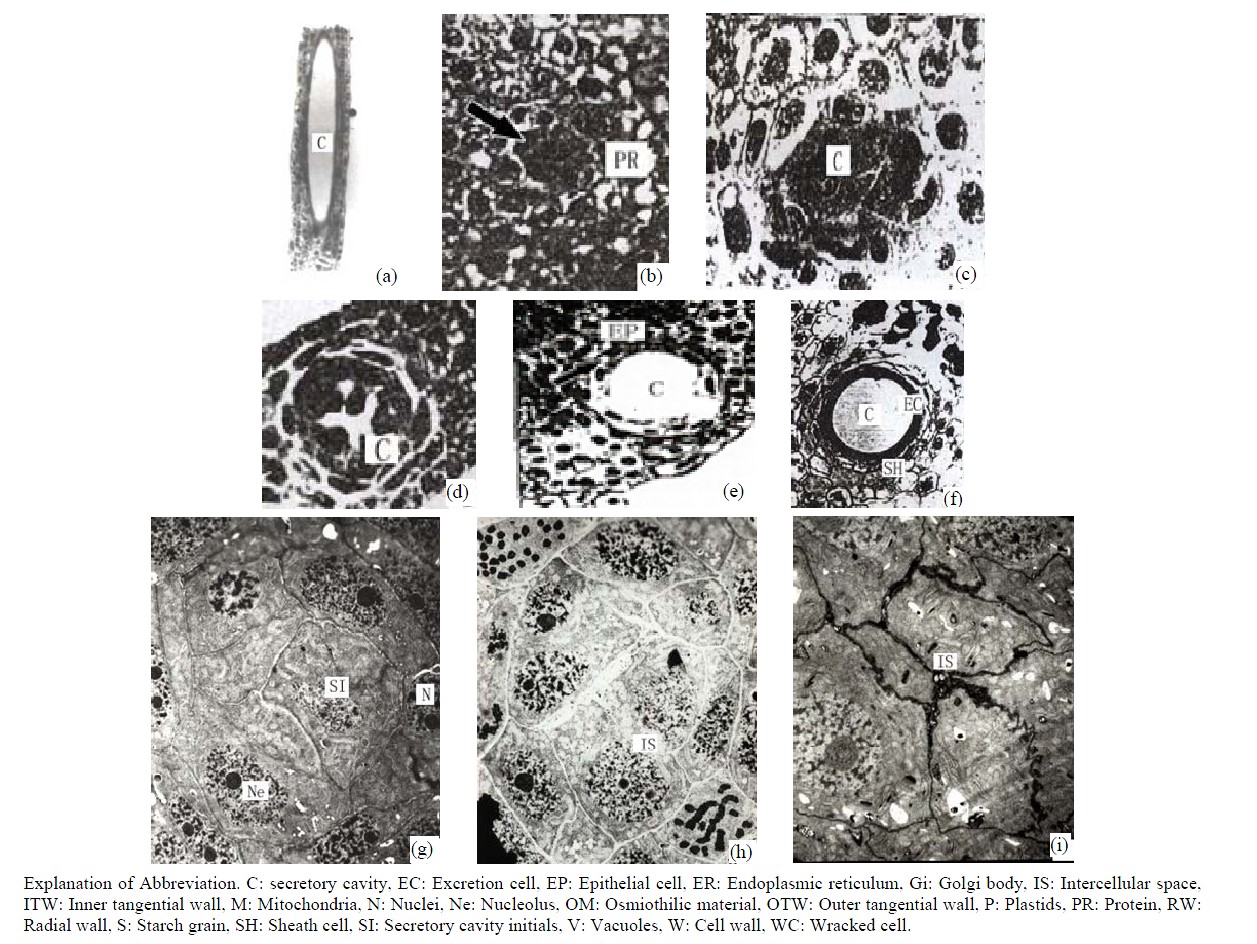
Figure 1. (a) The vertical section of secretory cavity in leaf (×40); (b) Secretory cavity initial cell clump (×66); (c) Showing the formation stages of intercellular space in the developing secretory cavity (×100); (d) Showing the secretory cavity in expending stages (×66); (e) Showing the secretory cavity in collapsing stages (×66); (f) Showing the secretory cavity in mature stages (×200); (g) Showing the ultrastructure of initial cell clump including the cell nuclei, plastids and mitochondria (×2000); (h) Showing the formation of the intercellular space between the central secretory cells (×2000); (i) Showing the division of intercellular space in radial and tangential (×2000).
form 2 - 3 layers of sheath cells elongating in tangential direction with a few small vacuoles. At this stage thenuclei are flat and arranged in tangential directions. It was also observed that at this stage some plastids contained acerose starch grains. The central original cells are polygonal with large round shaped nuclei, dense protoplasm and a few small vacuoles. These original cells are identified as the central secretory cells. Where there were plenty of organelles, such as abundant Mitochondrions with deeply stained stroma and obvious cristae, the Endoplasmic Reticulum (ER) had spread through the whole cytoplasm. The ER was in nature predominantly smooth although there was evidence of coarse ER and affluent cytoplastides in all secretory cell clumps. The cells also possessed different shapes, namely round, elliptical, dumbbell and clubbed. The lamellar structure of plastids hadn’t developed at this stage or had little inner membrane, therefore lacking starch grains. There were a small quantity of Golgi bodies near the cell wall at at this stage no secretory vacuoles in either end were observed (Figure 1(g)).
3.2.2. Intercellular Space Formation Phase
When the secretory cavity cells differentiated into sheath cells and central cells, the middle lamellars of the central adjacent cells began to expand and dissolve. These then formed the intercellular spaces along all directions of the cell wall. Later with the dissolution of the middle lamellars the cells separated from each other and the secretory cavities continuously enlarged (Figures 1(h)-(i)). When the secretory cavities had extended along all the directions of the cell wall the diameter of intercellular space increased. The structure of the central cells also changed and began to possess the features of an imminent dissolving and degrading cell. In effect the cells at this stage were deeply stained and the organelles weren’t obvious. However the plastids and mitochondrion could be observed with the plastid stroma having dissolved and the structure of the inner membrane having disappeared. During this stage the nuclei degraded causing the nuclear membrane to disappear. The result was that the whole nucleoplasm became loose, and the nucleus could be clearly observed (Figures 2(a)-(b)). At this stage there were plenty of black osmiophilic materials in the cytoplasm, plastid and vacuole of the degraded cells. With the enlarging of the secretory cavities, the secretory cells were arranged in radial columns (Figure 2(a)). The middle lamellars of the radial wall expanded and dissolved and the substance on the inside of wall was released into the secretory cavity (where there was evidence of a large quantity of black osmiophilic substance). Meanwhile the secretory cells elongated in tangential direction to increase the diameter
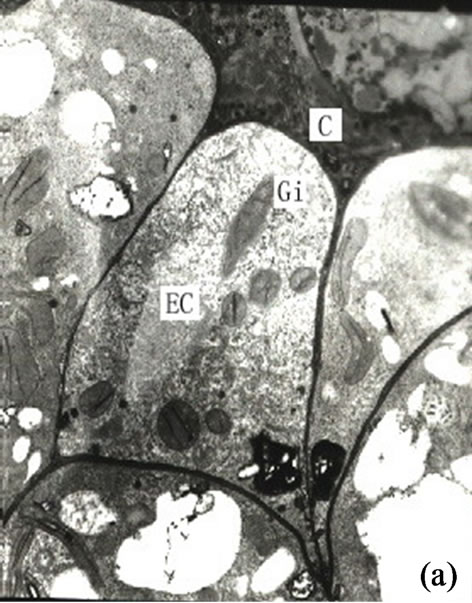
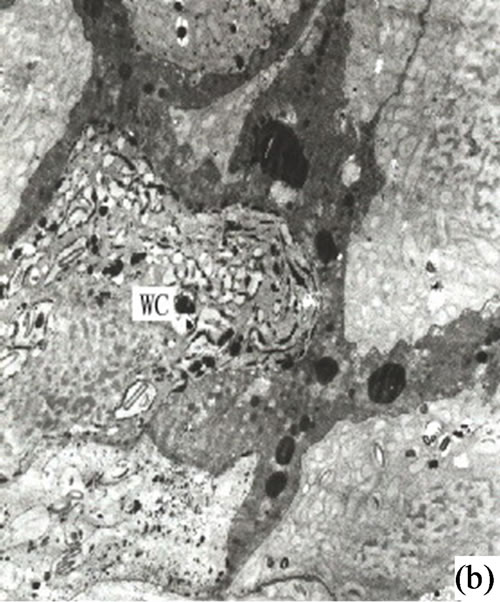
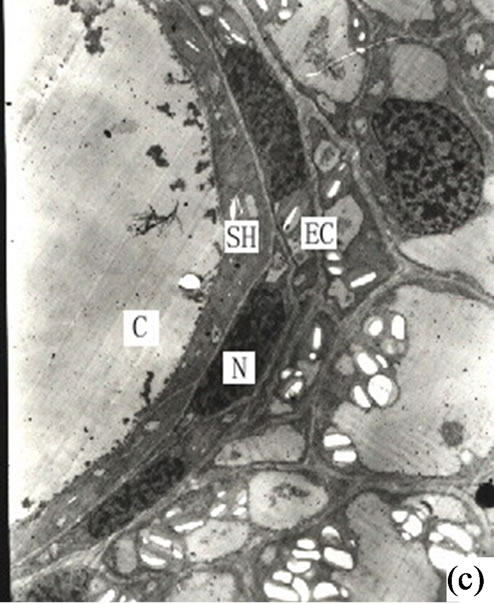
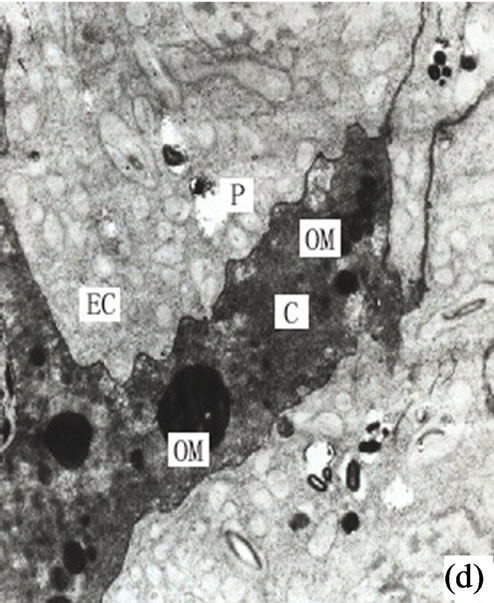
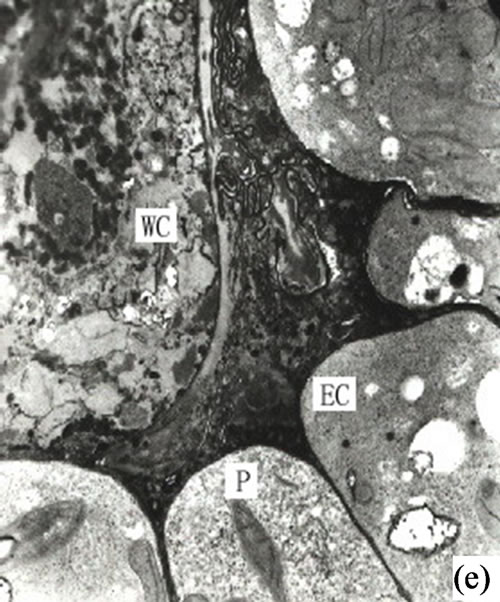
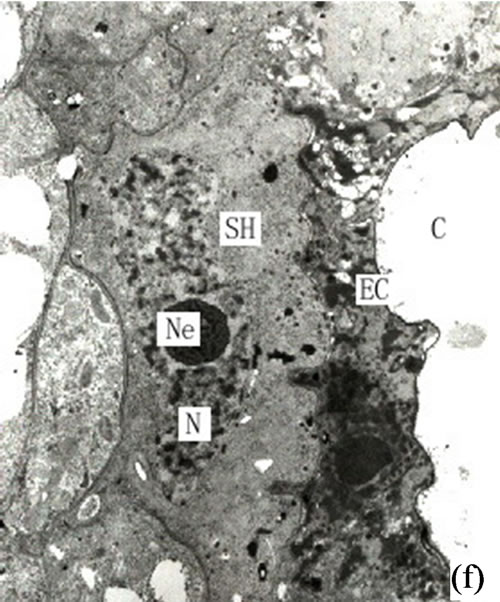
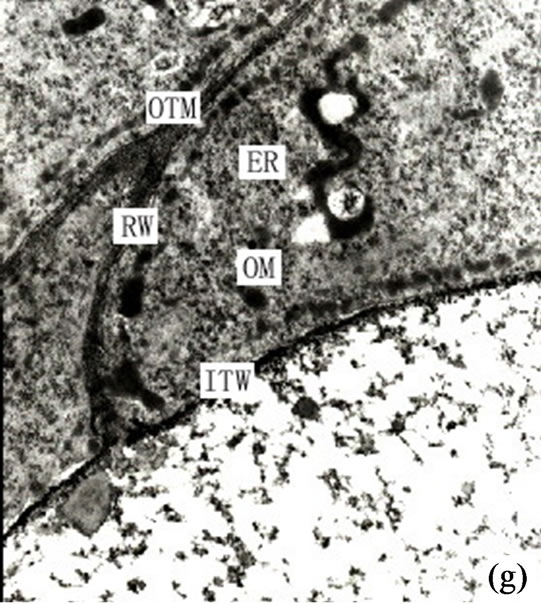
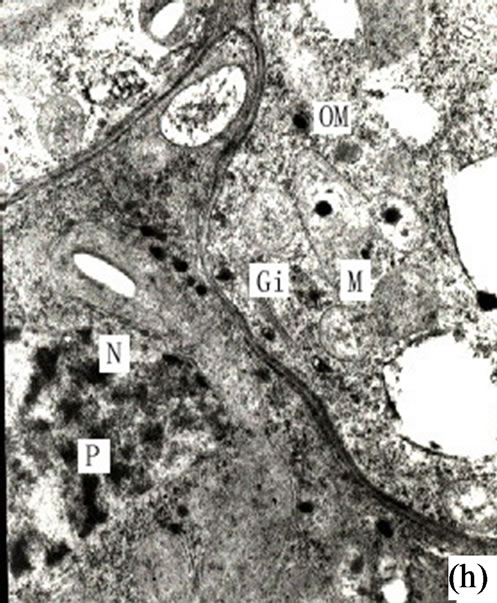
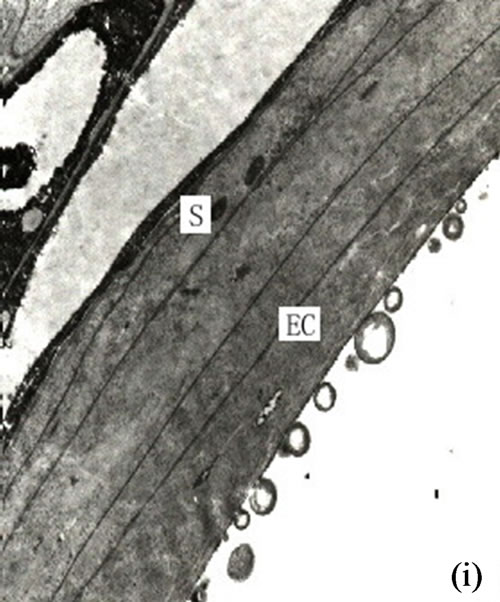
Figure 2. (a) Showing the secretory cells arranged tangentially (×4000); (b) Showing the autolysis of central secretory cells and the expending of cell space (×3000); (c) The mature development stages of the secretory cavity (×2500); (d) Showing the osmiophilic pellets in the secretory cavity (×6000); (e) Showing the slough off of secretory cells in the mature secretory cells in the mature secretory cavity (×3000); (f) Showing the neighboring cells in the secretory cavity (×3000); (g) Showing the secretory vesicles between the radial wall of secretory cells (×15000); (h) Showing the osmiophilic pellets in mitochondria and endoplasmic reticulum approaching to plasmalimma and plastids (arrowhead) (×10000); (i) Showing the endoplasmic reticulum in the mature secretory cavity (×6000).
of the cavity. The cells contained osmiophilic materials distributed near the inner and outer side of the secretory cell near cavity (Figures 2(g)-(h)). When the diameter of the secretory cell reached a critical point the peripheral cells of the secretory cavities divided into 3 layers. The innermost cells were narrower in radial direction but deeply dyed. The 1 - 3 outer layer of peripheral cells were vacuolized and lightly stained. Some of the inner layer cells, whose structures degraded and whose black osmiophilic substance had increased, and the inner and outer tangential wall cells as well as the outward irregular secretory cavities and the sheath cells were dissolved and degraded. (Figures 2(f)-(h)).
3.2.3. Secretory Cavities Maturity Phase
When the secretory cavities matured the cells stopped dissolving and the secretory cavities reached their largest diameter. The mature secretory cavity was made up of a near-round secretory cavity surrounded by 4 - 6 layers of flat secretory cells elongating in tangential directions. It was observed that the tangential length of some of the secretory cells amounted to 80 - 120 µm. At this stage the 2 - 3 layers of peripheral sheathe cells resembled the secretory cells in shape (Figure 2(c)). At this time there was a quantity of dust-colored substance stained by osmium acid in the cavity. The stained color of the secretory cells was at this stage quite deep although by this stage the organelles had been reduced in number. In addition on the side of the secretory cell closest to the secretion, there was an abundance of endoplasmic reticulum (ER) whilst the vacuoles had appeared in the cells. Also in evidence in the plastids was a small quantity of starch grains while the osmiophilic materials had reduced considerably. The sheath cells had at this time vacuolized and there were more starch grains in plastids. Except for the sheath cells the other cells were typically parenchyma in nature (Figure 3(a)).
3.3. Ultrastructure Features & Secretory Cell Secretion Relationship
It is our contention that the content of the secretion of the secretory cavity was connected with the cell ultrastructure development stage. The ultrastructure features of the secretory cells possessed different characteristics during different the development stages. At the early development stage of the secretory cavity there were a number of plastids distributed in the secretory cells with a variety of cell shapes (oval, round, dumbbell, stick-like) (Figures 1 (h)-(i) and 2(a)). However with the development of the secretory cavity the shape of the plastids was generally round or oval with only a few cells being distorted or irregular in shape. At this stage a small number of long thin starch grains appeared in the stroma (Figures 2(b)- (d)) while at the same time osmiophilic materials near plastids were evident in the stroma (Figures 3(c)-(d)). The number of the layers of the flat and sacciform structure of Golgi body reduced and ranged closely together. The secretory vacuolars around the Golgi body were absent at the early stage (Figure 2(a)). However when the secretion levels increased the number of Golgi body and its vacuolar all increased and black osmiophilic substances increased inside the flat vesica of the Golgi body (Figure 3(a)). Mitochondrion was also found in the secretory cell at this stage. During the most productive secretory stage the cristae of mitochondrion were evident and the black osmiophilic materials were contained in its cytoplasm (Figure 2(h)). With the maturing of the secretory cavity, although there still were a large number of mitochondrions, the osmiophilic materials in stroma reduced (Figure 3(d)). Endoplasmic Reticulum (ER) was also present in the cytoplasm (Figure 2(i)) and was joined with plastids and plasmalemma (Figure 3(a)). The ER was concentrated in greater quantities in the cytoplasm of the secretory cell near the side of the cavity and the sheath cells near the side of the secretory cell (Figures 2(g) and 3(h)). The cell wall of the central secretory cell of the secretory cavity was filmy and possessed hoar stained by osmic acid (Figures 1(g)-(h)). When the intercellular cavity appeared the electron density of the opposite wall on the side of secretory cavity increased with black (Figures 2(e) and 3(h)), while the color of the outer tangential wall and the radial wall of the secretory cell was still uneven and fibrous. With the maturing of the secretory cavity, the electron density of secretory cell wall recovered to its normal level. However the cell wall was still thin (on the secretory cavity side) while the thickness of the outer tangential wall and the sheath cell wall increased (Figure 2(c)). The secretory cell wall facing the side of the cavity was reticular in appearance. After the secretions penetrated through the wall, they accumulated again and formed into a vacuolar structure (Figures 3(a)-(f)). An amount of the secretion moved to the center of the secretory cavity (Figure 3(g)) while the small vacuole secretions syncretized each other then converged with the central big vacuolized secretions.
4. Discussion
4.1. Origin & Development of Ginkgo biloba Secretory Cavities
As mentioned at the start of this paper the origin and development of the secretory cavities of the different organs of Ginkgo biloba has been in dispute up to now with the conclusions recently drawn using electron microscopy still inconsistent in theme. This study along with earlier related research has shown that the formation mode of the secretory cavities of stem, leaves, buds and
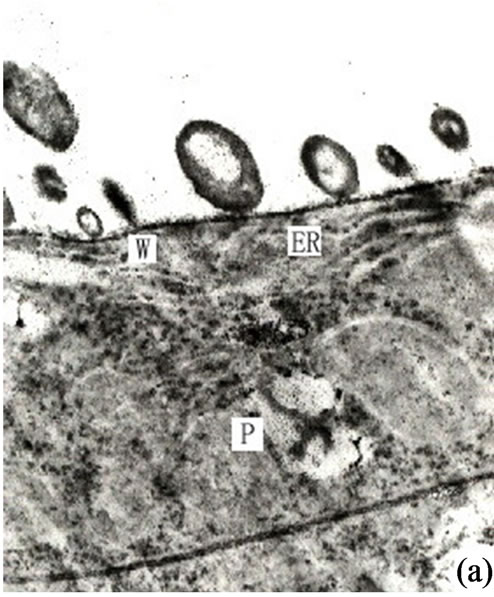
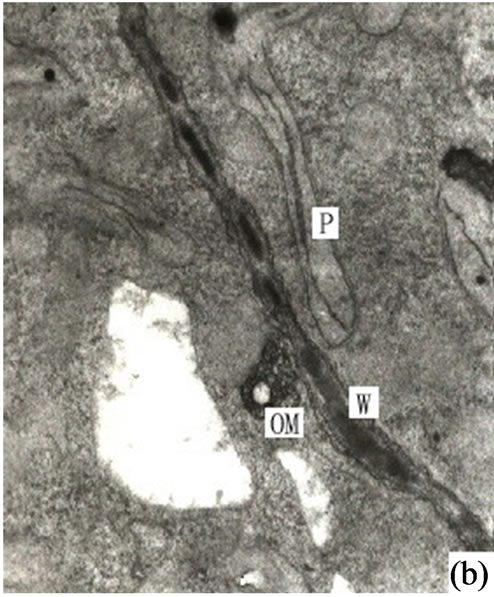
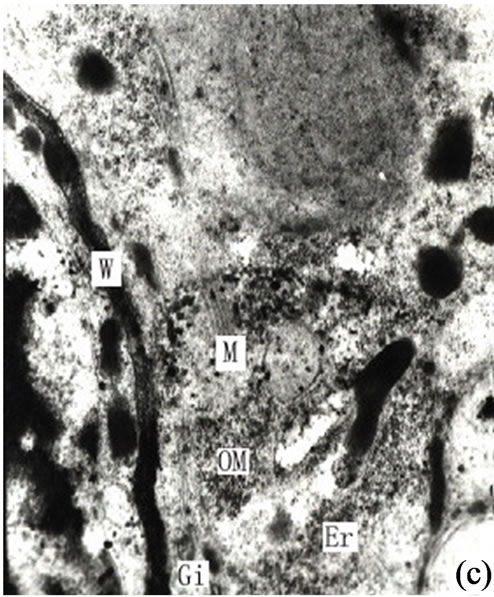
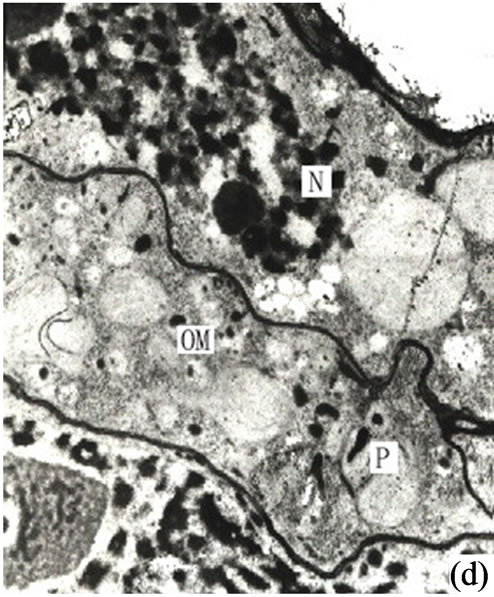
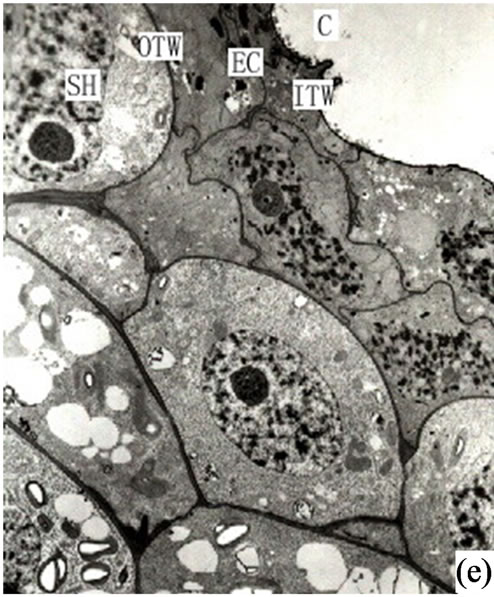

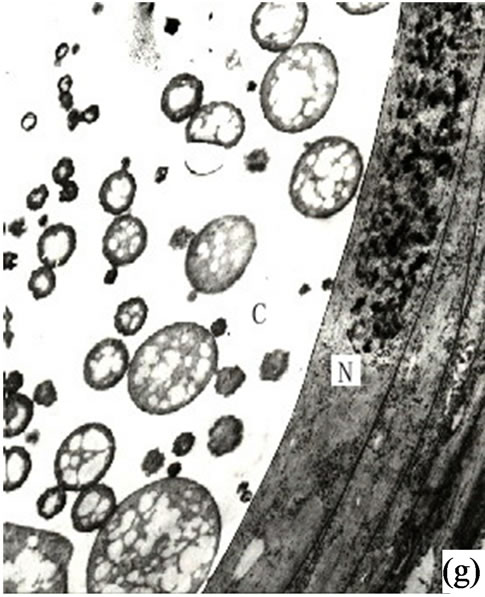
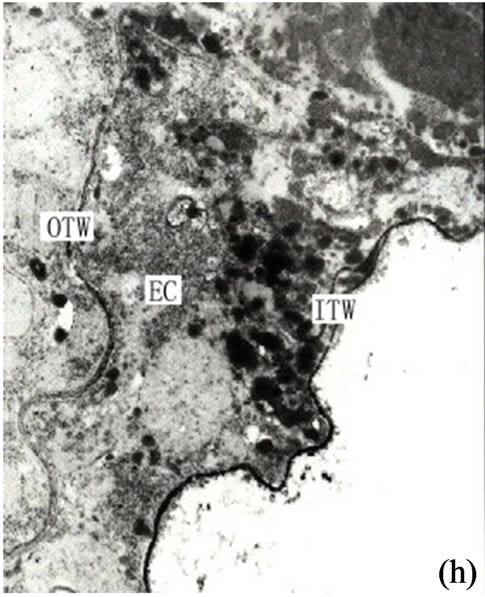
Figure 3. (a) Showing the secretions in endoplasmic reticulum, plastids of mature secretory cells and secretory cavity (×15000); (b) Showing the osmiophilic pellets in cell wall (×15000); (c) Showing the osmiophilic pellets near the Golgi body, endoplasmic reticulum and mitochondria (×20000); (d) Showing the osmiophilic pellets in the plastids (×10000); (e) Showing the out layer cell character of secretory cavity (×2000); (f) Showing the secretions directly percolate through the wall toward secretory cavity (×20000); (g) Showing a lot of secretions moving to secretory cavity (×4000); (h) Showing the different electronic density between the inner tangential wall and outer tangential wall (×800).
other organs in Ginkgo biloba was schizo-lysigeneous [4]. Our research leads us to the conclusion that the fundamental process relating to ultrastructure cell development is characterized by the separation of the central cells of the original cell clump from each other producing an intercellular space. This space is extended and enlarged in all directions causing a significant number of the central secretory cells to dissolve. These central secretory cells then degrade and separate off in the cell lumen.While some central secretory cells are degrading the remaining secretory cells are elongating tangentially with the outer sheath cells. As a result the secretory cavities are gradually enlarged. This conclusion correlates to the findings reported by Alain (1990). However Ameele (1980) reported that the cause of the secretory cavity of the microsporophyll in Ginkgo biloba was lysigneous. He contended that this resulted from the different formation modes of the various organs in Ginkgo biloba. Ameele reasoned that this was because different secretory cells in the same secretory cavity were in different states. While some secretory cells were in a state of disintegration others still remained in a normal state and continued to perform their secretory function. The conclusion of these findings was that the disorganized and disintegrating state of the central secretory cells was a normal and fundamental component of the development process of the secretory cell.
4.2. Synthesis of Secretory Cell Secretion
The chemical components of the secretions in the secretory cavity of Ginkgo biloba had been investigated by Scholz [5]. His findings were that the main secretions in Ginkgo biloba leaves were fats, lipids and resins. Appling histochemical analysis Ameele drew the conclusion that main sections were lipid compounds and pectin, and the substance of the secretory cavity would become black when met with osmic acid, as lipid compounds were of lipophilic characteristics [2]. Later research found that the lipophilic substances could be fixed by osmic acid when making electron microscope sections. Alain put forward that the theory that the synthesis of the lipophilic substance took place in the plastids and ER and was determined by the sedimentation positions of the osmiophilic substance when Ginkgo biloba tissues were fixed with osmic acid [1]. Through the observation of the secretory cavity of Ginkgo biloba it was evident that there was a significant amount osmiophilic substance in both the secretory and sheath cells. In addition the density of the latter was higher, and most of the osmiophilic substance was present in the cytoplasm on the side of the secretory cells (Figure 2(a)).The osmiophilic substance was distributed not only in the cytoplasm, but also in the plastids, mitochondrion, Golgi body, ER and small vacuole (Figures 3(b)-(d)). Analyzed microscopic photos illustrated that it was the plastids that was of closest correlation with the osmiophilic substance (which was the most affluent organelle in the secretory cells and sheath cells). Plastids were found in greater quantities in the original cells of the secretory cavities, with low density and a small amount of inner membrane (Figure 1(g)). With the enlargement of the secretory cavity the electron density of the plastids of the secretory cells increased causing some osmiophilic materials to appear. (As a side note the plastids where some osmiophilic materials were distributed were surrounded by ER). Simultaneously some starch grains and a large amount of osmiophilic substance appeared in the plastids of degrading cells (Figures 2(b)-(c)). When the secretory cavities matured a significant amount of starch grains appeared in the plastids, and the inner membranes disappeared. It was noted that Mitochondrions were always present in significant amounts throughout the whole development process of the secretory cavity. In particular they were found in the cytoplasm of the sheath cells near the secretory cells as well as in clusters on the side of the secretory cavity. (Figures 2(f)-(h) and 3(c)). Our contention is that the Mitochondrions offered energy for the degradation of the intercellular layers of the cell wall or the synthesis and transportation of secretions. The stroma of the mitochondrion contained osmiophilic materials (Figure 2(h)), which suggest that the mitochondrion were possibly involved in the synthesis of lipids. The ER was also distributed widely in the cytoplasm in the form of segments where there were osmiophilic materials. Finally it was also noted that there were also osmiophilic materials in the thylakoids of the Golgi body and nearby. We believe that the synthesis and transportation of the secretions of Ginkgo biloba is related to all organelles.
4.3. Mode of Secretion in Secretory Cells
Research into the submicroscopic structure of laticifer by Rhus, Zhao concluded that the raw lacquer was produced on the ER and entered the Golgi body in the form of droplets [6]. The raw lacquer was then packed and transported by Golgi body to the plasmalemma by the vacuolar of Golgi body and ER. Finally it was fused with the plasmalemma and released with the osmiophilic substance out of the plasmalemma. Later research stated that in all kinds of cell secreting lipids there were large amounts of ER which played a fundamental role in the synthesis and transportation of secretions [7-12]. Through observing the secretory cells of Ginkgo biloba it was found that ER was a key element in the transportation of not only the plastids but also plasmalemma, mitochondrion and Golgi body.
It is our contention that the secretions were synthesized in the plastids and transported to ambient ER through plasmalemma, then routed to the Golgi body in the form of vacuolar (Figures 2(f)-(g)) or directly transported to the plasmalemma (Figures 3(a)-(f)). The secretions were then fused with the plasmalemma and the osmiophilic materials were released to the area between the plasmalemma and the cell wall. Most of the osmiophilic droplets firstly decomposed into small granules. At the same time the cell walls on the side of the secretory cavity developed a loose fibril structure to support transport channels of the osmiophilic granules. Subsequently these granules permeated through the cell wall and entered into the secretory cavity by diffusion (Figures 3(a)-(f)). The granules were then reunited in the cavity to form a vacuolar structure which continuously moved to the center of the secretory cavity to form a bigger reticular structure surrounded by membrane (Figure 3(g)). In the electron microscopic photos the cell wall of the normal secretory cells and the parenchyma had the same electron density ranked in fibrous layers. When the density of these wall layers on the side of the secretory cavity increased small osmiophilic droplets (granules) could be observed. Except for secretory cells the cell wall had a high density of sheath cells containing plenty of osmiophilic substances (Figures 2(g) and 3(h)). This characteristic had been noticed when Wooding et al. (1963) studied the secretory cells of resin canal in Italian Pines [3]. Their research found that the walls on the side of the resins gradually developed a loose febrile mesh and the resin droplets of the plasmalemma passed through the cell walls by osmosis. Our own observations in Ginkgo biloba reached a similar conclusion. In summary the osmiophilic materials both in the secretory cells and sheath cells of secretory cavity in Ginkgo biloba are secreted by both diffusion and osmosis.
5. Acknowledgements
This work was supported by the National Science Foundation of China (No. 30872055). Thank Huang Jin-Sheng and Zhou Jian for their assistance.
REFERENCES
- C. Alain, G. Bourgeois and J. P. Balz, “The Secretory Apparatus of Ginkgo biloba: Structure, Differentiation and Analysis of the Secretions,” Trees, Vol. 4, No. 4, 1990, pp. 171-178. doi:10.1007/BF00225312
- R. J. Ameele, “Development Anatomy of Secretory Cavities in the Microsporophylls of Ginkgo biloba,” American Journal of Botany, Vol. 67, No. 6, 1980, pp. 912-917. doi:10.2307/2442432
- F. B. P. Wooding and D. H. Northcote, “The Fine Structure Resin Canals in Pinus pinca,” Journal of Ultrastructure Research, Vol. 13, 1963, pp. 233-244.
- F. R. Peng, J. Guo and J. Zou, “Anatomic Study on the Origin and Development of Secretory Cavities of Ginkgo biloba,” Journal of Nanjing Forestry University, Vol. 25, No. 4, 2001, pp. 64-68.
- R. Scholz, “Microchemical Studies of the Changes during Vernal Activity in Ginkgo biloba,” Journal of the Elisha Mitchell Scientific Society, Vol. 48, 1932, pp. 133-137.
- G. F. Zhao and Z. H. Hu, “Studies on the Relation of the Ultrastructure of the Secretory Cells of Laticiferous Canals and the Producing of Raw Lacquer in Toxicodendron Verniciflua,” Acta Botanica Sinica, Vol. 33, 1990, pp. 26-30.
- J. Benagoun and A. Fahm, “Intracellar Transport and Elimination of Resin from Epithelial Duct Cells of Pinus Balepensis,” Annals of Botany, Vol. 43, 1979, pp. 135- 142.
- W. H. Liu and Z. H. Hu, “The Secretory Structure of Hypericum Perforatum and Its Relation to Hypericin Accumulation,” Acta Botanica Sinica, Vol. 41, No. 4, 1999, pp. 369-372.
- H. F. Lv, W. H. Liu and Z. H. Hu, “Studies on the Relation between Secretory Structures and Its Secontary Product in Hypericum Sampsonii,” Acta Botanica Sinica, Vol. 19, No. 6, 1999, pp. 111-115.
- G. X. Shi, X. S. Xu and W. P. Chen, “Studies on Secretory Ducts in Stolons of Sagittaria Trifolia,” Acta Botanica Sinica, Vol. 8, 1998, pp. 92-98.
- A. Fahn and J. Benagoun, “Ultrastructure of Resin Ducts in Pinus balepensis: Development, Possible Sites of Resin Synthesis, and Mode of Its Elimination from the Protoplast,” Annals of Botany, Vol. 40, 1976, pp. 56-63.
- A. Fahn, “Secretorv Tissues in Plant,” Academic Press, London, 1979, pp. 217- 220.

