Surgical Science
Vol. 3 No. 5 (2012) , Article ID: 19340 , 5 pages DOI:10.4236/ss.2012.35055
Epithelial-Myoepithelial Carcinoma of Parotid Gland— A Surprising Clinical Entity
Post Graduate Institute of Medical Education and Research, Chandigarh, India
Email: drjayabakshi@ymail.com
Received February 13, 2012; revised April 17, 2012; accepted April 20, 2012
Keywords: Myoepithelial; Parotid; Carcinoma; Pleomorphic Adenoma
ABSTRACT
Objective: Objective of this study is to highlight the importance of considering clinical and radiological findings in parotid tumors before operating tumors with high suspicion of malignancy though reported as benign on histopathology. Material and Methods: 20 years old female patient underwent radical parotidectomy for epithelial-myoepithelial carcinoma of the left parotid gland which was diagnosed on FNAC. Contrast CT and contrast MRI also showed a tumor involving left parotid gland with destruction of ramus of mandible and loss of tissue planes medially & laterally. Facial nerve was infiltrated by tumor, thus sacrificed. Post operatively, patient had left facial palsy and was discharged after 7 days without any other sequelae. Postoperative histopathology showed pleomorphic adenoma with positive resection margins medially, superiorly & posteriorly. No further treatment was given in view of the benign nature of the tumor. Results: Patient was kept on monthly follow up and was disease free at one year follow up. Conclusion: Aggressive surgical treatment should be planned for malignant parotid tumors to give a better tumor free survival. Misdiagnosis can occur on FNAC as it can hit only one part of the tumor thus missing a malignant tumor or can interpret pleomorphic adenoma as epithelial-myoepithelial carcinoma.
1. Introduction
Pleomorphic adenoma is the most common neoplasm of both the major and minor salivary glands. It is composed of an array of tissue types, of both epithelial and mesenchymal origins, and is regarded as benign in clinical behavior, but has the potential to undergo malignant transformation. Epithelial-myoepithelial carcinoma (EMC) is a rare biphasic tumor of the salivary glands typically arising in the parotid. Fine needle aspiration cytology is widely used in the initial investigation of salivary gland swellings and whilst the cytological features of this tumour have been described they are not well recognized. Epithelial-myoepithelial carcinoma (EMC) is a rare type of malignant tumor, accounting for about 1% of all salivary gland tumors [1,2] and recognizes a typical biphasic pattern: a central ductular structure, often containing eosinophilic material surrounded by clear cells of myoepithelial origin. It was firstly described as a glycogenrich or clear cell adenoma because of the clear cell component. Donath et al. [3] noted that the myoepithelial component was an integral part of the tumor and in 1972 he introduced the term epithelial-myoepithelial carcinoma while describing this entity where he described the clinicopathological features of a case of epithelial-myoepithelial carcinoma of the parotid gland and highlighted the importance of awareness of this tumor in the differential diagnosis of biphasic tumors on Fine needle aspiration cytology. In 1991, the WHO recognized EMC as a distinct entity and subtype of salivary gland adenocarcinoma, and it became part of the new classification system [4]. It is currently felt that most malignant salivary gland tumors arise from either the excretory duct or the intercalated duct reserve cell. Either of these two cells have the potential for differentiation into a variety of epithelial tumor. EMEC arises most commonly in the parotid gland but has also been described in the submandibular gland, in the minor salivary glands and palate [4,5]. There is a female predominance, with a peak occurrence in the seventh decade [4]. EMEC is a lowgrade malignant tumor that may commonly recur locally after resection. Distant metastasis rarely occurs [3-5]. The lesion may be encapsulated, but the capsule may be incomplete and tumor nodules extend through it. Nuclear pleomorphism and metastasis are rare, although perineural and vascular invasion may occur. Because of cytological bland appearance; this tumor has been described as a type of adenoma. Prognosis is usually favorable.
2. Case Report
2.1. History
A 22 years old woman presented to our institution with a 6 months history of a painless swelling in the region of the left parotid gland. On examination, a 7 cm diameter, firm tumor mass was palpable in the gland. The growth was well demarcated, extending from zygomatic arch to angle of mandible with a smooth external appearance. It had a moderately firm consistency with fixity to neighboring tissues. It was non-tender with no associated facial weakness or cervical lymphadenopathy. The oral cavity was normal on inspection.
Ultrasonography done at another center revealed normal parotid glands with hypoechoic thickening with amorphous calcification seen deep to the gland. Fine needle aspiration done in private hospital showed the presence of round cells with moderate to abundant cytoplasm, which was vacuolated in few cells. Nuclei were round to oval with finely stippled cytoplasm. Some of the cells were binucleate. The cytological interpretation was not specific. Possibility of osteogenic, chondrogenic or reticuloendothelial cell origin was suggested.
Fine needle aspiration cytology done at our institute showed predominant scattered and occasional clusters of malignant cells, the tumor cells were round to oval with eccentrically placed nuclei, prominent nucleoli and moderate to abundant amount of basophilic cytoplasm. Many multinucleated giant cells, atypical mitotic figures were also noticed. Overall features were suggestive of myoepithelial, epithelial carcinoma (Figure 1).
MRI done at our institute-revealed a 7 × 5 × 5 cm lobulated soft tissue mass extending on both sides of left
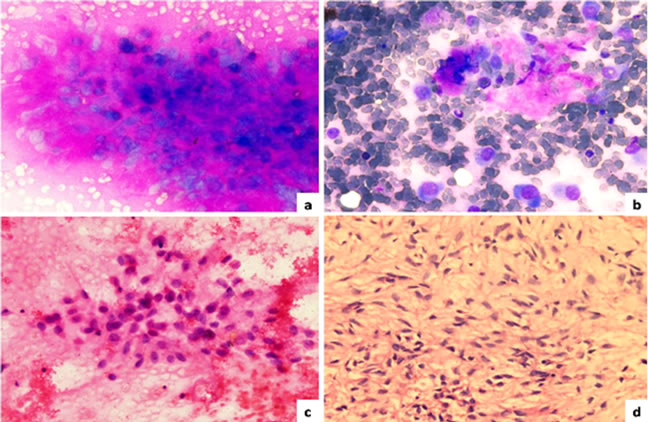
Figure 1. FNAC findings of left pre-auricular swelling; (a) Myoepithelial cells embedded in metachromatically staining chondromyxoid material (May Grünwald Giemsa stain 40×); (b) Scattered plasmacytoid type of myoepithelial cells (MGG 40×); (c) myoepithelial cells with eccentrically placed nuclei (Hematoxylin and Eosin 40×); (d) Cell block showing plasmacytoid to spindle cells in a myxoid background (H&E 40×).
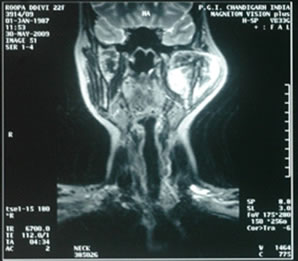
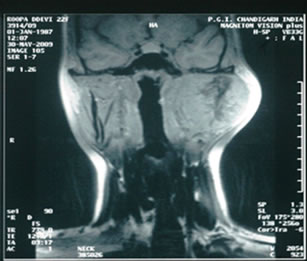
mandible, heterogeneously hyper-intense on T2 (Figure 2), iso-intense on T1 (Figure 3) showing sunray type of pattern with few hypointense areas suggestive of necrosis. Laterally the mass was displacing and compressing the parotid gland posterolaterally with maintained fat planes and masseter muscle anterolaterally, superiorly mass was involving both coronoid and condylar processes of mandible. Medially it was indenting the lateral wall of left parapharyngeal space with normal nasopharynx. No significant lymphadenopathy was seen. With a radiological impression of query Osteogenic carcinoma or Sarcoma.
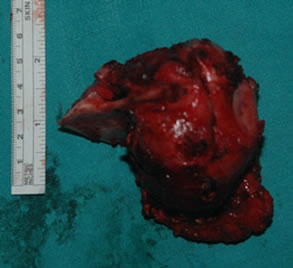
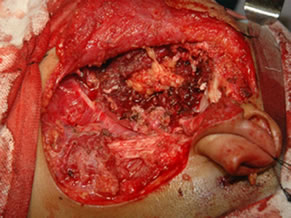
Under general anesthesia, 7 × 5 cm hard left parotid mass (Figure 4) was removed along with the submandibular gland and left level Ib lymphnode, the tumor was involving both superficial and deep lobe of the parotid with infiltration into the ramus of mandible from angle to coronoid process of mandible. It was extending up to zygomatic arch and medially into parapharyngeal space, with infiltration into the pterygoid muscles and masseter. Left TMJ joint was also infiltrated by the tumor and was removed. Tumor was removed completely with facial nerve and mandibulectomy was done 2 cm anterior to angle of mandible (Figure 5).
Macroscopically, the tumor was solid and well encapsulated, multilobulated with foci of hemorrhage. There was no capsular breach. Histopathologic examination showed that the encapsulated tumor was having an extensive chondromyxoid background, within which islands and trabeculae of tumor cells were present. Cells had
 (a)
(a)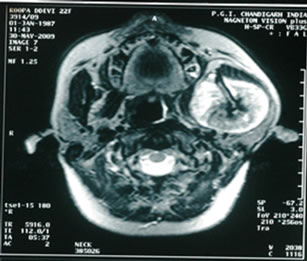 (b)
(b)
Figure 2. T2W images showing heterogeneously hyper-intense lobulated mass lesion involving the coronoid process of mandible.
 (a)
(a)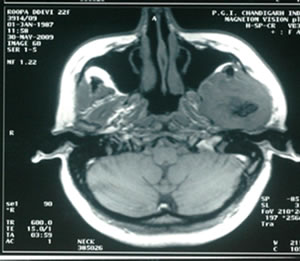 (b)
(b)
Figure 3. T1W images showing iso-intense lobulated mass with sunray appearance and hypo-intense areas suggestive of necrosis.
 (a)
(a)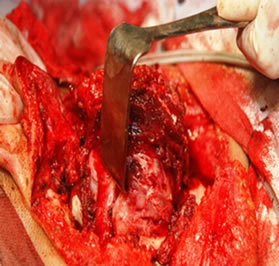 (b)
(b)
Figure 4. 7 × 5 cm hard mass involving both lobes of parotid and mandible. (a) Showing the tumour mass intra operatively; and (b) Showing the resected specimen.
 (a)
(a)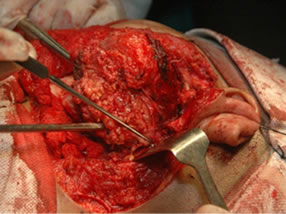 (b)
(b)
Figure 5. (a) Showing the facial nerve with skin hook as a pointer; and (b) Showing the bed after resection.
moderate amount of cytoplasm with peripherally pushed nucleus and fine nuclear chromatin. Numerous mitotic figures were seen with tumor infiltration into the underlying mandible, the medial, posterior and superior resection limits formed by the tumor, the inferior, lateral and anterior resection limits were free. Overall features were those of pleomorphic adenoma (Figure 6). The submandibular gland, lymph node and TMJ capsule were free of the tumor.
Postoperative period was uneventful with facial paralysis. There was no evidence of disease at one year follow-up.
3. Objective
To highlight the importance of considering clinical and radiological findings in parotid tumors before operating tumors with high suspicion of malignancy though reported as benign on histopathology.
4. Discussion
Approximately 1% of salivary gland tumors are epithelial myoepithelial carcinomas. Although they have predilection for parotid gland they may arise in minor salivary glands and rarely in extra oral sites such as the paranasal sinuses, pharynx, bronchus, palate. It is seen predominantly in elderly women with peak incidence in 7th decade.
Clinical presentation is not specific and is usually that of a long-standing and progressively enlarging painless mass. Facial nerve palsy occurs only rarely [6]. Though the tumor may appear well-circumscribed at surgery it is
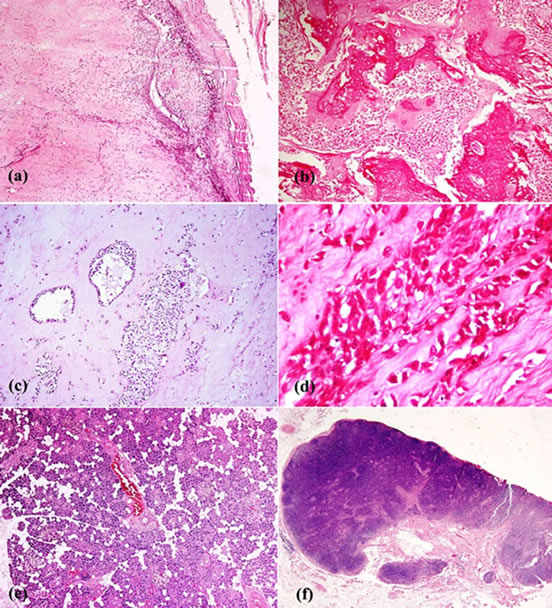
Figure 6. A well encapsulated (a) tumor arising in the deep lobe of the parotid gland, with large areas of osseous (b) and cartilaginous stroma (c) with scattered clusters and cords of epithelial cells (a), (b), (c), (d). The epithelial cells have scanty cyto-plasm, moderately pleomorphic nuclei and areas where cells show prominent nucleoli (d). There was no capsular invasion, mitosis or any necrosis. The superficial lobe ofthe parotid gland was normal (e) and the lymph nodes (f) showed only reactive hyperplasia.
not encapsulated and may be multinodular. Microscopically on histological examination the tumor is biphasic and characterized, as in this case, by tubules lined by an inner layer of cytokeratin-positive bland cuboidal epithetlial cells surrounded by an outer layer of S100 positive myoepithelial cells.
Characteristic extracellular globules of hyaline basement membrane material are present [7,8]. Typically the tumor nodules have an infiltrative margin and perineural and vascular invasion are present. In keeping with other biphasic tumors the diagnosis maybe difficult histologically and other salivary gland tumors such as pleomorphic adenoma, adenoid cystic carcinoma and also tumors with a predominant clear cell population such as clear cell carcinoma and sebaceous carcinoma should be considered before rendering the diagnosis. Fine needle aspiration cytology is a baseline investigative tool in the assessment of patients with salivary gland swellings. Whilst it is an accurate method of distinguishing neoplastic from non-neoplastic lesions it may not be possible to always accurately predict a specific tumor type due to the overlapping spectrum of cytological appearances found in a wide variety of salivary gland neoplasms. This can result in a differential diagnosis given in the cytology report in those tumors not characteristic of a specific entity. Four cell types are identified in myoepithelial salivary gland neoplasms: namely, plasmacytoid, spindle, epithelioid and clear cell types. Of these, the clear cell type is the least frequently encountered [9,10].
A review of the world literature in 2000 disclosed approximately 75 cases of myoepithelial carcinoma [11]. Some of these carcinomas originated de novo [11,12], whereas others were classified myoepithelial carcinoma expleomorphic adenoma [11-17] or as arising in benign myoepithelioma [11,16].
There are few reports on the cytological features of EMC [18-23] and in only one of these was the diagnosis considered prior to surgery [18]. A combination of three dimensional well-defined cell clusters, often surrounded by homogenous acellular material, clear cytoplasm in the peripheral cells and fragments of acellular hyaline nonfibrillary material have been suggested as being sufficiently characteristic to suggest the diagnosis on aspirates [19]. Histological features that may predict a worse prognosis include solid growth pattern, nuclear atypia, DNA aneuploidy and a high proliferative activity [7]. There are few reports on the effectiveness of different treatment modalities, although wide surgical excision is considered the treatment of choice [24]. Adjuvant radiotherapy maybe of use in preventing local recurrence [25,26]. The role of chemotherapy is uncertain.
5. Conclusion
This is an interesting case in which pleomorphic adenoma of the parotid mimicked as carcinoma clinically and radiologically. Though, epithelial-myoepithelial carcinoma is very rare salivary gland tumor, it should not be missed as under treatment may lead to recurrence and can reduce the survival in such patients. Radical surgery followed by radiotherapy remains the treatment of choice for this cancer.
REFERENCES
- J. G. Batsakis, A. K. El-Naggar and M. A. Luna, “Epithelial-Myoepithelial Carcinoma of the Salivary Glands,” Annals of Otology, Rhinology and Laryngology, Vol. 101, No. 6, 1992, pp. 540-542.
- T. A. Morrow, T. Chun and N. Mirani, “Epithelial Myoepithelial Carcinoma of the Parotid Gland,” Ear, Nose & Throat Journal, Vol. 69, No. 9, 1990, pp. 646-648.
- K. Donath, G. Seifert and R. Schmitz, “Zur Diagnose und Ultrastruktur des Tubularen Speichelgangkarzinoms,” Virchows Archiv, Vol. 356, No. 1, 1972, pp. 16-31. doi:10.1007/BF00543554
- G. Seifert and L. H. Sobin, “Epithelial-Myoepithelial Carcinoma, in World Health Organization International Histological Classification of Tumours,” Histological Typing of Salivary Gland Tumours, 2nd Edition, Springer-Veflag, Berlin, 1991, pp. 23-24.
- L. Barnes, B. N. Appel, H. Perez and A. M. El-Attar, “Myoepithelioma of the Head and Neck: Case Report and Review,” Oncology & Radiotherapy, Vol. 28, No. 1, 1985, pp. 21-28. doi:10.1002/jso.2930280107
- R. L. Corio, J. J. Sciubba, R. B. Brannon and J. G. Batsakis, “Epithelial-Myoepithelial Carcinoma of Intercalated Duct Origin. A clinicopathologic and Ultrastructural Assessment of Sixteen Cases,” Oral Surgery, Oral Medicine, Oral Pathology, Vol. 53, No. 3, 1982, pp. 280-287. doi:10.1016/0030-4220(82)90304-8
- V. Tralongo and E. Daniele, “Epithelial-Myoepithelial Carcinoma of Salivary Glands: A Review of Literature,” Anticancer Research, Vol. 18, No. 1998, pp. 603-638.
- R. H. W. Simpson, “Salivary Gland Tumours,” In: P. P. Anthony, R. N. M. MacSween and D. Lowe, Eds., Recent Advances in Histopathology, Churchill Livingstone, Edinburgh, 1997, pp. 167-190.
- M. Michal, A. Skalova, R. H. Simpson, V. Rychterova and I. Leivo, “Clear Cell Malignant Myoepithelioma of the Salivary Glands,” Histopathology, Vol. 28, No. 4, 1996, pp. 309-315. doi:10.1046/j.1365-2559.1996.d01-439.x
- G. L. Ellis and P. L. Auclair, “Tumors of the salivary glands,” In: J. Rosai and L. H. Sobin, Eds., Atlas of Tumor Pathology, Armed Forces Institute of Pathology, Washington DC, 1995. pp. 337-343.
- A. T. Savera, A. Sloman, A. G. Huvos and D. S. Klimstra, “Myoepithelial Carcinoma of the Salivary Glands: A Clinicopathologic Study of 25 Patients,” American Journal of Surgical Pathology, Vol. 24, No. 6, 2000, pp. 761-774. doi:10.1097/00000478-200006000-00001
- T. Nagao, I. Sugano, Y. Ishida, Y. Tajima, O. Matsuzaki, A. Konno, et al., “Salivary Gland Malignant Myoepithelioma: A Clinicopathologic and Immunocytochemical Study of Ten Cases,” Cancer, Vol. 83, No. 7, 1998, pp. 1292-1299.
- S. Di Palma and M. Guzzo, “Malignant Myoepithelioma of Salivary Glands: Clinicopathological Features of Ten Cases,” Virchows Archiv, Vol. 423, No. 5, 1993, pp. 389-396. doi:10.1007/BF01607152
- M. E. Tortoledo, M. A. Luna and J. G. Batsakis, “Carcinomas Ex Pleomorphicadenoma and Malignant Mixed tumors: Histomorphologic Indexes,” Archives of Otolaryngology, Vol. 110, No. 3, 1984, pp. 172-176. doi:10.1001/archotol.1984.00800290036008
- R. Singh and R. A. Cawson, “Malignant Myoepithelialcarcinoma (Myoepithelioma) Arising in a Pleomorphic Adenoma of the Parotid Gland. An Immunohistochemical Study and Review of the Literature,” Oral Surgery, Oral Medicine, Oral Pathology, Vol. 66, No. 1, 1988, pp. 65-70. doi:10.1016/0030-4220(88)90069-2
- L. Alos, A. Cardesa, J. A. Bombi, C. Mallofre, A. Cuchi and J. Traserra, “Myoepithelial Tumors of Salivary Glands: A Clinicopathologic Immunohistochemical, Ultrastructural, and Flow-Cytometric Study,” Seminars in Diagnostic Pathology, Vol. 13, No. 2, 1996, pp. 138-147.
- V. Tralongo, V. Rodolico, F. Burruano, S. Tortorici, A. Mancuso and E. Daniele, “Malignant Myoepithelioma of the Minor Salivary Glands Arising in a Pleomorphic Adenoma,” Anticancer Research, Vol. 17, No. 4A, 1997, pp. 2671-2675.
- V. K. Arora, K. Misra and A. Bhatia, “Cytomorphologic Features of the Rare Epithelial-Myoepithelial Carcinoma of the Salivary Gland,” Acta Cytologica, Vol. 34, No. 2, 1990, pp. 239-242.
- R. Carillo, E. Poblet, A. Rocamora and J. L. RodriguezPeralto, “Epithelial-Myoepithelial Carcinoma of the Salivary Gland. Fine Needle Aspiration Cytologic Findings,” Acta Cytologica, Vol. 34, No. 2, 1990, pp. 243-247.
- G. Kocjan, C. Milroy, E. W. Fisher and J. W. Eveson, “Cytological Features of Epithelial-Myoepithelial Carcinoma of Salivary Gland: Potential Pitfalls in Diagnosis,” Cytopathology, Vol. 4, No. 3, 1993, pp. 173-180. doi:10.1111/j.1365-2303.1993.tb00083.x
- L. J. Layfield and B. J. Glasgow, “Aspiration Cytology of Clear-Cell Lesions of the Parotid Gland: Morphologic Features and Differential Diagnosis,” Diagnostic Cytopathology, Vol. 9, No. 6, 1993, pp. 705-711. doi:10.1002/dc.2840090621
- T. Wax and L. J. Layfield, “Epithelial-Myoepithelial Cell Carcinoma of the Parotid Gland: A Case Report and Comparison of Cytologic Features with Other Stromal, Epithelial and Myoepithelial Cell Containing Lesions of the Salivary Glands,” Diagnostic Cytopathology, Vol. 14, No. 4, 1996, pp. 298-304. doi:10.1002/(SICI)1097-0339(199605)14:4<298::AID-DC4>3.0.CO;2-C
- C. J. R. Stewart, S. Hamilton, I. L. Brown and K. Mackenzie, “Salivary Epithelial-Myoepithelial Carcinoma: Report of a Case Misinterpreted as Pleomorphic Adenoma on Fine Needle Aspiration (FNA),” Cytopathology, Vol. 8, No. 3, 1997, pp. 203-209. doi:10.1046/j.1365-2303.1997.4375043.x
- D. P. C. Lau, M. J. Goddard, I. D. Bottrill and D. A. Moffat, “Epithelial-Myoepithelial Carcinoma of the Parotid Gland. An Unusual Cause of Ear Canal Stenosis,” Journal of Laryngology & Otology, Vol. 110, No. 5, 1996, pp. 493-495. doi:10.1017/S0022215100134085
- R. H. Simpson, T. J. Clarke, P. T. Sarsfield and P. G. Gluckman, “Epithelial-Myoepithelial Carcinoma of Salivary Glands,” Journal of Clinical Pathology, Vol. 44, No. 5, 1991, pp. 419-423. doi:10.1136/jcp.44.5.419
- M. A. Luna, N. G. Ordonez, B. Mackay, J. G. Batsakis and O. Guillamondegui, “Salivary Epithelial-Myoepithelial Carcinomas of Intercalated Ducts: A Clinical, Electron Microscopic, and Immunocytochemical Study,” Oral Surgery, Oral Medicine, Oral Pathology, Vol. 59, No. 5. 1985, pp. 482-490.

