Pharmacology & Pharmacy
Vol.3 No.3(2012), Article ID:20612,7 pages DOI:10.4236/pp.2012.33043
Effect of Biejiajian Oral Liquid on the Expression of RAAS in Hepatic Fibrosis Rats
![]()
Pharmacy College of Zhejiang Chinese Medical University, Hangzhou, China.
Email: *ylyj@mail.hz.zj.cn
Received March 14th, 2012; revised April 26th, 2012; accepted May 29th, 2012
Keywords: Hepatic Fibrosis; RAAS; Biejiajian Oral Liquid; Serum Pharmacology; Rat
ABSTRACT
Objective: To study the effect of Biejiajian Oral Liquid (BOL) on the rennin angiotensin aldosterone system (RAAS) in plasma of hepatic fibrosis rats and in hepatic stellate cell (HSC) of normal rats. We explore the mechanism of BOL on inhibiting the activation of HSC and illustrate its mechanism of anti-hepatic fibrosis further. Methods: SD Rats were divided into 5 groups randomly: normal control group, model group, Enalapril group and BOL groups with different concentration (2.0 g/ml or 1.0 g/ml). Every group was administered with CCl4 and olive oil solution to induce hepatic fibrosis except normal one. Each group was treated with the respective drug for 5 weeks and then got the blood. The level of renin, angiotensin II and aldosterone in the plasma of liver fibrosis rats were detected by the radioimmunoassay. By using reverse transcription-polymerase chain reaction (RT-PCR) to measure the gene expression of the rennin, ACE, angiotensinogen, AT1R and ALD. The AT1R gene expression in normal HSC was determined by the immunohistochemical measurement. Results: BOL could effectively reduce the activity of the PRA, AngIIand ALD, which showed a significant effect on the inhibition of the AngII (P < 0.01). Meanwhile, compared with the normal control group, there was a notable inhibitory action on the PRA of HSC which was administrated by serum containing BOL (P < 0.05). And yet, drug applied group showed no difference with the model group for other factors of the RAAS. Conclusion: BOL can inhibit the expression of RAAS in the rat plasma and can inhibit the expression of the mRNA of renin in the normal HSC, which could be the mechanism of anti-hepatic fibrosis.
1. Introduction
Hepatic fibrosis is a common pathological process of chronic liver diseases, which character is the excess accumulation of the extracellularmatrix (ECM) with the main part of collagen in the liver. It’s the early and necessary stage of cirrhosis and can be reversed at certain conditions [1].
The occurrence of liver fibrosis has a close relationship with the hepatic stellate cell (HSC). The activation and proliferation of HSC as well as the increase of synthesis of ECM will eventually lead to the hepatic fibrosis [2]. Recent studies have shown that there is reninangiotensinaldosterone system (RAAS) in HSC. The appearance and development of hepatic fibrosis is closely related to the RAAS [3]. The local liver RAAS participates in the development of hepatic fibrosis [4]. Angiotensin II (Ang II) is one of the most important bioactive peptides, which is able to induce the activation of HSC, promoting the secretion of transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF) and other cytokines and increasing the expression of collagens in type I and II, and then to promote the formation of hepatic fibrosis [5-7]. Some studies have also shown that angiotensin-convertase inhibitor (ACEI) and Ang II receptor type 1 blocker (ARB) can significantly attenuated the development of hepatic fibrosis, and possibly become a potential new potent drug for hepatic fibrosis [8-10].
Our previous research had proved that BOL could anti-hepatic fibrosis effectively. Being observed under the electronic microscope, the fat droplets in liver cell of the high-dose BOL group decreased, the structure of nucleus was clear and the mitochondria were tumid lightly compared with the model group [11,12]. A further study is necessary on whether the action of BOL inhibiting the proliferation of HSC is concerned with the expression of RAAS in HSC. Our research aims to study the effect of BOL on the expression of RAAS in the plasma of rats with hepatic fibrosis and in normal HSC so as to illustrate its mechanism of anti-hepatic fibrosis further.
2. Materials and Methods
2.1. Animal Modeling and Samples Preparation
2.1.1. Animal Modeling
Sixty male Sprague-Dawley (SD) rats (provided by the Zhejiang Academy of Medical Sciences SCXKC-Zhe- 2003-0001, Zhejiang, China) were used in this study. Liver fibrosis was induced in rats using a protocol described previously [12].
The hepatic fibrosis rats were randomly divided into 4 groups i.e. model group (n = 12), Enalapril group (n = 12), BOL high dosage group (n = 13) and low dosage group (n = 13). The normal group and the model group ig 0.9% NaCl (NS) 10 ml/kg. The Enalapril group ig Enalapril 10 mg/kg. BOL low and high dosage group ig BOL 10 ml/kg (containing raw drug 1.0 and 2.0 g/ml respectively). All groups had been treated once a day for 5 weeks and took water and fodder ad lib. Venous blood was collected from the abdominal cavity vein of anesthetized rat intraperitoneal injection of 3% pentobarbital sodium at 40 mg/kg. Separated plasma was used to detect renin activity, level of Ang II and aldosterone by radioimmunoassay. Serum was separated to detect the activity of ACE.
2.1.2. Preparation of Different Concentration BOL
BOL was an improved preparation from Biejiajian Pill that was recorded in Jinkui Yaolue (Zhang Zhongjing, Eastern Han Dynasy). It contained 2 g/ml raw drug. The dosage for rats was 20 g/kg for high dosage and 10 g/kg for low dosage respectively.
2.2. The Preparation of the Serum Containing BOL
Thirty two male SD rats of 280 - 320 g were randomly divided into 4 groups i.e. NS group, Enalapril (10 mg/kg) group, groups receiving high (20 g/kg) and low (10 g/kg) dosages of BOL. The rat was ig twice a day and last for 7 days. Venous blood was collected from inferior vena cava under asepsis 1 h after the last ig, then serum was separated (3000 r/min, 20 min, 4˚C), inactivated at 56˚C for 30 min, and stored at –70˚C for use.
2.3. The Culture of HSC
The HSC was provided by Professor Zhishan Ding of College of Life Science at Zhejiang Chinese Medical University. The maintenance of HSC had been described previously [13].
2.4. Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
A total of 2 × 105/cm2 HSC were inoculated into culture flasks and incubated in DEME containing relative drug serum for 48 h. Total RNAs from HSC were isolated. Semi-Quantitative RT-PCR was performed using an oligo dT primer and SuperScript One-Step RT-PCR system (Invitrogen Corp), according to the manufacturer’s instructions. The following primer sets were used in the RT-PCR:
Agt (F 5’-GCACGACTTCCTGACTT-3’, R 5’-TGACGCTGGCCTTGT-3’);
ACE (F 5’-AAACATTGGCTACACTGTAAGT-3’, R 5’-TTCGTGGAGGAGTATGACC-3’);
Agtr1 (F 5’-GAGGCGGACTACTACGCCAA-3’, R 5’-TAGCGCCAGGAATTGTTGCT-3’);
Ren (F 5’-AAAGTTGCCCTGGTAATG-3’, R 5’-GTGCTAAAGGAGGAAGTGTT-3’);
Cyp11b (F 5’-CTCCTCGTCATATCCATCTG-3’, R 5’-GCAGCCAATCAGAAATGTGG-3’);
Actb (F 5’-ACTGCCGCATCCTCTTCCTC-3’, R 5’-ACTCCTGCTTGCTGATCCACAT-3’).
The mRNA levels of genes of interest were normalized to that of Actb mRNA expression.
2.5. Statistical Analysis
Data was reported as mean ± standard deviation (SD). Statistical analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) with the post-hoc Bonferroni adjustment was used for comparisons between multiple groups. In cases when there were only two groups for comparison, the two-tailed Student’s t test was used. All P values were two-sided and the level of statistical significance was set P < 0.05.
3. Results
3.1. The Effect of BOL on RAAS in Plasma of Hepatic Fibrosis Rats
Compared with normal control group, ACE (P < 0.05), PRA and Ang II (P < 0.01) increased significantly in the model group. It indicated that liver fibrosis model was established successfully. Compared with model group, after being treated once a day for 5 weeks, PRA, Ang II and ACE (P < 0.01) decreased significantly in Enalapril group. Ang II and ACE (P < 0.01), PRA and ALD (P < 0.05) decreased significantly in BOL high dosage group. PRA and Ang II (P < 0.05) also decreased significantly in BOL low dosage group. But ALD and ACE (P > 0.05) of BOL low dosage group didn’t decrease significantly (Table 1).
3.2. The Effect of BOL on RAAS in HSC
Compared with normal control group, expressions of mRNA of PRA, Prehypertensin, ACE, CYP (P < 0.05) and AT1R (P < 0.01) were down regulated significantly by administering with Enalapril. Expressions of mRNA of PRA were down regulated significantly both in BOL high and low dosage groups (P < 0.05), but there was no significant difference between them. Expressions of mRNA of Prehypertensin, ACE, CYP and AT1R in BOL high and low dosage groups showed no significant difference (P > 0.05) (Table 2).
3.3. Expression of AT1 Receptor in HSC
Immunohistochemical detection demonstrated that the expression of AT1R in HSC could be found. According to our result, there is no significant difference between normal control group and other administration groups (P > 0.05) (Table 3).
4. Discussion
4.1. The Effect of BOL on RAAS of the Hepatic Fibrosis Rats Plasma and the Normal HSC
4.1.1. The Effect of BOL on RAAS of the Hepatic Fibrosis Rats Plasma
Recent studies had demonstrated that there was independent RAAS in the liver, which could be abnormally stimulated during the hepatic fibrosis. There had positive correlation between Ang II level and the fibrotic degree [14]. This study demonstrated that in vivo experiment, the content of PRA, Ang II and ACE in plasma were obviously increased as the hepatic fibrosis forming, which showed a dramatic difference compared with the normal group (P < 0.01).
The above-mentioned indexes in positive control group were obviously reduced (P < 0.01), which was consistent with relative literature. In BOL high concentration group, the content of PRA, Ang II, ALD and ACE were all decreased, and PRA and ACE showed significant difference (P < 0.01). The content of PRA and Ang II in BOL low concentration group showed difference significantly (P < 0.05). These results hinted that inhibiting the expression of RAAS in plasma of rats was probably the mechanism of BOL to prevent the denaturation and necrosis of hepatic cells as well as anti-hepatic fibrosis.
BOL could significantly down regulate the RAAS and effect on multi-links in RAAS, blocking various links of RAAS activation effectively, which showed advantages of multitarget compared with AT-1 receptor antagonist Candesartan and ALD antagonist Antisterone [15,16].
4.1.2. The Inhibition Effect of BOL on Local RAAS in HSC of Normal Rats
HSC occupied for about 5 percent of the entire hepatic cells, which existed in Space of Disse. Under normal condition, HSC could promote the split and hyperplasia of hepatic cells and synthesize extracellular matrix [17-19]. HSC could also express CYPII β2, which was the key enzyme for synthesizing aldosterone and increases gradually in the formation of hepatic fibrosis [20].
This study had shown that there was an obvious inhibitive effect of serum containing BOL on the expression of rennin mRNA of normal HSC (P < 0.05), meanwhile, showing no significant difference on the expression of other genes of RAAS compared with normal group.
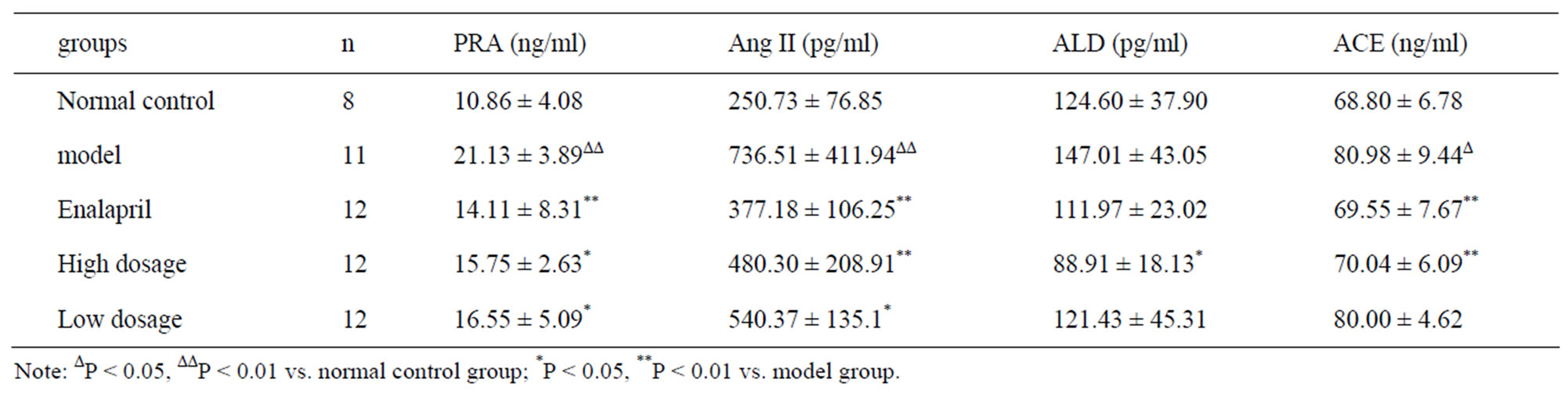
Table 1. The effect of BOL on PRA, Ang II, ALD and ACE in plasma of hepatic fibrosis rats (X ± S).
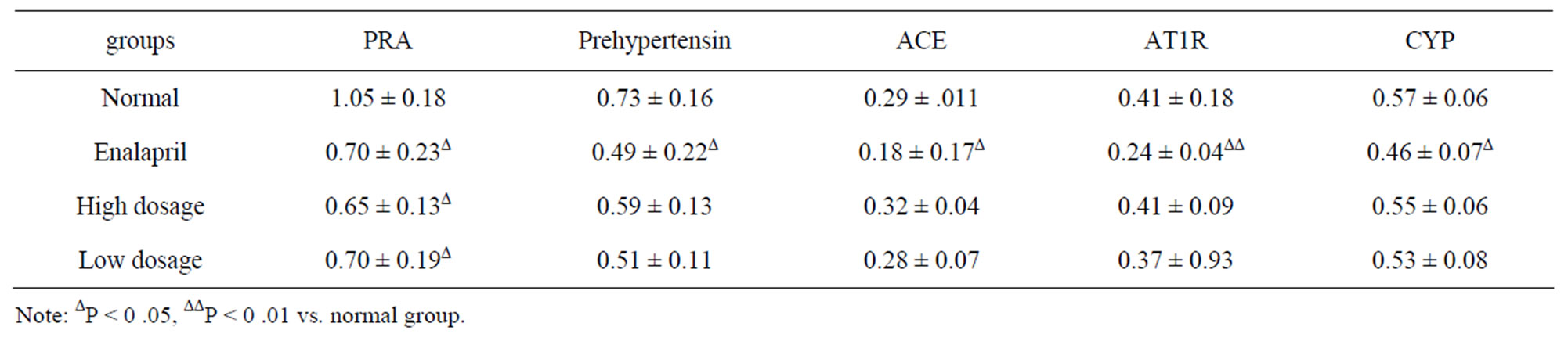
Table 2. The effect of BOL on expressions of RAAS mRNA in HSC (X ± S, n = 6).
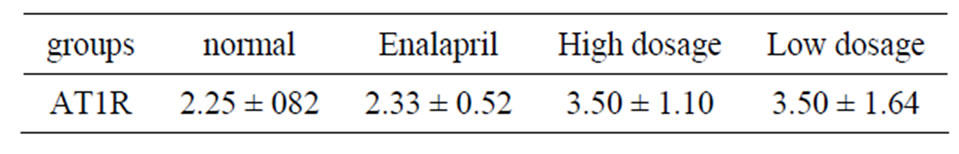
Table 3. Expression of AT1 receptor in HSC (X ± S, n = 6).
According to our study, the inhibitive effect of BOL on the signal pathway of RAAS of HSC was mainly focused on the process of synthesizing the rennin, thus blocking the development of hepatic fibrosis further [21-23]. BOL serum reduced the synthesis of rennin of RAAS in HSC, inhibiting the transformation of angiotensinogen induced by rennin, and might make further influence on the Ang II level, which ultimately inhibited the activation of HSC [24,25]. The effect of supporting the healthy energy of Traditional Chinese Medicine aimed to recover benign intracellular communication. For instance, any abnormity occurring in certain link of signal pathways might cause the uncontrollable development during the process of tumor generation. Traditional Chinese Medicine had the anti-tumor ability by influencing the cytokine and growth factor. This experiment revealed the target of BOL on depressing RAAS in HSC, which provided a novel way to explore theeffect of anti-hepatic fibrosis for Traditional Chinese Medicine [26].
However, BOL displayed an insignificant effect on other links of RAAS in HSC. The reasons might be interpreted as the following two aspects [27-30].
1) It was concerned with the two-way regulation effect of Traditional Chinese Medicine. The two-way regulation effect of Traditional Chinese Medicine could be considered as an integration role for multitarget in cellular signal pathways of multi-component in Chinese medicinal herbs, which mainly displayed in the aspects on immunization, endocrine, intestinal tract and temperature. Consequently, for HSC in various functional statuses, BOL might show different terminal effect. According to this experiment, the genes in positive control group were obviously inhibited (P < 0.05) while the genes in Chinese medicinal herbs groups were not obviously inhibited, which suggested that BOL had no effect on RAAS of normal HSC.
2) It was concerned with the effect time of BOL on HSC. In vivo study, the time for treatment of BOL was 5 weeks, which showed different down regulation effect on the expression of PRA, Ang II, ALD and ACE in plasma. Meanwhile, only the expression of rennin mRNA in normal HSC had been reduced by serum containing BOL. Thus, it was presumed that the regulation effect of BOL on expressions of indexes in RAAS of HSC was concerned with the effect time of serum containing BOL. Further experiment should be launched to explore whether serum containing BOL had the effect on regulating mRNA expression of angiotensinogen, ACE and AT1R in HSC as well as the influence from the drug action time.
4.2. The Mechanism of BOL on Anti-Hepatic Fibrosis by Regulating RAAS
RAAS performed a key role in formation of high blood pressure of human and animal model. Lately, the focus of interest on the role of RAAS in the pathophysiology of hypertension and organ injury had changed to a major emphasis on the role of the local RAS in specific tissues [31,32]. It was thought previously that RAAS belonged to endocrine system. As the development of molecular biology, rennin and some other compounds of RAAS had been discovered in extra-renal tissues and organs, which suggested that RAAS was not only a cyclical endocrine system, but also probably a general distributed paracrine, autocrine or endocrine system [33]. Recently, some studies on the endogenous RAAS had shown that angiotensin had direct effect on the proliferation of cells in artery and heart, which was achieved by different angiotensin in different tissues and organs [34]. Ang II receptor was the major receptor of angiotensin, which was classified into AT1 and AT2. AT1 performed a critical role in a variety of physiological functions and pathophysiological state [35-37]. It was considered that after binding with AT1 receptor, angiotensin regulated the phospholipase through GTP binding protein, hydrolyzing phosphatidylinositol-4,5-bisphosphate into diacylglycerol (DG) and inositol triphosphate (IP3), thus forming a set of second messenger system based on phosphoinositide, which showed close relationship with the regulation of cell proliferation [38]. Recently, it had been reported that the endogenous RAAS promoted fibrosis of myocardium, vessel and renal interstitium. It was proved that Ang II could stimulate the synthesis and deposit of extracellular matrix of vascular smooth muscle cell, resulting in vessel wall fibrosis, which inferred to the similar function in other organs [39]. Lim et al. got obvious effect by using AT-1 receptor antagonist Losartan to treat hypertrophic cardio myopathy rats. The expressions of mRNA of type I collagen and TGF-β were decreased more than 50% [40]. By now, the effect of RAAS on hepatic fibrosis had been paid more attention. Yoshiji et al., found that AT-1 receptor antagonist Candesartan could alleviate hepatic fibrosis of rats, reducing the expressions of serum markers of hepatic fibrosis and TGF-β mRNA [41]. Xishan Yang, et al., proved that the expression of aldosterone synthase gene CYPIIβ2 mRNA was enhanced during the formation of hepatic fibrosis [42]. Aldosterone antagonist Spironolactone had inhibitive effect on the early stage of hepatic fibrosis. AT-1 receptor was found on HSC, which had been confirmed by sequencing.
This study found that BOL could inhibit the expression of rennin mRNA in RAAS of normal HSC. Besides, it had inhibitive effect on several links of cyclical RAAS, inhibiting hepatic fibrosis through multitarget [34]. Consequently, the mechanism of BOL on inhibiting the expression of RAAS and anti-hepatic fibrosis was probably to reduce the activity of rennin in organs, blocking AT1 receptor and ALD receptor simultaneously, inhibiting the activity of ACE and reducing the Ang II level in liver, so as to inhibit the activation of HSC. BOL could decrease the level of Ang II and ALD in circulatory system. The reduction of Ang II and block of AT-1 receptor could effectively inhibit the secretion of TGF-β. TGF-β was the key factor for HSC on promoting proliferation and transforming into fibroblasts, which performed vital role in preventing and treating hepatic fibrosis and regulating the activity and function of target cells.
BOL was an improved formulation of Biejiajian pills recorded in Jinkui Yaolue (Zhang Zhongjing, Eastern Han Dynasy), having ability to reduce the content of Laminin (LN) and Hyaluronic Acid (HA) in hepatic fibrosis serum [11]. This study showed that RAAS in liver was significantly activated during the formation of hepatic fibrosis. After being administrated with BOL, the activity of RAAS in rat serum and the degree of hepatic fibrosis were obviously reduced; this indicated that local RAAS in liver participated in the occurrence of hepatic fibrosis.
BOL could effect on several links of RAAS. Compared with the single target drug AT-1 receptor antagonist Candesartan and ALD antagonist Spironolactone, BOL owned multitarget advantage, which showed better prospect for application and more suitable for hepatopath.
REFERENCES
- T. Lamireau, A. Desmouliere, P. Bioulac-Sage, et al., “Mechanisms of Hepatic Fibrogenesis,” Archives of Pediatrics, Vol. 9, No. 4, 2002, pp. 392-405. doi:10.1016/S0929-693X(01)00800-4
- S. Kanzler, A. W. Lohse, A. Keil, et al., “TGF-beta 1 in Liver Fibrosis: An Inducible Transgenic Mouse Model to Study Liver Fibrogenesis,” American Journal of Physiology, Vol. 276, No. 4, 1999, pp. 1059-1068.
- G. Paizis, R. E. Gilbert, M. E. Cooper, et al., “Effects of Angiotensin II Type 1 Receptor Blockade on Experimental Hepatic Fibrogenesis,” Journal of Hepatology, Vol. 35, 2001, pp. 376-385. doi:10.1016/S0168-8278(01)00146-5
- H. Yoshi Ji, J. Yoshii, Y. Ikenaka, et al., “Inhibition of Renin-Angiotensin System Attenuates Liver EnzymeAltered Preneoplastic Lesions and Fibrosis Development in Rats,” Journal of Hepatology, Vol. 37, 2002, pp. 22- 30.
- E. Albanis and S. L. Friedman, “Hepatic Fibrosis. Pathogenesis and Principles of Therapy,” Clinics in Liver Disease, Vol. 5, No. 2, 2001, pp. 315-334. doi:10.1016/S1089-3261(05)70168-9
- T. Lamireau, P. Desmouliere Bioulac-Sage, et al., “Mechanisms of Hepatic Fibrogenesis,” Archives of Pediatrics, Vol. 9, No. 4, 2002, pp. 392-405. doi:10.1016/S0929-693X(01)00800-4
- S. L. Friedman, “Molecular Regulation of Hepatic Fibrosis, an Integrated Cellular Response to Tissue Injury,” Journal of Biological Chemistry, Vol. 275, 2000, pp. 2247-2250. doi:10.1074/jbc.275.4.2247
- J. R. Jonsson, A. D. Clouston, Y. Ando, et al., “Angiotensin-Converting Enzyme Inhibition Attenuates the Progression of Rat Hepatic Fibrosis,” Gastroenterology, Vol. 121, 2001, pp. 148-155. doi:10.1053/gast.2001.25480
- H. S. Wei, D. G. Li, H. M. Lu, et al., “Effects of AT1 Receptor Antagonist, Losartan, on Rat Hepatic Fibrosis Induced by CCl4,” World Journal of Gastroenterology, Vol. 6, No. 4, 2000, pp. 540-545.
- R. Bataller, P. Gines, J. M. Nicolas, et al., “Angiotensin II Induces Contraction and Proliferation of Human Hepatic Stellate Cells,” Gastroenterology, Vol. 118, No. 6, 2000, pp. 149-1156. doi:10.1016/S0016-5085(00)70368-4
- L. Yao, Z.-M. Yao and Y. Tao, “Therapeutic Research of Biejia Jian Oral Liquid in Hepatofibrotic Rats,” Pharmacology and Clinics of Chinese Materia Medica, Vol. 18, 2002.
- L. Yao, Z.-M. Yao and Y. Tao, “Influence of BOL on Hyaluronic Acid, Laminin and Hyperplasia in Hepatofibrotic Rats,” World Journal of Gastroenterology, Vol. 7, No. 6, 2001, pp. 872-875.
- N. D. D’Ambrosio, J. L. Walewski, R. D. Clugston, et al., “Distinct Populations of Hepatic Stellate Cells in the Mouse Liver Have Different Capacities for Retinoid and Lipid Storage,” PLoS One, Vol. 6, No. 9, 2011, p. e24993. doi:10.1371/journal.pone.0024993
- M. Carlos and M. D. Ferrario, “Addressing the Theoretical and Clinical Advantages of Combination Therapy with Inhibitors of the Renin-Angiotensin-Aldosterone System: Antihypertensive Effects and Benefits beyond BP Control,” Life Science, Vol. 86, No. 9-10, 2010, pp. 289-299. doi:10.1016/j.lfs.2009.11.020
- C. W. Mende, “Application of Direct Renin Inhibition to Chronic Kidney Disease,” Cardiovascular Drugs and Therapy, Vol. 24, No. 2, 2010, pp. 139-149. doi:10.1007/s10557-010-6232-1
- T. K. W. Ma, K. K. H. Kam, B. P. Yan, et al., “ReninAngiotensin-Aldosterone System Blockade for Cardiovascular Diseases: Current Status,” British Journal of Pharmacology, Vol. 160, No. 6, 2010, pp. 1273-1292. doi:10.1111/j.1476-5381.2010.00750.x
- A. Leask, S. Q. Chen, D. Pala, et al., “Regulation of CCN2 mRNA Expression and Promoter Activity in Activated Hepatic Stellate Cells,” Journal of Cell Communication and Signaling, Vol. 2, No. 1-2, 2008, pp. 49-56. doi:10.1007/s12079-008-0029-z
- M. M. van Beuge, J. Prakash, M. Lacombe, et al., “Increased Liver Uptake and Reduced Hepatic Stellate Cell Activation with a Cell-Specific Conjugate of the Rhokinase Inhibitor Y27632,” Pharmaceutical Research, Vol. 28, No. 8, 2011, pp. 2045-2054. doi:10.1007/s11095-011-0430-9
- R. Raghow, “The Role of Extracellular Matrix in Postinflammatory Wound Healing and Fibrosis,” FASEB Journal, Vol. 8, 1994, pp. 823-831.
- Z.-Y. Ye, H. S. H. Houssein and R. I. Mahato, “Bioconjugation of Oligonucleotides for Treating Liver Fibrosis,” Oligonucleotides, Vol. 17, No. 4, 2007, pp. 349-404. doi:10.1089/oli.2007.0097
- F.-R. Yang, B.-W. Fang and J.-S. Lou, “Effects of Haobie Yangyin Ruanjian Decoction on Hepatic Fibrosis Induced by Carbon Tetrachloride in Rats,” World Journal of Gastroenterology, Vol. 16, No. 12, 2010, pp. 1458-1464. doi:10.3748/wjg.v16.i12.1458
- Y.-X. Zhou, J. Chen, J.-P. Li, Y.-L. Wang and X.-D. Jin, “Chinese Medicinal Herbs in Treating Model Rats with Hepatic Fibrosis,” African Journal of Traditional, Complementary and Alternative Medicines, Vol. 7, No. 2, 2010, pp. 104-108. doi:10.4314/ajtcam.v7i2.50862
- M. Buck and M. Chojkier, “A Ribosomal S-6 KinaseMediated Signal to C/EBP-β Is Critical for the Development of Liver Fibrosis,” PLoS One, Vol. 2, No. 12, 2007, p. e1372. doi:10.1371/journal.pone.0001372
- E. Novo and M. Parola, “Redox Mechanisms in Hepatic Chronic Wound Healing and Fibrogenesis,” Fibrogenesis Tissue Repair, Vol. 1, 2008, p. 5. doi:10.1186/1755-1536-1-5
- A. Sommerfeld, R. Reinehr and D. Häussinger, “Bile Acid-Induced Epidermal Growth Factor Receptor Activation in Quiescent Rat Hepatic Stellate Cells Can Trigger Both Proliferation and Apoptosis,” Journal of Biological Chemistry, Vol. 284, No. 33, 2009, pp. 22173-22183. doi:10.1074/jbc.M109.005355
- X. Shu, M. McCulloch, J. Gao, et al., “Chinese Herbal Medicine and Chemotherapy in the Treatment of Hepatocellular Carcinoma: A Meta-Analysis of Randomized Controlled Trials,” Integrative Cancer Therapies, Vol. 4, No. 3, 2005, pp. 219-229. doi:10.1177/1534735405279927
- S. John, C. B. Lubel, J. T. Herath, et al., “Angiotensin- (1-7), an Alternative Metabolite of the Rennin-Angiotensin System, Is Up-Regulated in Human Liver Disease and Has Antifibrotic Activity in the Bile-Duct-Ligated Rat,” Clinical Science, Vol. 117, 2009, pp. 375-386.
- R. M. Pereira, R. A. S. dos Santos, F. L. da Costa Dias, et al., “Renin-Angiotensin System in the Pathogenesis of Liver Fibrosis,” World Journal of Gastroenterology, Vol. 15, No. 21, 2009, pp. 2579-2586. doi:10.3748/wjg.15.2579
- G. Son, I. N. Hines, J. Lindquist, et al., “Inhibition of Phosphatidylinositol 3-Kinase Signaling in Hepatic Stellate Cells Blocks the Progression of Hepatic Fibrosis,” Journal of Hepatology, Vol. 50, No. 5, 2009, pp. 1512- 1523. doi:10.1002/hep.23186
- Y. Nishikawa, N. Ohi, A. Yagisawa, et al., “Suppressive Effect of Orthovanadate on Hepatic Stellate Cell Activation and Liver Fibrosis in Rats,” The American Journal of Pathology, Vol. 174, No. 3, 2009, pp. 881-890.
- J. M. Abrams and J. W. Osborn, “A Role for BenzamilSensitive Proteins of the Central Nervous System in the Pathogenesis of Salt-Dependent Hypertension,” Clinical and Experimental Pharmacology and Physiology, Vol. 35, No. 5-6, 2008, pp. 687-694. doi:10.1111/j.1440-1681.2008.04929.x
- A. Whaley-Connell, M. S. Johnson and J. R. Sowers, “Aldosterone: Role in the Cardiometabolic Syndrome and Resistant Hypertension,” Progress in Cardiovascular Diseases, Vol. 52, No. 5, 2010, pp. 401-409. doi:10.1016/j.pcad.2009.12.004
- R. H. Pichler and I. H. de Boer, “Dual Renin-Angiotensin-Aldosterone System Blockade for Diabetic Kidney Disease,” Current Diabetes Reports, Vol. 10, No. 4, 2010, pp. 297-305. doi:10.1007/s11892-010-0126-2
- W.-L. Wang, C. A. Haller, J. Wen, et al., “Decoupled Syndecan-1 mRNA and Protein Expression Is Differentially Regulated by Angiotensin II in Macrophages,” Journal of Cellular Physiology, Vol. 214, No. 3, 2008, pp. 750-756. doi:10.1002/jcp.21271
- K. Sasaki, et al., “Cloning and Expression of a Complementary DNA Encoding a Bovine Adrenal Angiotensin II Type-1 Receptor,” Nature, Vol. 351, 1991, pp. 230-233. doi:10.1038/351230a0
- J. H. Henriksen, M. Kiszka-Kanowitz and F. Bendtsen, “Review Article: Volume Expansion in Patients with Cirrhosis,” Journal of Alimentary Pharmacology & Therapeutics, Vol. 16, No. 5, 2002, pp. 12-23. doi:10.1046/j.1365-2036.16.s5.3.x
- C. Duvoux, D. Zanditenas, C. Hezode, et al., “Effects of Noradrenalin and Albumin in Patients with Type I Hepatorenal Syndrome: A Pilot Study,” Journal of Hepatology, Vol. 36, No. 2, 2002, pp. 374-380. doi:10.1053/jhep.2002.34343
- G. Paizis, M. E. Cooper, J. M. Schembri, et al., “Up-Regulation of Components of the Renin-Angiotensin System in the Bile Duet-Ligated Rat Liver,” Gastroenterology, Vol. 123, No. 5, 2002, pp. 1667-1676. doi:10.1053/gast.2002.36561
- M. Mifune, H. Sasaxnura, H. Shimizu, et al., “Angiotensin II Type 2 Receptors Stimulate Collagen Synthesis in Cultured Vascular Smooth Muscle Cells,” Hypertension, Vol. 36, No. 5, 2000, pp. 845-850. doi:10.1161/01.HYP.36.5.845
- D. Lim, S. Lutueuta, P. Bachireddy, et al., “Angiotensin II Bloekade Reverses Myoeardial Fibrosis in a Transgenic Mouse Model of Human Hypertrophic Cardiomyopathy,” Journal of Circulation, Vol. 103, No. 6, 2001, pp. 789- 791. doi:10.1161/01.CIR.103.6.789
- H. Yoshi Ji, J. Yoshii, Y. Ikenaka, et al., “Inhibition of Renin-Angiotensin System Attenuates Liver EnzymeAltered Preneoplastic Lesions and Fibrosis Development in Rats,” Journal of Hepatology, Vol. 37, No. 1, 2002, pp. 22-30.
- Y. Xi-Shan, L. Xu, W. Ping-Sheng, et al., “CYP II β2 Expression in Rat Liver and the Effect of Spironolactone on Hepatic Fibrogenesis,” Journal of Hormone Research, Vol. 33, No. 6, 2000, pp. 288-293.
NOTES
*Corresponding author.

