Journal of Sustainable Bioenergy Systems
Vol.4 No.1(2014), Article ID:44315,16 pages DOI:10.4236/jsbs.2014.41010
Application of Aquatic Plants for the Treatment of Selenium-Rich Mining Wastewater and Production of Renewable Fuels and Petrochemicals
Ana F. Miranda1, Nazim Muradov2, Amit Gujar2, Trevor Stevenson1, Dayanthi Nugegoda1, Andrew S. Ball1, Aidyn Mouradov1*
1Royal Melbourne Institute of Technology University, Melbourne, Australia
2University of Central Florida, Florida Solar Energy Centre, Cocoa, USA
Email: *aidyn.mouradov@rmit.edu.au
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 30 January 2014; revised 28 February 2014; accepted 15 March 2014
ABSTRACT
Aquatic plants aggressively colonising wetlands are widely used for the biosorption of the soluble contaminants from wastewater and represent an attractive feedstock for biofuel production. Three common Australian aquatic plants, duckweed (Landoltia punctata), elodea, (Elodea canadensis) and water clover (Marsilea quadrifolia), colonizing different depths of wetlands were tested for their ability to treat the selenium-rich mining wastewater and for their potential for production of petrochemicals. The results showed that these plants could be effective at biofiltration of selenium and heavy metals from mining wastewater accumulating them in their fast growing biomass. Along with production of bio-gas and bio-solid components, pyrolysis of these plants produced a range of liquid petrochemicals including straight-chain C14-C20 alkanes, which can be directly used as a diesel fuel supplement or as a glycerine-free component of biodiesel. Other identified bio-oil components can be converted into petrochemicals using existing techniques such as catalytic hydrodeoxygenation. A dual application of aquatic plants for wastewater treatment and production of value-added chemicals offers an ecologically friendly and cost-effective solution for water pollution problems and renewable energy production.
Keywords:Biofuel; Bioremediation; Pyrolysis; Mining Wastewater; Thermochemical Conversion

1. Introduction
Phytoremediation, defined as the use of green plants to remove pollutants from the environment or to render them harmless represents one of the most globally researched issues and is becoming a subject of intense public and scientific interest and a topic of many recent reviews [1] -[3] . Phytoremediation includes several processes namely, phytoextraction, phytodegradation, phytofiltration, rhizodegradation, phytostabilization and phytovolatilization. More than 400 plant species from at least 45 plant families have been identified as hyperaccumulators of different contaminants [4] -[9] . Aquatic plants rapidly colonizing lakes and waterways attract significant attention because of their unprecedented growth rates, bioremediation (phytofiltration) capacity and easy maintenance. The annual average yield of duckweed is 39.2 - 105.9 t/ha, yr which is much higher than annual yields of the main bioenergy grasses, switchgrass (5.2 - 26 t/ha, yr), bermuda grass (6.1 - 27 t/ha, yr) and miscanthus (5.0 - 44 t/ha, year) [10] [11] . Some aquatic plants can accumulate heavy metals 100,000 times greater than in the associated water [12] .
Selenium (Se) is an essential element for animals and humans health. It provides protection against diseases such as infertility, liver necrosis, muscular dystrophy, Keshan disease and white muscle in livestock [13] -[15] . For Se, the margin between nutritional requirement and toxicity is unusually narrow and depends on the individual species and circumstance. The minimum nutritional level of Se for animals is about 0.05 - 0.10 mg Se g−1 in dry forage feed. Higher levels of Se, however, (2 - 5 mg Se g−1) may cause toxic effects in livestock [16] and in humans [17] . In the environment Se occurs in a variety of oxidation states, some of which are water soluble, gaseous, or mineralized. In most seleniferous soils and agricultural drainage waters Se exists in a forms of two soluble molecules, selenate (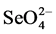 , Se6+) and selenite (
, Se6+) and selenite (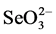 , Se4+). Both of these molecules are of major concern because they are toxic and known to bioaccumulate in different tissues [5] [18] [19] .
, Se4+). Both of these molecules are of major concern because they are toxic and known to bioaccumulate in different tissues [5] [18] [19] .
Removal of Se has been very difficult because of its low concentrations in the parts-per-billion range, its chemical similarity to sulphur, which can be present at more than a million fold higher concentrations [6] [7] . A number of physical, chemical, and biological treatment technologies for the removal of Se from contaminated soils and waters have been investigated [8] . Most of the biological processes investigated for the removal of Se from agricultural wastewater utilize bacterial populations to reduce 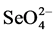 and
and 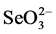 into insoluble elemental Se (Se0) [9] . Alternatively Se can be removed from agricultural drainage water through its volatilization producing methylate Se after the addition of various carbon sources [9] . Selenium can be removed by plants by phytoaccumulation and plant volatilization, rhizofiltration and rhizodegradation [20] [21] . Since plants can accumulate up to 80 µg/g of Se in their dry weight [20] a value lower than the minimum nutritional level of Se for animals [16] , selenium-enriched plant biomass can be used as organic selenium fertilizer and/or animal feed adding them to forage crops.
into insoluble elemental Se (Se0) [9] . Alternatively Se can be removed from agricultural drainage water through its volatilization producing methylate Se after the addition of various carbon sources [9] . Selenium can be removed by plants by phytoaccumulation and plant volatilization, rhizofiltration and rhizodegradation [20] [21] . Since plants can accumulate up to 80 µg/g of Se in their dry weight [20] a value lower than the minimum nutritional level of Se for animals [16] , selenium-enriched plant biomass can be used as organic selenium fertilizer and/or animal feed adding them to forage crops.
The substantial biomass of aquatic plants together with the relatively high levels of starch, cell wall carbohydrates and lipids make them the feedstock of choice for bioethanol production [22] [23] . Their lack of or low level of lignin can also improve the economics of bio-ethanol production, reducing the pre-treatment and enzyme dosages significantly. The saccharification of cell walls for the production of reduced sugars for conversion to value-added products or ethanol has been well described [10] [24] [25] . Duckweed, the most investigated aquatic macrophyte can produce a theoretical ethanol yield reaching 6.42 × 103 L∙ha−1, 50% more ethanol than maize which is the main ethanol producing feedstock in many countries [26] .
Pyrolysis represents thermal decomposition (400˚C - 550˚C) of organic compounds in the absence of air/oxygen. All three pyrolysis products, gas, bio-oil and bio-solids (char and mineral ash) can be converted into petrochemicals and value products. In addition the potential to concentrate the toxic contaminants accumulated in feedstocks, such as heavy metals in the solid fractions such as a char and/or ash makes this technology more preferable for the production of value-added products. Most of the pyrolysis studies so far have been carried out with cellulosic feedstocks, which are mainly composed of cellulose, hemicelluloses and lignin [27] -[29] . Recently, we have optimized conditions for pyrolysis of Lemna minor which showed great potential as a feedstock for the production of bio-oil and bio-char [30] [31] .
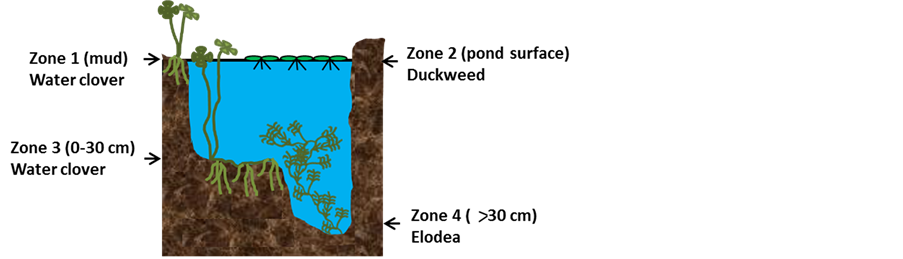
This study aimed to assess common Australian representatives of aquatic macrophytes: duckweed elodea and water clover for their ability to uptake Se and heavy metals from Se-rich mining wastewater. Growth of these plants species cover different depth zones of water ponds and wetlands: water clover being both aquatic and semi-aquatic can grow in dense clumps in mud (zone 1, Figure 1) or be fully submerged in shallow water with some of the leaves extending to float on the water surface (zone 3); duckweed floats on the surface (zone 2), and
 (a)
(a) (b)
(b)
Figure 1. (a) Growing zones of duckweed, elodea and water clover in wetland; (b) Growth of duckweed (left), elodea (middle) and water clover (right) in 250 ml plastic containers. Scale bars = 1 cm.
elodea grows fully submerged in deep water with roots growing in the mud at the bottom of the water (zone 4). All of these plants can grow and reproduce aggressively even outside their native habitat, colonizing large water reservoirs and representing an attractive feedstock for biofuel production. These plants are also known for their potential to uptake microelements and nutrients from wastewaters [12] [32] -[35] . For the first time L. punctata duckweed species, elodea and water clover were assessed for the bioremediation of Se-rich mining wastewater and as feedstock for production of value products such as bio-gas, bio-solids and bio-liquid using pyrolysis technology.
2. Materials and Methods
2.1. Growing Duckweed, Elodea and Water Clover
The plants selected were aquatic species common to Australian wetlands. Duckweed was acquired from Robert Bell, Bio-Tech Waste Management Pty Ltd. Elodea and water clover were grown in the RMIT University’s collection. The plants were collected, rinsed in deionized water and any unwanted debris removed. Experiments were carried out in plastic containers (250 mL) filled with 200 mL of SMW or 50% SMW diluted in 1/2 Hoagland’s medium. Control experiments were conducted in sterile 1/2 Hoagland’s medium. The samples were placed in growth chambers (23˚C) with 16 h photoperiod and a photosynthetic photon flux density of 50 µm/m2-s provided by three fluorescent tubes. Three replicates were included for each treatment. Destructive sampling was conducted to evaluate the Se and heavy metal concentrations in plants at day 7. The medium in each container were mixed every day. Growth was monitored after 7-day test period by weighting fresh weight using the following equation: μ = (lnNtn − lnNt0)/(tn − t0), where Ntn was the fresh weight at day 7, and Nt0 was the fresh weight at day 0.
2.2. Selenium Extraction and Measurements
Water samples from different dilutions of SMW were collected at the beginning and at the end of the experimental period, acidified with concentrated HNO3 to pH 2 and kept at 4˚C. Plants from each treatment were rinsed with Milli-Q water, blotted on filter paper and dried at 70˚C overnight. Dried samples were then ground using a mortar and pestle and aliquots (100 mg) were weighed into a glass tubes and digested with a HNO3 (68.5%):HClO4 (70%) mixture (1 mL, 10:1, v/v) in a dry heating block at 100˚C for 30 min [36] . After cooling to room temperature samples were filtered using a Whatman number 42 (11 cm) filter paper and diluted to 10 mL with Milli-Q water. Plant extracts and wastewater were analysed for total selenium concentration by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent Technologies, Model 4500 series 300).
2.3. Pyrolysis Experiments
Pyrolysis experiments were carried out in a quartz tube reactor with a diameter of 12 mm. The samples were dried at 110˚C overnight before experimental runs. Heating of the quartz tube reactor was carried out using a tube furnace which was controlled by a temperature controller (Omega CSC32, Omega Engineering, Inc., USA). Two thermocouples (one external to the tube and one internal) were used to monitor pyrolysis temperature. The furnace (and the quartz reactor) was vertically aligned, so that the liquid products dripped into the condenser assembly which was chilled using ice. After condensation of the liquid product, the gas passed through a glass wool filter before being collected in a gas-sampling bag. The condenser was weighed before and after the reaction to obtain the weight of the liquid product (bio-oil) collected. The solid product bio-char was dislodged from the pyrolysis reactor after the experiment and weighed. The volume of the pyrolysis gas was measured and the gas sample was analyzed by gas-chromatography (GC). Three replicates were conducted for each treatment.
2.4. Thermogravimetric Analysis
Thermogravimetric (TGA) and differential thermogravimetric (DTG) analyses of the plant samples were performed using the PerkinElmer Diamond TG/DTA instrument. The samples were used “as is” without drying. The samples were loaded into the instrument and heated from 50˚C to 950˚C at a heating rate of 20˚C/min. TGpyrolysis (using He as carrier gas) was used to determine the moisture content, volatiles and fixed carbon. TG-combustion (using air as carrier gas) was used to determine the ash content. The loss of weight between 25˚C and 120˚C was used to calculate the moisture content of the sample. The volatile content of the samples was determined from the loss of weight between 120˚C and 650˚C (primary volatiles), and 650˚C and 950˚C (secondary volatiles). The ash content of the sample was determined from the amount of solids remaining at the end of the combustion run. Fixed carbon was calculated by subtracting the ash content from the solids remaining at the end of the pyrolysis run.
2.5. Analysis of Bio-Oil
The liquid product of pyrolysis (bio-oil) was analyzed by gas chromatography—mass spectrometry (GC-MS). The sample of bio-oil was dissolved in aliquot amounts of dichloromethane (DCM) and injected into an Agilent 6890N (Agilent Technologies, Santa Clara, CA, USA) coupled to a JEOL GCMate-II (JEOL Ltd., Tokyo, Japan) GC-MS. Typical GC-MS parameters used in the analyses were as follows: helium as carrier gas at a flow rate of 2 mL/min, column: HP-5ms (60 m ´ 0.32 mm ´ 0.25 µm), injection port temperature: 300˚C, GC-MS interface temperature: 250˚C, a sample injection volume: 10 mL, split ratio: 50:1.
2.6. GC Analysis of Gaseous Products of Pyrolysis
Pyrolysis gas analysis was performed using a Varian 450 (Varian Inc., USA) GC with thermal conductivity detector (TCD) for permanent gases and flame ionization detector (FID) for hydrocarbon gases. Argon was used as carrier gas and three columns were used for separationPLOT alumina/KCL, Molecular sieve 5A and Haysep Q.
2.7. Statistical Analysis
All treatments in this study were conducted in triplicate. The experimental data were subjected to the one-way analysis of variance (ANOVA) as implemented in the GraphPad InStat 3 statistics platform. Tukey simultaneous tests were conducted to determine the statistical differences between treatments. In order to ascertain that the observed variations in growth rates, efficiency of nutrients uptake and the yield of pyrolysis products are statistically significant, the probability (P) values were determined. A 95% confidence level (P ≤ 0.05) was applied for all analysis.
3. Results and Discussion
3.1. Application of Duckweed, Elodea and Water Clover for Selenium-Rich Mining Wastewater Treatment
The ability of plant cultures of duckweed, elodea and water clover to uptake total selenium (TSe) from the selenium-rich mining wastewater (SMW) is shown in Table1 All plants showed the capacity to reduce the concentration of TSe in wastewater and accumulate TSe in their tissues after one week. Water clover and elodea showed 10.9% and 14.6% of TSe removal from 100% SMW (12.5% and 18.7% TSe uptake per g DW, respectively), which correlated with 0.63 µg/g DW and 0.79 µg/g DW of TSe accumulation in their tissues, respectively. These relatively low uptake rates can be explained by sensitivity of both plants to undiluted wastewater and/or by their naturally low rates of TSe uptake. There was low level of increase in fresh weight in 100% SMW for these plants (Figure 2). Growth rates of their fresh biomass after one week in 100% SMW were 0.013 (9% increase) for elodea and 0.009 (4% increase) for water clover (Figure 2). An approximate 2 fold increase in fresh biomass was observed in control experiments for both plants, with growth rates of 1.13 and 1.12 for elodea and water clover, respectively. Growing in 100% SMW duckweed showed up to 30% of TSe uptake (23.1% uptake per g of DW), accumulating 2.57 µg/g DW TSe in its dry biomass. This high efficiency of phytoextraction can be explained by higher tolerance of duckweed to undiluted SMW showing up to a 1.5 fold increase (growth rate of 0.07) in fresh biomass (Figure 2) and also by its higher capacity for TSe uptake.
Table 1. TSe removal efficiency from SMW and TSe accumulation in duckweed, elodea and water clover.
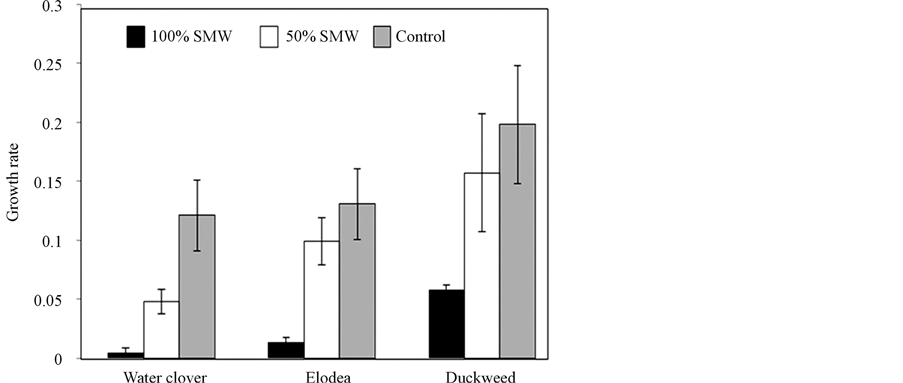
Figure 2. Growth rates of fresh biomass of duckweed, elodea and water clower in 100% SMW and 50% SMW. C-control.
Dilution of SMW significantly improved all plants growth rates which in turn enhanced their TSe uptake capacities. Fresh biomass of water clover and elodea in 50% SMW was almost doubled, showing growth rates of 0.04 and 0.09, respectively (Figure 2). As a result, rates of TSe removal by water clover and elodea were increased to 30.4% and 39.1%, respectively (Table 1). Accumulation of TSe in plant biomass was also increased to 1.1 µg/g DW and 1.35 µg/g DW, respectively. Duckweed showed up to 55% uptake of TSe from 50% SMW, accumulating 3.6 µg/g of TSe in its biomass. Because of the increased growth rates of all plants, the values of TSe accumulation (per g DW) were not significantly increased. The values of TSe removal (per g DW) in the case of elodea and duckweed was even decreased, showing 14.5%/g DW, 16.2%/g DW and 17.8%/g DW for water clover, elodea and duckweed, respectively.
Previously, muskgrass (Chara canescens)-containing microcosms grown in Se-rich drainage water showed a similar efficiency of Se removal (39.8%) [37] , which was lower than Se removal rates observed for other aquatic plants, such as cattail (up to 68%), duckweed (up to 99%), hydrilla (up to 100%), and swamp lily (up to 61%) [20] . This could be explained by the fact that sodium selenite solutions were used in these experiments as a source of Se, whereas the mining wastewater used in our experiments and Se-rich drainage water [37] contained a large amount of heavy metals and other microelements whose uptake by plants could compete with TSe bioremediation.
Table 2 shows the efficiency of uptake of different microelements including heavy metals from 100% SMW by the duckweed, elodea and water clover plants. The heavy metals identified in the SMW, Fe, Co, Cd, Cu, Ni, Mn, Cr, Mo, Zn we represented in a range of concentrations from 0.21 µg/L (Co) to 1009 µg/L for Fe. These metals, except Cd, play a number of crucial roles in many biological functions as cofactors for metalloproteins and biomolecules. Cd which is not used by plant cells in any way and known to be extremely toxic [38] is present in SMW in small amounts (0.21 µg/L). Fe and Ni are among most represented heavy metals found in SMW. Similar to Se these two metals are essential at low concentrations for living cells being part of the metalloenzymes that catalyze processes including the biosynthesis of hormones, the metabolism of drugs, DNA and RNA base repair and, the biosynthesis of antibiotics [39] .
Duckweed showed highest efficiency of uptake of most of the heavy metal contaminations found in 100% SMW, with almost complete (99%) removal of Cu and 87% uptake of Co. Concentrations of Ni, Zn and Cd
*heavy metals.
were reduced at lower efficiencies (61%, 58% and 57%, respectively). Both elodea and water clover showed lower levels of heavy metals uptake than duckweed. Elodea showed highest uptakes for Cr (66%) and Zn (46%). Water clover showed highest uptakes for Fe (36%), Mo (35%) and Zn (35%).
In spite of the fact that duckweed have been used for over 30 years to recover heavy metals most of the published experiments were conducted with representatives of Lemna genus, Lemna minor and Lemna gibba [12] [40] -[46] . In most cases these plants showed almost complete removal of most of the heavy metals from the individual solutions and significant reduction of their concentrations from different types of wastewaters. Relatively little information is available regarding the effects of heavy metals uptake and their removal from industrial and residential wastewater by duckweed. Representatives of Spirodela genus, which is phylogenetically close L. punctata, S. punctata and S. polyrhiza showed some tolerance to the Cr showing 97% absorption in 20 ppm sodium chromate solution [47] [48] . We also found one paper describing removal of Pb and Zn by unidentified representative of Spiradella genus. This plant showed up to 60% removal of both heavy metals after 14 days [47] [48] . The effective absorption of Zn, Cr, Cu, Pb and Cd from water solutions by elodea was previously shown by Wilson and Moore, 1997 [49] , who showed that elodea was not adversely affected by the heavy metals.
3.2. Thermochemical Conversion of Duckweed, Elodea and Water Clover into Bio-Gas, Bio-Liquid and Bio-Solids.
The three aquatic macrophyte representatives used in this work for mining wastewater treatment represent distant species colonizing different zones of wetland: water clover growth in mud and shallow water (zones 1 and 3, Figure 1), duckweed growth on water surface (zone 2), and elodea growth in deeper water (zone 4). Together these plants could cover most of the wetland water reservoirs. In this work we analysed thermochemical conversion of duckweed, elodea and water clover biomass collected after the recycling of Se and heavy metals from the mining wastewater into three major pyrolysis products, bio-gas, bio-oil and bio-char. Previously we have shown the significant potential of other duckweed species, Lemna minor as a feedstock for the production of bio-oil and bio-char [10] [26] .
Thermogravimetric Analysis of Duckweed, Elodea and Water Clover
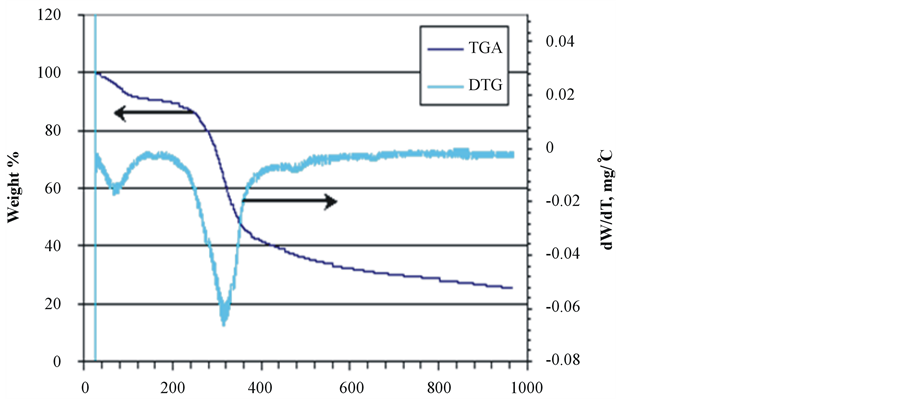
Investigation of the thermal behavior of duckweed, elodea and water clover over a wide range of temperatures (25˚C - 950˚C) was carried out using a thermogravimetric analyzer (TGA). The results of the proximate analysis of the samples, including the content of primary and secondary volatiles, fixed (solid) carbon and ash (i.e., inorganic components of the samples) are summarized in Table3 Total volatiles collected at 120˚C - 950˚C represent 63% - 75% of total product, with the highest level observed in water clover, which also contain the highest proportion of primary volatiles (73%) collected at 120˚C - 650˚C.
Duckweed and elodea showed much higher proportion of secondary volatiles (650˚C - 950˚C). Duckweed showed a slightly reduced proportion of fixed carbon (14.7% vs 18.5% and 17.4% in elodea and water clover, respectively), but greater amounts of ash (12.7% vs 7.9% and 3% in elodea and water clover, respectively). Thermogravimetric analyses of the pre-dried samples of duckweed, elodea and water clover at a temperature range of 25˚C - 950˚C and a heating rate of 20˚C/min are depicted in Figure 3.
The results of the TG/DTG analyses of the samples are consistent with the complex structure of aquatic biomass species, comprising several classes of natural compounds: proteins, lipids, and carbohydrates. TGA/DTG patterns of all analyzed samples show three distinctive zones of thermal degradation. It the first dehydration zone (20˚C - 190˚C), water molecules adsorbed on the samples’ surface as well as those bound within the inner cells of the biomass species are released at 20˚C - 100˚C and 100˚C - 190˚C, respectively. All the tested samples
 (a)
(a)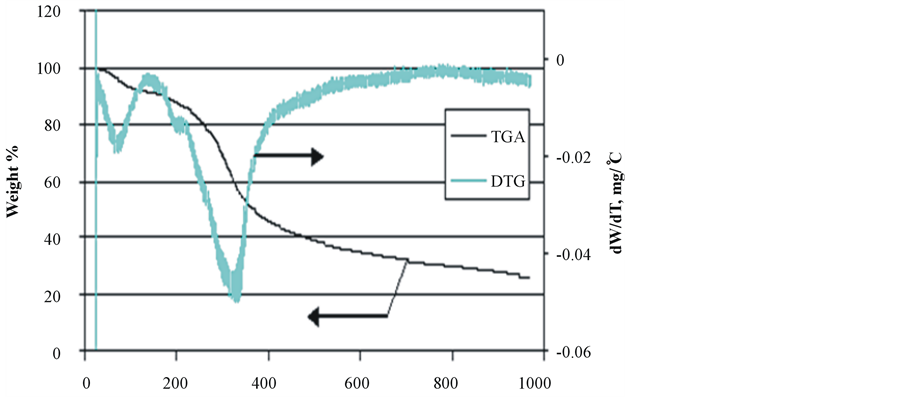 (b)
(b)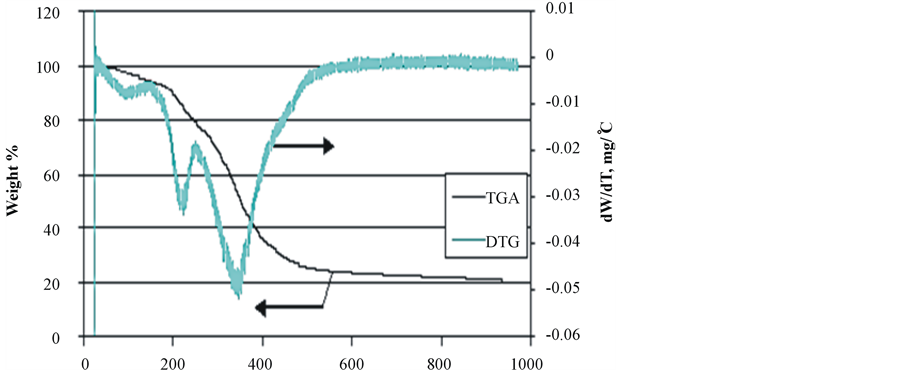 (c)
(c)
Figure 3. TGA/DTG patterns of biomass species. (a) Duckweed; (b) Elodea; (c) Water clover.
showed a similar pattern in this zone of thermal degradation. In the second zone (190˚C - 400˚C), the majority of volatile compounds were released, with the maximum release occurring at the temperature range of 300˚C - 350˚C. During this stage of thermal degradation, the chemical bonds of different bio-polymers start breaking with less thermally stable compounds degrading first. The intensive peak at 320˚C - 340˚C in the DTG curves of all species can be attributed to hemicellulose [50] [51] . The cellulose molecules typically degrade in the temperature range of 350˚C - 370˚C, thus, it is probable that its peak is overlapped with that of hemicellulose. A similar DTG profile for the macroalgae species has been reported by other authors, who showed that hemicellulose is thermally degraded faster than cellulose with the latter breaking between 250˚C and 407˚C and higher [51] .
It can be seen that the DTG thermogram of water clover markedly differs from that of both duckweed and elodea, in that it exhibits two peaks in the low and high temperature ranges of the second degradation zone. The peak in the low temperature range (200˚C - 220˚C) could be attributed to degradation and evaporation of oily compounds and other thermally unstable macromolecules. This behavior is in agreement with observations reported by other authors, who stated that prior to decomposition of hemicellulose in water clover, oily compounds degraded and evaporated from the sample at the temperature range of 170˚C - 230˚C [52] .
In the third zone (450˚C - 700˚C), the degradation of thermally stable bio-polymers occurs. A very weak peak at 480˚C in the DTG of duckweed and elodea samples may point to the presence of small amounts of lignin-like compounds. It has been reported that thermal degradation of lignin in macroalgae [53] and plants [50] [53] [54] showed peaks in this temperature range. Weak peaks at very high temperature range (about 680˚C - 700˚C) could be attributed to carbonization of char and thermal transformations in inorganic ash [52] .
3.3. Pyrolysis of Duckweed, Elodea and Water Clover
3.3.1. Distribution of Pyrolysis Products
The distribution of biomass pyrolysis products: gas, liquid and solids (char) is shown in Figure 4. No significant differences were observed in the distribution of gas, liquid and solid products between duckweed and elodea. These plants showed good mass balances (greater than 93%) of the major pyrolysis products: gas, liquid and solids (char) and their distribution. Char was the main product of duckweed and elodea pyrolysis (46.9% and 49.7%, respectively), whereas, water clover produced higher yield of the liquid fraction (49.3%) with correspondingly lower yield of gaseous (10.4%) and solid (25.1%) products.
3.3.2. Analysis of Gaseous Products of Pyrolysis
Table 4 shows the results of GC analysis of pyrolysis gaseous products, consisting mainly of CO and CO2 and small amounts of H2 and light (C1-C4) hydrocarbons: methane, ethane, ethene, propane, propene and butanes/ butenes. CO2 is the predominant component of the pyrolysis gas in all samples, with similar proportions observed in duckweed and elodea (71% - 86%, respectively) and 62% for water clover. Lower percentages of CO were found in elodea (11%) compared to duckweed (21%) and water clover (25%). Water clover showed significantly higher proportion of gaseous hydrocarbons, methane (C1), ethane/ethene (C2), propane/propane (C3)
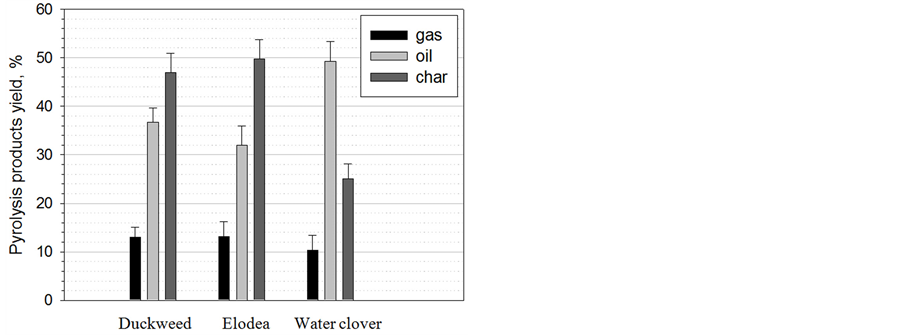
Figure 4. Production of bio-oil, bio-gas and bio-char after pyrolysis of duckweed, elodea and water clover.
Table 4. GC analysis of gaseous products of duckweed, elodea and water clover (in vol%).
Total C4: butane + iso-butane + sec-butanol + tert-butanol.
and butane/iso-butane/sec-butanol/tert-butanol (C4) compare to duckweed and elodea.
3.3.3. Analysis of Bio-Liquid Products of Pyrolysis
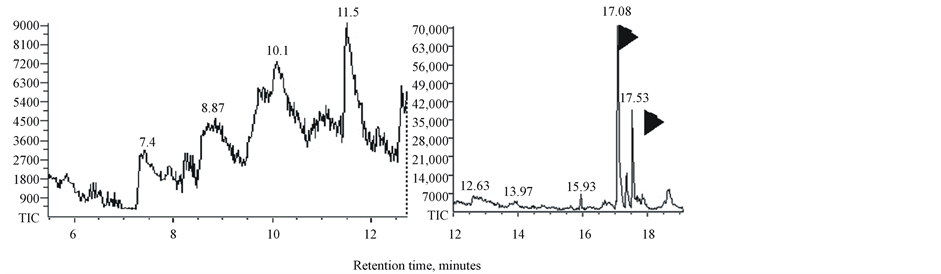
Bio-oils produced by pyrolysis of duckweed, elodea and water clover are dark-brown viscous liquids (not shown). GC-MS chromatograms of dichloromethane-dissolved bio-oil samples and retention times of the peaks of individual components of bio-oils are shown in Figure 5. The peak assignments and the list of molecules identified by search-match feature of the MS software are summarized in Table5 Only those peaks with a high degree of certainty (over 90%) are included in this list. In general, all analysed samples produced a mixture of low molecular weight compounds representing derivatives of aromatic and non-aromatic heterocyclic and oxygenated compounds such as phenols, pyrrole, indolizine, indole and piperidine. Water clover showed a larger spectrum of the derivatives of phenols, pyrroles, indoles and piperidines than duckweed and elodea. Most of these products were previously identified in pyrolysis products of another duckweed representative, L. minor [30] [31] . Characteristically, liquid composition of both of these duckweed species (L. minor and L. punctata) showed no other hydrocarbons than phytol. Most of the molecules identified in these duckweed species can potentially be converted into “green” gasoline and diesel fuel using existing techniques, such as catalytic hydrodeoxygenation. Most of these molecules were also found after pyrolysis of micro and macroalgae [22] [55] . Unlike duckweed, bio-oil produced from elodea and water clover samples showed the presence of diesel-range long chain saturated and mono-unsaturated alkanes (parafins) and their derivatives sizing from C14 to C20, tetradecane, pentadecane, hexadecane, octadecane, 9-eicosane, 9-nonadecene, phytol (3,7,11,15-tetramethyl-2-hexadecen-1-ol, acyclic diterpene alcohol) and 3,7,11,15-tetramethyl-2-hexadecene (Table 5 and Table 6). The bio-oil contained a mixture of the similar long chain lipid-derived alkanes were found in pyrolysis products of some algal strains [22] [54] [56] [57] .
Phytol, the product of chlorophyll molecule’s degradation represents the most abundant pyrolysis product and also was detected in all analysed samples (Table 6). A derivative of phytol, 3,7,11,15-tetramethyl-2-hexadecene (15.5 - 15.93 min retention time) was also detected in all samples but at lower concentrations. Phytol is commercially used as a precursor for the manufacture of synthetic forms of vitamin E [58] and vitamin K1 [59] Phytol is also used in the fragrance industry and used in cosmetics, shampoos, toilet soaps, household cleaners and detergents [60] . Its worldwide use has been estimated to be approximately 0.1 - 1.0 metric tons per year [www.ifraorg.org/view_document.aspx?docId=22594]. Phytol is being explored by Argonne national laboratory for the production of drop-in biofuels
(http://www.transportation.anl.gov/engines/multi_dim_model_biofuels.html). Its physical and chemical properties such as density, cetane number and heat of combustion are close to that of diesel fuel.
3.3.4. Analysis of Solid Products of Pyrolysis (Bio-Char and Ash)
Plant biomass is a resource for the production of value-added bio-solids such as bio-chars and ashes. Both products have been used for decades as soil amending agents enhancing moisture retention and nutrient holding capacity. Biochar has traditionally been used for the production of activated carbons and other carbonaceous products [61] . Because of its availability, stability and low cost activated carbons can be used as adsorbents, catalyst supports, or utilized directly as catalysts, replacing conventional expensive catalysts such as noble metals, Pt, Rh, Ir or Ni that can be easily poisoned by aggressive substances such as sulphurous, phosphorus and nitrogenous compounds [57] . We have previously shown that CO2-treated bio-char exhibited appreciable initial catalytic activity in biogas reforming with production of syngas[31] .
The yield of bio-char was higher for elodea (50%) and duckweed samples (48%), and lower for water clover samples (25%) (Figure 4). Based on the thermogravimetric patterns obtained in flowing air atmosphere, the ash
 (a)
(a)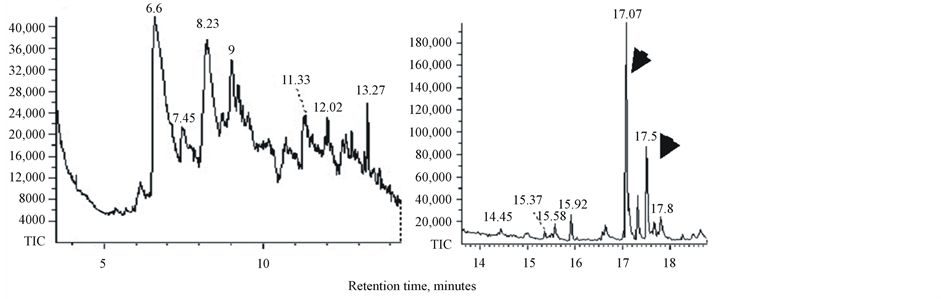 (b)
(b)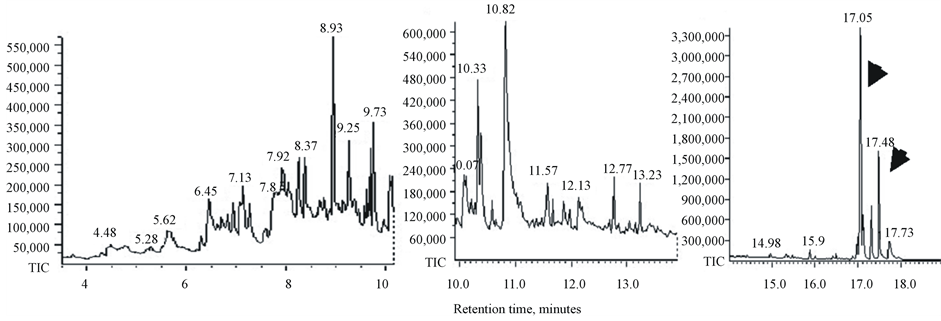 (c)
(c)
Figure 5. GC-MS chromatograms of bio-oil from duckweed, elodea and water clover.
content of duckweed and elodea bio-chars were estimated at a weight fraction of 12.7% and 7.9%, respectively (Table 3). Water clover showed a weight fraction of 3% which is similar for typical plant biomass (typically less than weight fraction of 2% [31] ).
4. Conclusions
For the first time three common Australian representatives of aquatic macrophytes, duckweed, (), elodea () and water clover were used for bioremediation of the Se-rich mining wastewater. Growing and colonising different depths of water reservoirs these plants represent attractive components for bio-filtration. Generated substantial biomass represents an attractive feedstock for biofuel production. The main conclusions from this research include:
Table 5. GC-MS analysis of bio-oil products of duckweed, elodea and water clover pyrolysis.
Table 6. Chemical formulas of selected chemicals.
1) Duckweed elodea () and water clover could be effective at removing TSe and heavy metals from the Se-rich mining wastewater.
2) The highest efficiency of SMW phytoremediation by duckweed is a result of its tolerance to SMW and efficiency of TSe and heavy metal’s uptake and accumulation.
3) Along with efficient biofiltration of microelements duckweed has generated a greatest biomass after one week growing in SMW.
4) Analysis of pyrolysis products showed that bio-oils contain a mixture of promising precursors of renewable fuels and petrochemicals. Chemical composition of liquid fraction duckweed showed no hydrocarbons and was different from the spectrum of chemicals found in elodea and water clover, which petrochemicals and straightchain C14-C20 alkanes. These hydrocarbons can be directly used as a diesel fuel supplement, or as a glycerinefree component of biodiesel. Pyrolysis products of duckweed can potentially be converted into gasoline and diesel biofuel using different existing techniques, such as catalytic hydrodeoxygenation.
Analysis of pyrolysis products showed that char was the main product of duckweed and elodea pyrolysis, whereas, water clover produced higher yields of the liquid fraction.
Acknowledgements
The authors are gratefully thankful to Airlie and Robert Bell from Bio-Tech Waste Management Pty Ltd for providing the duckweed culture and helpful suggestions on their growth; Mr Hendrik Denkhaus, DAAD “RISE worldwide” program (Germany) for involvement in sample preparation for analysis and Paul Morrison for analysis of selenium in plants and wastewater. This work was supported by the College of Science, Engineering and Health and the School of Applied Sciences of Royal Melbourne Institute of Technology University.
References
- Salt, D.E., Smith, R.D. and Raskin, I. (1998) Phytoremediation. Annual Review of Plant Physiology and Plant Molecular Biology, 49, 643-668. http://dx.doi.org/10.1146/annurev.arplant.49.1.643
- Dushenkov, S. (2003) Trends in Phytoremediation of Radionuclides. Plant and Soil, 249, 167-175. http://dx.doi.org/10.1023/A:1022527207359
- Marmiroli, N., Marmiroli, M. and Maestri, E. (2006) Phytoremediation and Phytotechnologies: A Review for the Present and the Future. Soil and Water Pollution Monitoring, Protection and Remediation, 69, 403-416.http://dx.doi.org/10.1007/978-1-4020-4728-2_26
- Hasan, S.H., Talat, M. and Rai, S. (2007) Sorption of Cadmium and Zinc from Aqueous Solutions by Water Hyacinth (Eichchornia crassipes). Bioresource Technology, 98, 918-928. http://dx.doi.org/10.1016/j.biortech.2006.02.042
- Weres, O., Jaouni, A.R. and Tsao, L. (1989) The Distribution, Speciation and Geochemical Cycling of Selenium in a Sedimentary Environment, Kesterson Reservoir, California, USA. Applied Geochemistry, 4, 543-563. http://dx.doi.org/10.1016/0883-2927(89)90066-8
- Fan, T.W.M., The, S.J., Hinton, D.E. and Higashi, R.M. (2002) Selenium Biotransformations into Proteinaceous Forms by Foodweb Organisms of Selenium-Laden Drainage Waters in California. Aquatic Toxicology, 57, 65-84. http://dx.doi.org/10.1016/S0166-445X(01)00261-2
- Fang, W.X. and Wu, P.W. (2004) Elevated Selenium and Other Mineral Element Concentrations in Soil and Plant Tissue in Bone Coal Sites in Haoping Area, Ziyang County, China. Plant and Soil, 261, 135-146.http://dx.doi.org/10.1023/B:PLSO.0000035580.32406.
- Stewart, A.R., Luoma, S.N., Schlekat, C.E., Doblin, M.A. and Hieb, K.A. (2004) Food Web Pathway Determines How Selenium Affects Aquatic Ecosystems: A San Francisco Bay Case Study. Environmental Science & Technology, 38, 4519-4526. http://dx.doi.org/10.1021/es0499647
- Thompsoneagle, E.T. and Frankenberger, W.T. (1990) Protein-Mediated Selenium Biomethylation in Evaporation Pond Water. Environmental Toxicology and Chemistry, 9, 1453-1462. http://dx.doi.org/10.1002/etc.5620091204
- Xu, J. and Shen, G. (2011) Growing Duckweed in Swine Wastewater for Nutrient Recovery and Biomass Production. Bioresource Technology, 102, 848-853. http://dx.doi.org/10.1016/j.biortech.2010.09.003
- Cheng, J., Landesman, L., Bergmann, B.A., Classen, J.J., Howard, J.W. and Yamamoto, Y.T. (2002) Nutrient Removal from Swine Lagoon Liquid By Lemna Minor 8627. Transactions of the ASAE, 45, 1003-1010.http://dx.doi.org/10.13031/2013.9953
- Basile, A., Sorbo, S., Conte, B., Cobianchi, R.C., Trinchella, F., Capasso, C. and Carginale, V. (2012) Toxicity, Accumulation, and Removal of Heavy Metals by Three Aquatic Macrophytes. International Journal of Phytoremediation, 14, 374-387. http://dx.doi.org/10.1080/15226514.2011.620653
- Salonen, J.T., Alfthan, G., Huttunen, J.K. and Puska, P. (1984) Association between Serum Selenium and the Risk of Cancer. American Journal of Epidemiology, 120, 342-349.
- Zhu, L.Z., Yang, G.Q., Liu, S.J., Gu, L.Z., Qian, P.C., Huang, J.H. and Lu, M.O. (1987) Human Selenium Requirements in China. AVI Press, Westport.
- Ullrey, D.E. (1992) Basis for Regulation of Selenium Supplements in Animal Diets. Journal of Animal Science, 70, 3922-3927.
- Shortridge, E.H., O’Hara, P.J. and Marshall, P.M. (1971) Acute Selenium Poisoning in Cattle. New Zealand Veterinary Journal, 19, 47-50. http://dx.doi.org/10.1080/00480169.1971.33930
- Tan, J., Wang, W., Wang, D. and Hou, S. (1994) Adsorption, Volatilization, and Speciation of Selenium in Different Types of Soils in China. In: Frankenberger Jr., W.T. and Benson, S., Eds., Selenium in the Environment, Marcel Dekker, New York, 47-68.
- Ohlendorf, H.M., Hothem, R.L., Bunck, C.M., Aldrich, T.W. and Moore, J.F. (1986) Relationships between Selenium Concentrations and Avian Reproduction. Transactions of the North American Wildlife and Natural Resources Conference, 51, 330-342.
- Presser, T.S. and Ohlendorf, H.M. (1987) Biogeochemical Cycling of Selenium in the San-Joaquin Valley, California, USA. Environmental Management, 11, 805-821. http://dx.doi.org/10.1007/BF01867247
- Carvalho, K.M. and Martin, D.F. (2001) Removal of Aqueous Selenium by Four Aquatic Plants. Journal of Aquatic Plant Management, 39, 33-36.
- Terry, N. and Zayed, A.M. (1998) Environmental Chemistry of Selenium. Marcel Dekker, New York.
- Chow, M.C., Jackson, W.R., Chaffee, A.L. and Marshall, M. (2013) Thermal Treatment of Algae for Production of Biofuel. Energy & Fuels, 27, 1926-1950. http://dx.doi.org/10.1021/ef3020298
- Miao, X.L., Wu, Q.Y. and Yang, C.Y. (2004) Fast Pyrolysis of Microalgae to Produce Renewable Fuels. Journal of Analytical and Applied Pyrolysis, 71, 855-863. http://dx.doi.org/10.1016/j.jaap.2003.11.004
- Chen, Q., Jin, Y.L., Zhang, G.H., Fang, Y., Xiao, Y. and Zhao, H. (2012) Improving Production of Bioethanol from Duckweed (Landoltia punctata) by Pectinase Pretreatment. Energies, 5, 3019-3032. http://dx.doi.org/10.3390/en5083019
- Zhao, X., Elliston, A., Collins, S.R.A., Moates, G.K., Coleman, M.J. and Waldron, K.W. (2012) Enzymatic Saccharification of Duckweed (Lemna minor) Biomass without Thermophysical Pretreatment. Biomass & Bioenergy, 47, 354-361. http://dx.doi.org/10.1016/j.biombioe.2012.09.025
- Xu, J.L., Cui, W.H., Cheng, J.J. and Stomp, A.M. (2011) Production of High-Starch Duckweed and Its Conversion to Bioethanol. Biosystems Engineering, 110, 67-72. http://dx.doi.org/10.1016/j.biosystemseng.2011.06.007
- Wang, H.M., Male, J. and Wang, Y. (2013) Recent Advances in Hydrotreating of Pyrolysis Bio-Oil and Its Oxygen-Containing Model Compounds. ACS Catalysis, 3, 1047-1070. http://dx.doi.org/10.1021/cs400069z
- Wang, W.C. and Freemark, K. (1995) The Use of Plants for Environmental Monitoring and Assessment. Ecotoxicology and Environmental Safety, 30, 289-301. http://dx.doi.org/10.1006/eesa.1995.1033
- Wang, Z., Lin, W.G., Song, W.L. and Wu, X.X. (2012) Pyrolysis of the Lignocellulose Fermentation Residue by Fixed-Bed Micro Reactor. Energy, 43, 301-305. http://dx.doi.org/10.1016/j.energy.2012.04.026
- Muradov, N., Fidalgo, B., Gujar, A.C. and T-Raissi, A. (2010) Pyrolysis of Fast-Growing Aquatic Biomass—Lemna minor (duckweed): Characterization of Pyrolysis Products. Bioresource Technology, 101, 8424-8428. http://dx.doi.org/10.1016/j.biortech.2010.05.089
- Muradov, N., Fidalgo, B., Gujar, A.C., Garceau, N. and T-Raissi, A. (2012) Production and Characterization of Lemna minor Bio-Char and Its Catalytic Application for Biogas Reforming. Biomass & Bioenergy, 42, 123-131. http://dx.doi.org/10.1016/j.biombioe.2012.03.003
- Mohapatra, D.P., Ghangrekar, M.M., Mitra, A. and Brar, S.K. (2012) Sewage Treatment in Integrated System of UASB Reactor and Duckweed Pond and Reuse for Aquaculture. Environmental Technology, 33, 1445-1453. http://dx.doi.org/10.1080/09593330.2011.633103
- Rawat, S.K., Singh, R.K. and Singh, R.P. (2012) Remediation of Nitrite Contamination in Ground and Surface Waters Using Aquatic Macrophytes. Journal of Environmental Biology, 33, 51-56.
- Plangklang, P. and Reungsang, A. (2008) Effects of Rhizosphere Remediation and Bioaugmentation on Carbofuran Removal from Soil. World Journal of Microbiology & Biotechnology, 24, 983-989. http://dx.doi.org/10.1007/s11274-007-9562-9
- Rai, P.K. (2009) Heavy Metal Phytoremediation from Aquatic Ecosystems with Special Reference to Macrophytes. Critical Reviews in Environmental Science and Technology, 39, 697-753. http://dx.doi.org/10.1080/10643380801910058
- Singh, S.S., Singh, S.K. and Mishra, A.K. (2008) Na+ Regulation by Combined Nitrogen in Azolla pinnata-Anabaena azollae Symbiotic Association during Salt Toxicity. Ecotoxicology and Environmental Safety, 69, 32-38. http://dx.doi.org/10.1016/j.ecoenv.2007.04.001
- Lin, Z.Q., de Souza, M., Pickering, I.J. and Terry, N. (2002) Evaluation of the Macroalga, Muskgrass, for the Phytoremediation of Selenium-Contaminated Agricultural Drainage Water by Microcosms. Journal of Environmental Quality, 31, 2104-2110. http://dx.doi.org/10.2134/jeq2002.2104
- Morrow, H. (2000) Cadmium and Cadmium Alloys. John Wiley & Sons, Inc., Hoboken.
- Banci, L. and Bertini, I. (2013) Metallomics and the Cell: Some Definitions and General Comments. Metal Ions in Life Sciences, 12, 1-13. http://dx.doi.org/10.1007/978-94-007-5561-1_1
- Guimaraes, F.P., Aguiar, R., Oliveira, J.A., Silva, J.A.A. and Karam, D. (2012) Potential of Macrophyte for Removing Arsenic from Aqueous Solution. Planta Daninha, 30, 683-696. http://dx.doi.org/10.1590/S0100-83582012000400001
- Marchand, L., Mench, M., Marchand, C., Le Coustumer, P., Kolbas, A. and Maalouf, J.P. (2011) Phytotoxicity Testing of Lysimeter Leachates from Aided Phytostabilized Cu-Contaminated Soils Using Duckweed (Lemna minor L.). Science of the Total Environment, 410, 146-153. http://dx.doi.org/10.1016/j.scitotenv.2011.09.049
- Parra, L.M., Torres, G., Arenas, A., Sánchez, E. and Rodríguez, K. (2012) Phytoremediation of Low Levels of Heavy Metals Using Duckweed (Lemna minor). In: Ahmad, P. and Prasad, M.N.V., Eds., Abiotic Stress Responses in Plants, Springer, New York, 451-463.
- Rahman, M.A. and Hasegawa, H. (2011) Aquatic Arsenic: Phytoremediation Using Floating Macrophytes. Chemosphere, 83, 633-646. http://dx.doi.org/10.1016/j.chemosphere.2011.02.045
- Tel-Or, E. and Forni, C. (2011) Phytoremediation of Hazardous Toxic Metals and Organics by Photosynthetic Aquatic Systems. Plant Biosystems, 145, 224-235. http://dx.doi.org/10.1080/11263504.2010.509944
- Turker, O.C., Bocuk, H. and Yakar, A. (2013) The Phytoremediation Ability of a Polyculture Constructed Wetland to Treat Boron from Mine Effluent. Journal of Hazardous Materials, 252-253, 132-141. http://dx.doi.org/10.1016/j.jhazmat.2013.02.032
- Ucuncu, E., Tunca, E., Fikirdesici, S., Ozkan, A.D. and Altindag, A. (2013) Phytoremediation of Cu, Cr and Pb Mixtures by Lemna minor. Bulletin of Environmental Contamination and Toxicology, 91, 600-604. http://dx.doi.org/10.1007/s00128-013-1107-3
- Staves, R.P. and Knaus, R.M. (1985) Chromium Removal from Water by Three Species of Dyckweeds. Aquatic Botany, 23, 261-273. http://dx.doi.org/10.1016/0304-3770(85)90070-1
- Srivastav, R.K., Gupta, S.K., Nigam, K.D.P. and Vasudevan, P. (1993) USE of Aquatic Plants for the Removal of Heavy Metals from Wastewater. International Journal of Environmental Studies, 45, 43-50. http://dx.doi.org/10.1080/00207239308710877
- Wilson, J. and Moore, J. (1997) Chromium and Zinc Uptake in Elodea Densa and Ceratophyllum Demersum: Applications for Bioremediation. Bsc. Undergraduate Thesis, Oregon State University, Corvallis.
- Mészáros, E., Várhegyi, G., Jakab, E. and Marosvölgyi, B. (2004) Thermogravimetric and Reaction Kinetic Analysis of Biomass Samples from an Energy Plantation. Energy & Fuels, 18, 497-507. http://dx.doi.org/10.1021/ef034030+
- Skodras, G., Grammelis, P., Basinas, P., Kakaras, E. and Sakellaropoulos, G. (2006) Pyrolysis and Combustion Characteristics of Biomass and Waste-Derived Feedstock. Industrial & Engineering Chemistry Research, 45, 3791-3799. http://dx.doi.org/10.1021/ie060107g
- Daneshvar, S., Salak, F. and Otsuka, K. (2012) Pyrolytic Behavior of Green Macro Algae and Evaluation of Its Activation Energy. International Journal of Chemical Engineering & Applications, 3, 256-263.
- Trinh, T.N., Jensen, P.A., Sørensen, R.H., Dam-Johansen, K. and Søren, H. (2012) Flash Pyrolysis Properties of Algae and Lignin Residue. In: Krautkremer, B., Ed., 20th European Biomass Conference and Exhibition, JRC/IET, Milano, 966-972.
- Ranzi, E., Cuoci, A., Faravelli, T., Frassoldati, A., Migliavacca, G., Pierucci, S. and Sommariva, S. (2008) Chemical Kinetics of Biomass Pyrolysis. Energy & Fuels, 22, 4292-4300. http://dx.doi.org/10.1021/ef800551t
- Kebelmann, K., Hornung, A., Karsten, U. and Griffiths, G. (2013) Intermediate Pyrolysis and Product Identification by TGA and Py-GC/MS of Green Microalgae and Their Extracted Protein and Lipid Components. Biomass & Bioenergy, 49, 38-48. http://dx.doi.org/10.1016/j.biombioe.2012.12.006
- Ross, A.B., Anastasakis, K., Kubacki, M. and Jones, J.M. (2009) Investigation of the Pyrolysis Behaviour of Brown Algae before and after Pre-Treatment Using PY-GC/MS and TGA. Journal of Analytical and Applied Pyrolysis, 85, 3-10. http://dx.doi.org/10.1016/j.jaap.2008.11.004
- Muradov, N.Z. and Veziroglu, T.N. (2008) “Green” Path from Fossil-Based to Hydrogen Economy: An Overview of Carbon-Neutral Technologies. International Journal of Hydrogen Energy, 33, 6804-6839. http://dx.doi.org/10.1016/j.ijhydene.2008.08.054
- Netscher, T. (2007) Synthesis of Vitamin E. Vitamin E: Vitamins and Hormones Advances in Research and Applications, 76, 155-202.
- Daines, A.M., Payne, R.J., Humphries, M.E. and Abell, A.D. (2003) The Synthesis of Naturally Occurring Vitamin K and Vitamin K Analogues. Current Organic Chemistry, 7, 1625-1634. http://dx.doi.org/10.2174/1385272033486279
- McGinty, D., Letizia, C.S. and Api, A.M. (2010) Fragrance Material Review on Phytol. Food and Chemical Toxicology, 48, S59-S63. http://dx.doi.org/10.1016/j.fct.2009.11.012
- Azargohar, R. and Dalai, A.K. (2006) Biochar as a Precursor of Activated Carbon. Applied Biochemistry and Biotechnology, 131, 762-773.
NOTES

*Corresponding author.


