Advances in Microbiology
Vol.3 No.4(2013), Article ID:35536,9 pages DOI:10.4236/aim.2013.34053
Nutritional, Microbiological and Sensory Characteristics of Malted Soy-Kunu Zaki: An Improved Traditional Beverage
Department of Food Science and Technology, Bells University of Technology, Ota, Nigeria
Email: *bis_adek@yahoo.com
Copyright © 2013 Aminat O. Adelekan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 18 March, 2013; revised 8 April, 2013; accepted 8 May, 2013
Keywords: Nutritional; Microbiological; Malting; Sensory; Kunu-Zaki
ABSTRACT
The nutritional, microbiological and sensory characteristics of improved kunu-zaki produced from malted cereals enriched with malted soymilk at different substitution levels (0% - 30%) were investigated. Enrichment with soymilk and malting increased the protein, amino acid, ash and moisture content of the improved kunu-zaki. Malting resulted in a decrease in carbohydrate and fat content. The protein content of the malted samples ranged between 2.79% and 3.82% while that of unmalted was 2.36%. There was decrease in the concentration of phytic acid and trypsin inhibitor after malting but the concentration increased as soymilk was added. Microbiological analyses showed that microbial load of samples increased with the addition of soymilk. There was a significant difference (p < 0.05) in the sensory attributes, acceptability decrease with increase in soymilk addition because of the beany flavor.
1. Introduction
Kunu, a traditional non alcoholic beverage made mainly from millet, is of low viscosity and has a sweet-sour taste, milky cream in appearance and is popular with people of northern Nigeria [1]. Kunu is consumed on its own by adults as a thirst quencher or serves as refreshment in some communities. It is sometimes used as a weaning drink for infants. However, since this drink is produced from cereals, its protein is incomplete and needed to be supplemented [2]. The major important cereals which are used in the preparation of kunu are millet, maize, guinea corn, sorghum and rice. During the preparation of kunu, the ingredients needed are ginger (zingiber officinales), Aligator pepper (Afromonium melegueta), red pepper (Capsicum species), black pepper (Piper guinense) and kakandoru or Eru. All these ingredients perform one function or the other in the course of the preparation. The most abundant constituent of kunu is water and it acts as the medium in which all other constituents are dissolved and contain only traces amount of inorganic substances. The nutritive value of kunu is highly due to the presence of protein, carbohydrates and some vitamin especially the vitamin B [3].
Kunu-zaki however seem to be highly nutritious with relatively low cost of production. It is being prepared from local cereals which are very common and part of our stable food substances. Kunu produced from malted millet, a portion of the cereal is malted, dried, ground and then mixed with the uncooked portion. The mixture is then added to the cooked portion and stirred vigorously and allowed to ferment [4]. The hydrolytic enzyme (amylase) in the malted cereal aids in digesting the thick slurry thereby converting the complex carbohydrate to simple sugars. The final product is usually sweet. [4], reported that kunu-zaki produced with the addition of ground malted rice to either millet, sorghum or maize resulted in 33%, 44%, 63% increases in crude protein, fats and carbohydrate. Kunu is taken after a meal as a supplement or to quench thirst. It is widely accepted as food drink in some urban centre especially in the Hausa land [5]. It is greatly consumed by a large number of people ranging from market women and men including young and old at home. The quality and quantity of the products depend largely on the quality of the ingredients and its proper handling in the course of production by the producer. The products could be obtained qualitatively after 2 days and it could be stored for another 3 days when refrigerated [3].
Processing can play a prominent role in preventing postharvest losses. It may actually be seen as the most important component of any nation’s agro-industry. The establishment of processing facilities is, in itself, a first essential step in the stimulation of both consumer demand for the processed product, and of adequate supply of raw materials for processing. Processing of local food drinks is also governed by these principles. It can be observed that the volume of such drinks being produced at the cottage level seems to be relatively small, when compared with the potentials available.
Kunu is mostly taken by low class people and the beverage is becoming widely acceptable throughout Nigeria, so this research is embarked upon to improve the nutritional value and acceptability of this product in order to meet the daily dietary requirements of the consumers. Kunu-zaki is a cereal based product which is low in protein content, malting and fortification with soybeans will improve the nutritional quality of the beverage.
2. Materials and Method
The raw materials needed for this study were purchased at Sango market in Ogun State. The packaging material (PET) bottle was purchased from Poly Product Ota, Ogun State. All other chemicals and reagents used in this study were sourced from the Food processing and Central Research Laboratories of Bells University of Technology, Ota, Ogun State.
2.1. Production of Malted Cereals and Legumes
The flow chart for the production of malted cereals and legume is shown on Figure 1. The grains (millet, sorghum and soybeans) were washed separately with tap water and steeped for about 18 hours and drained. The drained grains were spread on a jute bag and kept in a

Figure 1. Flowchart for production of malted cereals.
dark cupboard ,watered at intervals for about 3 days at ambient temperature (25˚C - 30˚C) to germinate. The malted grains (millet sorghum and soybeans) were kilned at about 50˚C - 65˚C. After kilning, the grains were rubbed in between the palms to remove the rootlet.
2.2. Production of Soy Flour
Soy beans (3000 g) were steeped overnight. The rehydrated beans were rubbed in between the hand to remove the hull and washed with clean water. The bean seeds were drained and boiled for about 30 minutes at 100˚C and dried in the oven at 60˚C to obtain the unmalted soy bean. It was milled into flour and packaged. The malted soybeans (2350 g) were also milled and packaged for further processing. The flow chart is shown on Figure 2.
2.3. Soymilk Preparation
The soy flour obtained was processed into soymilk. The ratio of water to flour on a weight basis was 10:1. The resulting slurry was then brought to boil for 10 minutes. Then the mixture was filtered through a muslin cloth to remove the filter cake in order to obtain clear soy milk. The ratio of blended malted cereal and malted soymilk is shown on Table 1.
2.4. Preparation of Kunu-Zaki
The flow chart for preparing conventional kunu-zaki is shown on Figure 3. Sand and other solid impurities were removed from the cereals. Then it was soaked for about 48 hours after which the ingredients such as ginger, gar-

Figure 2. Flowchart for the production of soy flour.
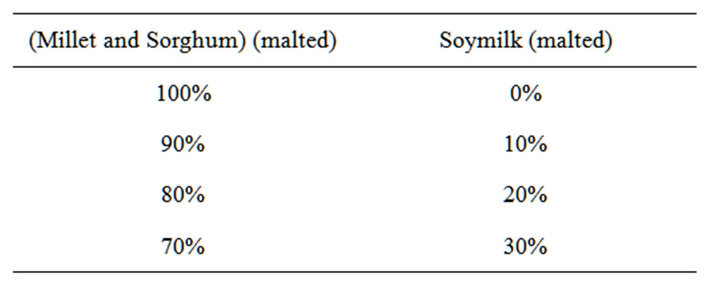
Table 1. Ratio of blended malted cereal and malted soymilk (%).

Figure 3. Flow chart for the production of conventional kunu-zaki.
lic, cloves and red pepper were added. It was grinded very well in a hygienic way and sieve with a very clean muslin cloth. It was allowed to settle down and the filtrate was fermented for 24 hours, during which the slurry was allowed to settle and sediment. The supernatant liquid was decanted and the residue was retained. The residue was divided into two. Two-third of the residue was boiled and the one-third was poured into it to produce kunu-zaki. Adequate water was added to meet the satisfactory level of the consumer i.e., not too watery and not too thick. The samples were sieved again in order to remove the soluble material. Sugar was added and was left to ferment for 8 hours. The improved kunu-zaki was mixed with the soymilk at different percentage and is ready for consumption. The malted cereal grains were also used in preparation of kunu-zaki as shown on Figure 4.
3. Physico-Chemical Analyses of Improved Kunu-Zaki
3.1. Proximate Analyses
The proximate analyses were carried out according to the method described by AOAC [6].
3.2. Determination of Total Titrable Acidity
This measures the acidity in samples. It was determined according to the procedure described by AOAC [7]. 10 ml of the sample was measured into a dried and weighed conical flask, and 50 ml of distilled water was added, followed by 0.5 ml phenolphthalein indicator and titrated against 0.1 M NaOH. The end point is reached when a faint pink colour is obtained.
3.3. Determination of Specific Gravity
This was determined using a density bottle. The samples were poured into a 50 ml density bottle and weighed. This is known as the mass. The mass was divided by the volume of the density bottle, to get the density.
3.4. Determination of Amino Acids Profile
Amino acid profile was carried using the ninhydrin colorimeter method of analysis of Rosen [8]. The extract was suitably diluted to 1 ml of this was added, 0.5 ml cyanide acetate buffer and 0.5 ml of 3% ninhydrin solution in methyl cellosolve. The mixtures was heated for 15 minutes in 100˚C water bath thereafter 5 ml isopropyl alcohol water mixtures as added and shaken vigorously. After cooling, the colour was read in a colorimeter at 570 nM. The concentration of amino acids was calculated from standard graph based on known concentration of various amino acids
3.5. Determination of Mineral Content
The dry ashing procedure was used for mineral content determination [6]. Five (5) grams of each of the samples were accurately weighed into porcelain crucibles and pre-ashed until the sample was completely charred on a hot plate. The pre-ashed samples were thereafter ashed in the muffle furnace at 500 degrees Celsius till the ash was white for about 2 hours. After ashing, the crucibles were transferred into the desiccator to cool and the reweighed. Each sample was quantitatively transferred into volumetric flasks by carefully washing the crucibles with 1 ml nitric acid, then with portions of dilute nitric acid.
All washings were transferred to individual volumetric flasks, repeating the washing procedure twice. The solutions were diluted to volume with deionized water, and
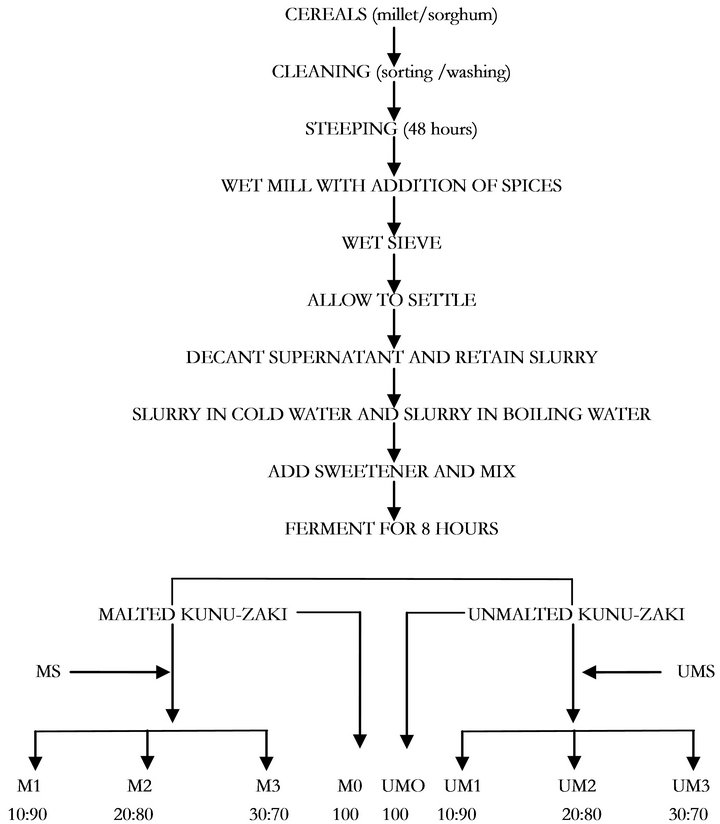
Figure 4. Flowchart for the Production of improved Kunu-Zaki. Legend: M1 = Malted kunu-zaki + malted soymilk (90:10) %; M2 = Malted kunu-zaki + malted soymilk (80:20) %; M3 = Malted kunu-zaki + malted soymilk (70:30) %; M0 = Malted kunu-zaki (100)%; UM1 = Unmalted kunu-zaki + unmalted soymilk (90:10) %; UM2 = Unmalted kunu-zaki + unmalted soymilk (80:20) %; UM3 = Unmalted kunu-zaki + unmalted soymilk (70:30) %; UM0 = Unmalted kunu-zaki (100) %; MS = Malted soymilk; UMS = Unmalted soymilk.
were used for individual mineral determination using the appropriate standards and blank. The content of the minerals; Calcium, Magnesium, and Iron were determined with the Atomic Absorption Spectrophotometer (Buck Scientific, Model 210).
3.6. Determination of Nutritional Inhibitors Determination of Phytate
The method described by AOAC [7] was used for phytate determination. Sample (2 g) was weighed into 250 mls conical flask. 100 mls of 2% concentrated hydrochloric acid was used to soak each of the samples in conical flasks for 3 hours and then filtered through a double layer filter paper. 50 mls of each of the sample filtrates was placed in 250 ml beakers and 107 mls of distilled water was added to each of the samples to improve proper acidity. 10 mls of 0.3% Ammonium Thiocyanate solution was added to each sample solution as indicator and titrated with standard iron (III) chloride solution which contained 0.00195 g iron per ml. The end point was signified by brownish-yellow coloration that persisted for 5 minutes.
3.7. Determination of Trypsin Inhibitor
Samples (0.2 g) were weighed into screw cap centrifuge tubes. 10 mls of 0.1 Molar phosphate buffers were added into each and contents were shaken at room temperature for 1hour on a UDY shaker. The suspension obtained was thereafter centrifuged at 500 rpm for 5 minutes and filtered through Whatman No. 42 filter paper. The volumes of each were adjusted to 2 mls with phosphate buffer and the test tubes were placed in water bath maintained at 37 degrees Celsius. 6 mls of 5% TCA Solution was added to an empty tube to serve as the blank. 2 mls of casein solution was added to each of the tubes initially placed in the water bath followed by incubation for 20 minutes. 6 mls of TCA solution was added into the sample tubes 20 minutes after (so as to stop the reaction) and shaken. The reaction was allowed to proceed for 1 hour at room temperature and the mixture was filtered through Whatman No. 42 filter paper. The absorbance of sample filtrates and trypsin standard solutions were then read at 280 nm.
3.8. Microbiological Evaluation
3.8.1. Bacterial Load
Spread plate technique as described by Palczar and Chan, [9] was used. 1 ml of each sample was aseptically transferred to 9 ml of sterile water in a separate tube and mixed vigorously. 1 ml of the resulting mixture was transferred to 9 ml of sterile water in a separate tube. The process was continued until the 6th diluents (10−6). Nutrient Agar (NA) was inoculated with a 0.1 ml of appropriately diluted kunu-zaki (10−6) by spread-plating technique and incubated at 37˚C for 24 hours. Colonies were counted and multiplied by the dilution factor.
Bacterial load (cfu/g) = N × × D Where:
× D Where:
N = Numbers of colonies counted.
V = Volume of inoculums D = Dilution factor
3.8.2. Fungi Load Count
The fungal load was determined in a similar way as the bacterial load. However, Potato Dextrose Agar was used using spread plate technique. 0.1 ml of the 4th diluents was used and incubated 37˚C.
Fungi load (cfu/g) = N × × D Where:
× D Where:
N = Numbers of colonies counted.
V = Volume of inoculums D = Dilution factors
3.9. Sensory Evaluation
Evaluation was done by 20 judges selected randomly from Bells University of Technology Ota, Ogun. The nine (9) point hedonic scale was used (score “9” having excellent attribute and Score “1” indicating extremely dislike) Samples were coded with three digit random numbers and presented in random order. The characteristics evaluated, were appearance, taste, colour, mouth feel and overall acceptability [10]. The responses were statistically analyzed via analysis of variance (ANOVA).
4. Results and Discussion
4.1. Effect of malting and Soymilk Addition on the Proximate Composition of Kunu-Zaki
The proximate composition of the improved kunu-zaki is as presented on Table 2. The protein content of the improved kunu-zaki ranged from 2.36% to 3.90%, which shows significant difference among the samples (p< 0.05). Protein content of Kunu-zaki produced from malted cereals (millet and sorghum) and malted soymilk ranges from 2.79% to 3.90%, while the protein content of kunu-zaki produced from unmalted cereals and unmalted soymilk ranges from 2.47% to 3.82%. This was due to the effect of malting which increased the protein content and this was in accordance to Wang and Fields [11] that malting is a traditional processing technology is used to
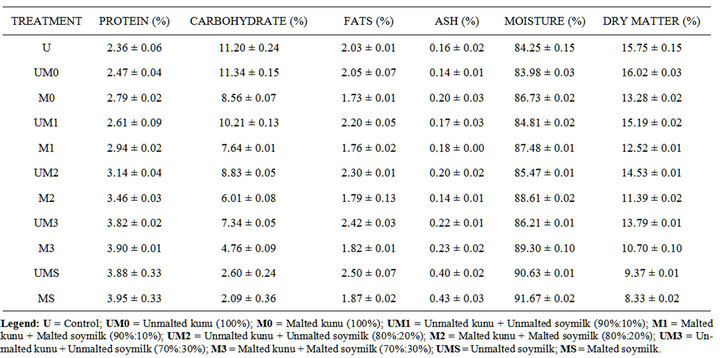
Table 2. Proximate compostion of improved kunu-zaki.
improve the nutritional quality of protein. This could be attributed to a synthesis of enzymatic proteins by germinating seed. Soymilk added at different percentages to Kunu-zaki may have had a significant effect on the protein content. The protein content increased due to fact that protein content of the soymilk is higher than that of kunu-zaki and it is noted that addition of legume to cereals gives a better protein value.
The carbohydrate content decreased with increased in addition of soymilk. Kunu-zaki with 30% of soymilk had the least carbohydrate (Table 3). It indicated that, the addition of the soymilk may have contributed to the decreased in carbohydrate content in the kunu-zaki. The fats content also increased significantly (p < 0.05) as soymilk was added to the kunu-zaki, 100% has the least fats content, unmalted kunu-zaki (2.05), malted kunu-zaki (1.73) and 30% having the highest, unmalted kuni-zaki + unmalted soymilk (2.42), and malted kunu-zaki + malted soymilk (1.82) The higher fat content of soymilk may have had effect on the fats content of the kunu-zaki as the percentage of soymilk increases.
Malting also had significant effect (p < 0.05) on the carbohydrate and fats content of the samples. The carbohydrate and fats content of the unmalted samples was higher than that of the malted samples, this was as a result of fats and carbohydrate used as energy for respiretion or metabolic activities during malting and this is in accordance with [12-14]. Ocheme and Chinma [15] reported that malting decrease fat contents of cereals.
The moisture content of the samples increased signifycantly (p < 0.05) as the level of the soymilk increases. This indicated that the soymilk had higher moisture content, so as the level of kunu-zaki to soymilk increases, the moisture content increases. The ash content also increased as soymilk was added to the kunu-zaki. Malting also had significant effect on the ash content. The soymilk had the highest ash content which had effect on the kunu-zaki as the soymilk was added.
4.2. Effect of Malting on the Physio-Chemical and Mineral Composition of Improved Kunu-Zaki
The pH of the kunu-zaki were significantly different (p < 0.05) (Table 3). The pH ranges from 3.56 to 4.49 and the pH of the control (U) is 3.75. The pH of the unmalted samples ranges from 3.56 to 4.54 while the pH of the malted samples ranges from 3.52 to 4.49 making it acidic. The acidity of “kunu-zaki” beverage has been noted to be a result of lactic acid production by some bacteria during fermentation [16] and thus, the drink becomes sour to taste and organoleptically unacceptable with time. Malting increased pH of the treatments and they were significant difference (p < 0.05).
Addition of soymilk also had affected on the pH content of the kunu-zaki, as the level of soymilk increases, the pH also increases. It indicated that the soymilk had higher pH than the kunu-zaki and neutralizes the effect of the pH of the kunu-zaki.
The specify gravity of the samples were significantly different (p < 0.05). As the addition of soymilk level increased, the specify gravity decreased, this indicated that the specify gravity of the soymilk is lower than the kunuzaki. The mineral content of the samples were signifi-

Table 3. Physio-chemical and mineral composition of improved kunu-zaki.
cantly different (p < 0.05).
4.3. Effect of Malting on the Essential Amino Acid Composition of Improved Kunu-Zaki
Eight essential amino acids were analyzed as shown on Table 4. It was shown that there were changes in the amino acid profile after malting and enrichment with soymilk (p < 0.05). This can be as a result of germination of grain where large molecules such as protein are broken down into smaller units of amino acid making the amino acid to be readily available in the germinated grain. As the level of soymilk addition increased, the essential amino acid also increased. Soymilk is rich in e3ssential amino acid. The amino acid profile of the improved kunu-zaki when compared with the FAO (Food and Agriculture Organization) reference protein showed that all the samples did not meet with the sample but enrichment with soymilk improved the amino acid profile as compared with those samples without soymilk.
4.4. Effect of Malting on the Anti Nutritional Factors of Improved Kunu-Zaki
The phytic acid and trypsin inhibitor were reduced after malting as showed on Table 5. The trypsin inhibitor concentration reduced from 0.067 mg/100 g in unmalted kunuzaki to 0.057 mg/100 g in malted kunu-zaki, the same trend was observed in the result of the phytic acid concentration. Germination has been reported to reduce the concentration of anti nutritional factors in grains [17].
4.5. Effect of Malting on the Anti Nutritional Factors of Improved Kunu-Zaki
It was observed that the addition of soymilk to the kunu increased its microbial load. There was significant difference (p < 0.05) in both the total bacterial load and total fungal load count of the improved kunu-zaki when stored at ambient temperature (Tables 6 and 7). The improved kunu-zaki enriched with 30% soymilk had the highest total bacterial and total fungal count while the kunu-zaki samples without soymilk had the lowest values. The soymilk addition may have contributed to the increase in microbial load because the pH of soymilk favours microbial growth and being a proteinuous liquid, it may serve as a medium for microbial growth. The storage of kunu-zaki samples at ambient condition may result in increased microbial load; this may be due to high moisture content of the product and its higher nutritional content which may be favourable to microorganisms. Lactic acid bacteria and yeast are associated with fermentation of cereal foods given them a sour taste [18,19].
4.6. Effect of Malting and Soymilk Addition on Sensory Qualities of Kunu-Zaki
Table 8 showed the sensory evaluation results. There
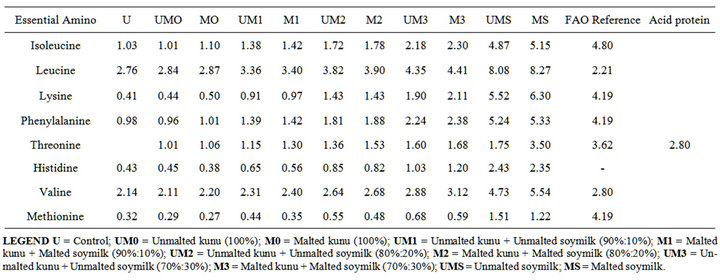
Table 4. Effect of malting and soymilk addition on the essential amino acid composition (g/16N).

Table 5. Effect of malting on the anti-nutritional factor of the improved kunu-zaki (mg/100 g).

Table 6. Total bacterial count at ambient temperature of improved kunu-zaki (cfu/ml).
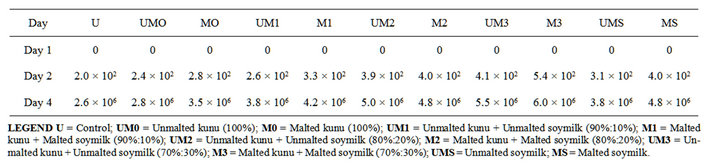
Table 7. Total fungal count at ambient temperature of improved kunu-zaki (cfu/ml).
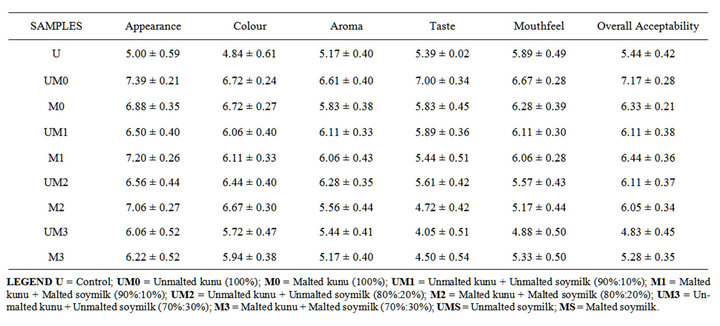
Table 8. Sensory evaluation of the improved kunu zaki.
were significant difference (p < 0.05) among the samples in apprearance, colour, taste, aroma and overall acceptability. There was no significant difference (p < 0.05) in mouth feel for all the samples. Ummalted kunu-zaki (100%) had the highest mean score in appearance, taste and overall acceptability while the unmalted kunu + unmalted soymilk had the least score. The kunu-zaki without soymilk were more acceptable and the acceptability decrease with increase in the addition of soymilk. This may be because of the beany taste of soybean which is not acceptable to people, although the addition of soymilk increased the nutritional content of kunu, the beany flavor made not to be well accepted.
5. Conclusions and Recommendation
Improved kunu-zaki enriched with soy milk gave a nutriatious beverage. As the level of soy milk addition increases, the amino acid profile and protein content increases. Malting of the cereal also shows an increase in amino acid profile and protein content of the beverage, also the pH of the kunu-zaki increased as the quantity of soy milk added increased. This may be attributed to the high pH of soy milk which will make the beverage to be suitable for ulcer patients.
Although enriching kunu-zaki with soy milk gave a nutritious beverage, but as the level of addition increased, the overall acceptability decreased as a result of the beany taste of the soy mlik.
It is therefore recommended that flavours can be added to reduce the beany taste of improved kunu-zaki, also preservatives should be added to the product to improve the shelf stability of the product and kunu-zaki should be refrigerated rather than storing at ambient temperature in order to increase the shelf life.
REFERENCES
- I. A. Adeyemi and S. Umar, “Effect of Method of Manufacture on Quality Characteristics of Kunu Zaki, a Milletbased Beverage,” Nigerian Food Journal, Vol. 12, 1994, pp. 34-41.
- S. R. Singh, K. O. Rachie and K. E. Dashieli, “Soybean Research Production and Utilization,” John Wiley and Sons Ltd., Hoboken, 1987, pp. 1-5
- A. C. Chapman, “Some Derivatives of Humulene,” Journal of the Chemical Society, 1982. pp. 1303-1306
- O. Akoma, S. A. Onuoha, M. O. Ajiboye, A. O. Akoma and A. M. Alawoki, “The Nutritional and Sensory Quality Characteistics of Kunun-Zaki Produced with the Addition of Hydrolytic Enzymes from Malted Rice (Oryza sativa),” Journal of Food Technology in Africa, Vol. 7, No. 1, 2002, pp. 24-26. doi:10.4314/jfta.v7i1.19314
- A. I. Ihekoronye and P. O. Ngoddy, “Integrated Food Science and Technology for the Tropics,” Macmillian Publishers Ltd., London, 1985, pp. 250.
- AOAC, “Official Methods of Analysis. Association of Analytical Chemists,” Washington DC, 1990.
- AOAC, “Official Methods of Analysis. Association of Analytical Chemists,” Washington DC, 2002.
- C. Magné and F. Larher, “Higher Sugar Content of Extract Interferes with the Colorimetric Determination of Free Amino Acids and Free Proline,” Analytical Biochemistry, Vol. 200, No. 1, 1957, pp. 115-118. doi:10.1016/0003-2697(92)90285-F
- M. J. Palczar and E. C. S. Chan, “Laboratory Exercise in Microbiology,” Black Dot Inc., New York, 1997.
- E. Larmond, “Laboratory Methods for Sensory Evaluation of Foods,” Department of Agric, Ottawa, 1977, pp. 18-46.
- Y. D. Wang and M. L. Fields, “Germination of Corn and Sorghum in the Home to Improve Nutritive Value,” Journal of Food Science, Vol. 43, No. 4, 1978, pp.1113-1115. doi:10.1111/j.1365-2621.1978.tb15247.x
- D. E. Briggs and J. MacDonald, “Patterns of Modification in Malting Barley,” Journal of the Institute of Brewing, Vol. 89, No. 4, 1983, pp. 260-273. doi:10.1002/j.2050-0416.1983.tb04184.x
- F. O. Sade, “Proximate, Antinutritional Factors and Functional Properties of Processed Pearl Millet (Pennisetum glaucum),” Journal of Food Technology, Vol. 7, No. 3, 2009, pp. 92-97.
- N. M. Nnam, “Evaluation of Effect of Sprouting on the Viscosity, Proximate Composition and Mineral Content of Hungary Rice, Acha (Digiteria exilis) Flour,” Nigerian Food Journal, Vol. 18, 2002, pp. 57-62.
- O. B. Ocheme and C. E. Chinma, “Effect of Soaking and Germination on Some Physic Chemical Properties of Millet Flour for Porridge Production,” Journal of Food Technology, Vol. 6, 2008, pp. 185-188.
- A. W. Ashiru, O. O. Olaleye and P. O. Egbenni, “Occurrence of pathogenic organisms in kunun drink sold within Lagos Metropolis,” Proceedings of the 27th Annual NIFST Conference, 2003, p. 84.
- T. Traore, C. Mouquet, C. Icard-Vaerniere, A. S. Traore, and S. Treche, “Changes in Nutrient Composition, Phytate and Cyanide Contents and Amylase Activity during Cereal Malting in Small Production Units in Ouagadougou (Burkina faso),” Food Chemistry, Vol. 99, No. 1, 2004, pp. 105-114. doi:10.1016/j.foodchem.2004.01.032
- I. A. Akinrele, “Fermentation Studies on Maize during Preparation of a Traditional African Starch-Cake Food,” Journal of the Science of Food and Agriculture, Vol. 21, No. 12, 1970, pp. 619-625. doi:10.1002/jsfa.2740211205
- S. A. Odunfa and S. Adeyele, “Microbiological Changes during the Traditional Production of Ogi-Baba, a West African Fermented Sorghum Gruel,” Journal of Cereal Science, Vol. 3, No. 2, 1985, pp. 173-180. doi:10.1016/S0733-5210(85)80027-8
NOTES
*Corresponding author.

