Advances in Microbiology
Vol. 3 No. 2 (2013) , Article ID: 32427 , 5 pages DOI:10.4236/aim.2013.32030
MBDS Solvent: An Improved Method for Assessment of Biofilms
Institute for Glycomics, Griffith University, Gold Coast, Australia
Email: c.day@griffith.edu.au
Copyright © 2013 Greg Tram et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received November 22, 2012; revised December 18, 2012; accepted January 17, 2013
Keywords: Crystal Violet; Pseudomonas aeruginosa; Microtitre Plate Assay
ABSTRACT
Biofilms are recognised as an important contributor to bacterial resistance towards traditional antimicrobial treatments. Assessment of biofilm formation currently relies on a 96 well microtitre plate assay, which usually involves the colourimetric detection of stain (typically crystal violet) removed from previously stained biofilm. The amount of crystal violet released is then used as a quantitative indicator of the amount of biofilm formed. Currently, this is achieved by solubilisation of the stain by ethanol which results in partial decolourisation of the crystal violet stained biofilm which impacts the accuracy and reproducibility of this method. Herein, we describe a modified biofilm dissolving solution (MBDS) which produces a more uniform and reproducible colour release from stained biofilm through solubilisation of the biofilm architecture itself. Here we use crystal violet stained biofilms of P. aeruginosa strain PA0-1, to demonstrate an approximate two fold increase in crystal violet release by MBDS, as compared to ethanol treatment. In addition, when ethanol decolourised biofilms were treated again with MBDS, an almost equal amount of remnant crystal violet was recovered by dissolving the biofilm and the stain trapped within it. These results were reflected in microscopic analysis of ethanol treated and MBDS treated biofilm. Similar results were obtained when MBDS was used to decolourise and dissolve the biofilms of a number of other bacterial species highlighting the advantages of MDBS as a universal solvent for the colour detection of biofilm.
1. Introduction
Biofilms are a mode of surface associated microbial growth which bacteria frequently employ in order to better deal with environmental stresses [1]. Biofilms also play an important role in the infection processes of a number of organisms by contributing strongly to factors such as antimicrobial resistance. Pseudomonas aeruginosa is a bacterium well documented to form biofilms on moist surfaces, medical instruments and in chronic respiratory infections, particularly in cystic fibrosis patients [2-4]. Biofilms of Streptococci and Staphylococci species have also been implicated in many nosocomial infections, such as encrustation of catheters and cases of endocarditis [5,6].
One of the main characteristics of bacterial biofilms is a complex extracellular matrix secreted around the adherent cells [7]. This extracellular matrix consists of a number of long chained polymeric molecules [8]. The general composition of these molecules can vary between species [1] but are primarily comprised of exopolysaccarides and lipopolysaccharides [7]. Within this matrix, bacteria are able to survive for a significantly longer time than their planktonic counterparts [9] due to the protection from adverse environments [10] including the protection from phagocytes and components of the host immune system [11].
Due to the importance of biofilms to the infective pathways of a number of organisms, there is a need to be able to accurately and effectively quantitate biofilm formation and to study the effects of agents which may be able to inhibit the processes of biofilm formation. Currently, many assays rely on the spectrophotometric quantitation of ethanol decolourisation of crystal violet stained biofilm [12,13]. This method results in a widely varying data due to ineffective decolourisation and solubilisation of the stained biofilm. Here, we describe an alternative method which relies on solubilisation of stained biofilm matrix, providing increased reliability and sensitivity.
2. Materials and Methods
2.1. Biofilm Formation
The P. aeruginosa strain PA0-1 was grown aerobically in liquid Luria Bertani media for 12 hours. A number of other bacterial species were used; Klebsiella pneumoniae strain GH-KP1, Enterobacter cloaca strain GH-EC1, Serratia marscescens strain GH-SM1, Clostridium perfingens strain GH-CP1 and Staphylococcus aureus strain GH-SA1. These bacterial strains form part of the Griffith University Culture Collection and were isolated from clinical sources from the Gold Coast Hospital. These bacterial strains were grown aerobically overnight on Columbia blood agar (Oxoid) supplemented with 5% defibrinated horse blood. Bacterial cultures were then diluted with fresh Luria Bertani broth (Oxoid) an optical density of 0.5 as assessed at 600 nm. 100 µL of this bacterial suspension was then individually inoculated into a minimum of 3 rows of 8 wells in polystyrene 96-well microtitre plates (Nunc). Bacterial suspension was also used to inoculate 24-well plates containing glass cover slips. The plates were incubated aerophilically at 37˚C for 24 hours as outlined [13].
2.2. Crystal Violet Staining and Solubilisation
The 96-well plates containing formed biofilm were then washed with distilled water three times to remove planktonic cells and media. A 1% crystal violet solution (Sigma) was then used to stain the remaining biofilm and allowed to penetrate for 15 minutes. The wells were then washed a further 3 times with distilled water until runoff was clear.
Four solutions were then assessed for the ability to extract crystal violet from the stained biofilm; 95% ethanol (Chem Supply) (a standard solution for crystal violet decolourisation of 96-well plate biofilm assays (12), a 5% sodium dodecyl sulphate (SDS, Amresco) solution, a modified biofilm dissolving solution (SDS dissolved to a final concentration of 10% with 80% ethanol in H2O) and distilled H2O. It was hypothesised that when combined with ethanol, SDS would be more able to break down the lipid rich architecture of the biofilm, liberating more crystal violet and providing a more complete assessment of biofilm formed.
Following staining of the biofilm in the 96-well plates, crystal violet was solubilised with 100 µL of solvent and mixed by pipetting and removed into a clean plate. The plate containing solubilised crystal violet was then read with a Victor X multilabel plate reader at a wavelength of 600 nm. A student’s t-test P value of ≤0.05 was used as a cut-off when testing for significant difference between absorbance readings.
2.3. Microscopic Analysis
The cover slips containing formed biofilm were removed from the 24-well plates and washed three times to remove planktonic cells. A 1% crystal violet solution was used to stain biofilm containing cover slips. These were then treated with either ethanol or MBDS. The cover slips were then re-stained for a further 15 minutes with crystal violet, dried and mounted onto glass microscope slides. The slides were then examined using a Nikon Eclipse E600 light microscope and images were taken using a Nikon DXM-1200C digital camera and Act-1 visualisation software.
3. Results and Discussion
3.1. Assessment of SDS Based Decolourises of Stained P. aeruginosa Biofilm
Following biofilm formation, each series of wells was stained with a 1% crystal violet solution. One of either ethanol (95%), MBDS, SDS solution (5%) or distilled H2O was then applied to determine the effectiveness in solubilising crystal violet from stained biofilm.
Upon treatment with each respective agent, MBDS and 5% SDS showed an almost 2 fold increase (p value > 0.05) in biofilm recovery compared to traditional ethanol treatment (Figure 1). In addition, a number of other differences were observed in the method of solubilisation. The 5% SDS solution showed a uniform colour, however, substantial foaming was produced, impacting on the accuracy of the spectrophotometric assessment of dissolved crystal violet. The MBDS demonstrated similar level of decolourisation to 5% SDS, however, the foaming of the solution was substantially reduced. Ethanol solubilisation was not effective in completely solubilising crystal violet, with many insoluble clumps of crystal violet stained material being present during treatment. The H2O control shows a negligible OD600 spectrophotometer reading of ≤0.05 suggesting that it is ineffective at liberating crystal violet from stained biofilm and was used as a control.

Figure 1. Spectrophotometry values quantifying the initial recovery of crystal violet from stained biofilm by different solvents.
Following the initial treatment with solvents, the remaining biofilm was again washed with water and a MBDS treatment was then applied to all wells to determine whether any remnant crystal violet could be extracted from the remnant of stained biofilm. Both MBDS and SDS showed similar recovery of stain following subsequent MBDS treatment (Figure 2). Stained biofilm previously treated with ethanol yielded further crystal violet recovery following the MBDS treatment equal to that detected by the initial spectrophotometer reading, suggesting that ethanol was only able to dissolve approximately 50% of the crystal violet penetrating the biofilm. The control H2O treated wells showed that almost none of the crystal violet was dissolved in the initial H2O treatment as resolubilisation by the MBDS showed a spectrophotometer reading similar to those shown in Figure 1 for initial solubilisation by the MBDS.
Following spectrophotometric assays, microscopic analysis was conducted in order to determine the effect of ethanol and MBDS treatment on formed P. aeruginosa biofilm. Prior to treatment, stained biofilm shows that much of the slide contains large aggregates of bacterial biofilm covering the microscope slide. Ethanol treatment of biofilm shows that large aggregates have detached, however, many of the smaller clusters of biofilm remain. Treatment of biofilm with the MBDS results in solubilisation of the majority of formed biofilm and little remains adhered (Figure 3). This was reflected with secondary MBDS treatment which demonstrates a marked decrease in stained biofilm of previously ethanol treated biofilms. This was not seen with secondary ethanol treatment of formed biofilm. This further demonstrates the solubilisation action of the MBDS. Although large aggregates can easily detach following ethanol treatment, much of the crystal violet remains trapped in smaller clusters which are solubilised following treatment with the MBDS.
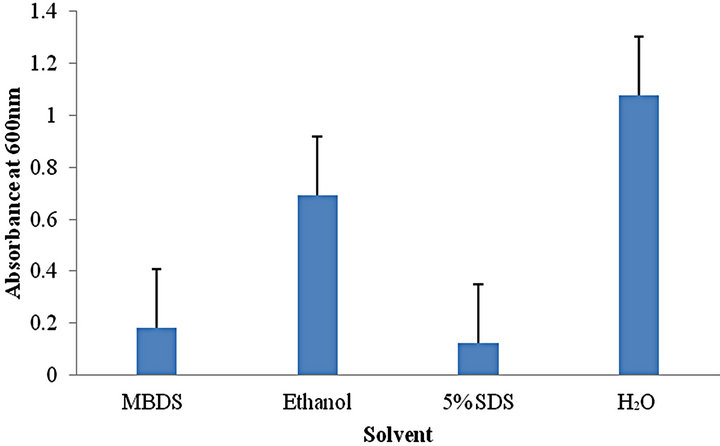
Figure 2. Spectrophotometry readings showing crystal violet recovery by secondary MBDS treatment of previously solvent treated biofilm.
3.2. Effectiveness of MBDS on Biofilms of Other Bacterial Species
Similar assays were conducted to evaluate the MBDS solvent for its ability to quantitate biofilms of a number of other Gram negative and Gram positive bacterial species. Formed biofilm was stained with crystal violet and either ethanol, MBDS, SDS or H2O were used to decolourise stained biofilm of four Gram negative organisms (S. marscescens, K. pneumoniae, E. cloacae and C. perfingens) and a Gram positive organism (S. aureus) (Figure 4). Gram negative organisms tested showed similar results to P. aeruginosa biofilm with an approximate two fold increase in the amount of stained biofilm recovered by MBDS when compared to ethanol (all p value > 0.05). The Gram positive organism S. aureus showed only a slightly increased OD600 when treated with MBDS (p value 0.3).

Figure 3. Comparisons of formed biofilm with ethanol or MBDS treatment. (A) Pre-ethanol treated biofilm; (B) Post-ethanol treated biofilm; (C) Pre-MBDS treated biofilm; (D) Post-MBDS treated biofilm. All images taken at 400× magnification.

Figure 4. Comparisons of the ability of MBDS and ethanol to recover crystal violet from stained biofilms of a number of bacterial species.
3.3. MBDS Dissolves Biofilm Rather than Decolourises
Ethanol is widely accepted to decolourise stained biofilm, leaving biofilm at least partially intact whilst extracting crystal violet. However, crystal violet stained portions of the biofilm, which may not be accessible by ethanol, remain. The MBDS appears to work through solubilisation of the biofilm itself. In order to confirm this, stained biofilm was treated and re-stained to determine whether there was any residual biofilm remaining in the wells of the microtitre tray.
When subjected to a secondary treatment, the MBDS shows that there is significantly less recoverable crystal violet upon re-staining and decolourising when compared to the initial stain and treatment (p value ≤ 0.05) then following the traditional ethanol treatment, suggesting that the biofilm itself may have been solubilised (Figure 5). Ethanol treatment following re-staining shows that the amount of crystal violet recovered is similar to that released by the initial treatment (p value > 0.05), suggesting that much of the original biofilm remains.
A large portion of the biofilm architecture of most organisms consists of lipid and glycan based polymeric molecules such as lipopolysaccharides [7]. Although widely used [12,13], traditional biofilm quantitation that involved staining the biofilm with crystal violet and then distaining it with 95% ethanol, is often unreliable due to partial decolourisation of the stain and presence of insoluble fragments of stained biofilm in solution. In contrast, the SDS component of the alternative solvent, MBDS, described in this study, appears to be able to solubilise the most of the biofilm matrix, as compared to traditional ethanol treatment, allowing more complete liberation of crystal violet from stained biofilm. In addition to providing greater recovery of crystal violet from biofilm, MBDS produces a more uniform colour, dissolving ethanol insoluble clumps of biofilm. This uniform colour provides a more accurate assessment via spectrophotometry. The dissolution of formed biofilm
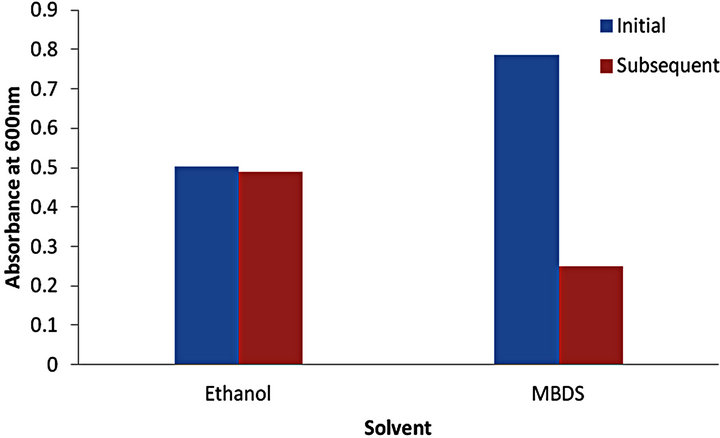
Figure 5. Spectrophotometer values showing recovered crystal violet from initial treatment and re-stained biofilm.
also provides a more efficient and accurate assessment of the amount of biofilm formed. Whilst the SDS component is responsible for the action of MBDS, when used alone as a solution, substantial foaming by the detergent is produced and affect the ability of traditional 96-well plate readers to accurately assess the optical density.
When MBDS was used to quantitate the biofilms of other Gram negative organisms, it yielded the same degree of biofilm solubilisation as for P. aeruginosa biofilm, whereas the recovery of stain from the biofilm of Gram positive S. aureus resulted in less significant difference to that of the traditional ethanol solubilisation. This may be due to dissimilarity in the components of Gram negative and Gram positive biofilm architecture. Gram positive organisms do not possess an extensive lipid profile when compared to Gram negative bacterium. In addition, Gram positive organisms have a decreased capacity to synthesise lipid molecules. This reduced lipid content compared to Gram negative organisms will enable ethanol to more effectively penetrate the biofilm leading to only a slight increase in MBDS recovered biofilm stain.
Biofilms are an important component involved in infections of many bacterial organisms and the need to find agents inhibiting biofilm formation will enable the prevention of many nosocomial infections such as those of P. aeruginosa. Novel antimicrobial agents are continuously being investigated for potential anti-biofilm activity and usually rely on the colourimetric microtitre plate method for assessment of biofilm formation. The use of an MBDS solution for assessment of biofilms, particularly when levels of the biofilm may be reduced, will provide a more accurate and reliable measurements to be achieved.
REFERENCES
- G. O’Toole, H. Kaplan and R. Kolter, “Biofilm Formation as Micrbobial Development,” Annual Review of Microbiology, Vol. 54, No. 4, 2000, pp. 49-79. doi:10.1146/annurev.micro.54.1.49
- R. Djeribi, W. Bouchloukh, T. Jouenne and B. Menaa, “Characterization of Bacterial Biofilms Formed on Urinary Catheters,” American Journal of Infection Control, Vol. 40, No. 9, 2012, pp. 854-959. doi:10.1016/j.ajic.2011.10.009
- J. Bryers, “Medical Biofilms,” Biotechnology and Bioengineering, Vol. 100, No. 1, 2008, pp.1-18. doi:10.1002/bit.21838
- T. Bjarnsholt, P. Jensen, M. Fiandaca, J. Pedersen, C. Hansen and C. Andersen, “Pseudomonas aeruginosa Biofilms in the Respiratory Tract of Cystic Fibrosis Patients,” Pediatric Pulmonology, Vol. 44, No. 6, 2009, pp. 547- 558. doi:10.1002/ppul.21011
- A. Agarwal, K. Singh and A. Jain, “Medical Significance and Management of Staphylococcal Biofilm,” Immunology and Medical Microbiolgy, Vol. 58, No. 2, 2009, pp. 147-160. doi:10.1111/j.1574-695X.2009.00601.x
- X. Ge, T. Kitten, Z. Chen, S. Lee, C. Munro and P. Xu, “Identification of Streptococcus Sanguinis Genes Required for Biofilm Formation and Examination of Their Role in Endocarditis Virulence,” Infection and Immunity, Vol. 76, No. 6, 2008, pp. 2551-2559. doi:10.1128/IAI.00338-08
- S. Branda, S. Vik, L. Friedman and R. Kolter, “Biofilms: The Matrix Revisited,” Trends in Microbiology, Vol. 13, No. 1, 2005, pp. 20-26. doi:10.1016/j.tim.2004.11.006
- D. Lopez, H. Vlamakis and R. Kolter, “Biofilms,” Cold Spring Harbour Laboratory Press, New York, 2010.
- M. Boyle, T. Ford, J. Maki and R. Mitchell, “Biofilms and the Survival of Opportunistic Pathogens in Recycled Water,” Waste Management and Research, Vol. 9, No. 5, 1991, pp. 465-470. doi:10.1016/0734-242X(91)90077-K
- Y. Shen, S. Stojicic and M. Haapasalo, “Bacterial Viability in Starved and Revitalized Biofilms: Comparison of Viability Staining and Direct Culture,” Journal of Endodontics, Vol. 36, No. 11, 2010, pp. 1820-1823. doi:10.1016/j.joen.2010.08.029
- K. Hughes, I. Sutherland and M. Jones, “Biofilm Susceptibility to Bacteriophage Attack: The Role of PhageBorne Polysaccharide Depolymerase,” Microbiology, Vol. 144, No. 11, 1998, pp. 3039-3047. doi:10.1099/00221287-144-11-3039
- J. Gooderham, M. Bains, J McPhee, I. Wiegand and R. Hancock, “Induction by Cationic Antimicrobial Peptides and Involvement in Intrinsic Polymyxin and Antimicrobial Peptide Resistance, Biofilm Formation, and Swarming Motility of PsrA in Pseudomonas aeruginosa,” Journal of Bacteriology, Vol. 190, No. 16, 2008, pp. 5624- 5634. doi:10.1128/JB.00594-08
- J. Overhage, A. Campisano, M. Bains, E. Torfs, B. Rehm and R. Hancock, “Human Host Defense Peptide LL-37 Prevents Bacterial Biofilm Formation,” Infection and Immunity, Vol. 76, No. 9, 2008, pp. 4176-4182. doi:10.1128/IAI.00318-08

