American Journal of Analytical Chemistry
Vol.5 No.2(2014), Article ID:42545,12 pages DOI:10.4236/ajac.2014.52016
Buried Hydrothermal Systems: The Potential Role of Supercritical Water, “ScriW”, in Various Geological Processes and Occurrences in the Sub-Surface
1Ambio Tech Team, Stavanger, Norway
2Lithicon, Trondheim, Norway
3Det Norske Oljeselskap ASA, Trondheim, Norway
Email: martin.hovland@ambio.no, hg.rues@gmail.com, hans.konrad.johnsen@detnor.no
Received November 28, 2013; revised December 29, 2013; accepted January 15, 2014
ABSTRACT
It is well known that seawater that migrates deep into the Earth’s crust will pass into its supercritical domain at temperatures above 407˚C and pressures above 298 bars. In the oceanic crust, these pressures are attained at depths of 3 km below sea surface, and sufficiently high temperatures are found near intruding magmas, which have temperatures in the range of 800˚C to 1200˚C. The physico-chemical behaviour of seawater changes dramatically when passing into the supercritical domain. A supercritical water vapour (ScriW) is formed with a density of 0.3 g/cc and a strongly reduced dipolar character. This change in polarity is causing the ScriW to lose its solubility of the common sea salts (chlorides and sulphates) and a spontaneous precipitation of sea salts takes place in the pore system. However, this is only one of many cases where the very special properties of ScriW affect its surroundings. The objective of this paper is to increase awareness of the many geological processes that are initiated and governed by ScriW. This includes interactions between ScriW and its geological surroundings to initiate and drive processes that are of major importance to the dynamics and livelihood of our planet. ScriW is the driver of volcanism associated with subduction zones, as ScriW deriving from the subduction slab is interacting with the mantle rocks and reducing their melting point. ScriW is also initiating serpentinization processes where olivines in the mantle rocks (e.g. peridotite) are transformed to serpentine minerals upon the uptake of OH-groups from hydrolysed water. The simultaneous oxidation of Fe2+ dissolved from iron-bearing pyroxenes and olivines leads to the formation of magnetite and hydrogen, and consequently, to a very reducing environment. ScriW may also be the potential starter and driver of the poorly understood mud and asphalt volcanism; both submarine and terrestrial. Furthermore, the lack of polarity of the water molecules in ScriW gives the ScriW vapour the potential to dissolve organic matter and petroleum. The same applies to supercritical brines confined in subduction slabs. If these supercritical water vapours migrate upwards to reach the critical point, the supercritical vapour is condensed into steam and dissolved petroleum is partitioned from the water phase to become a separate fluid phase. This opens up the possibility of transporting petroleum long distances when mixed with ScriW. Therefore, we may, popularly, say that ScriW drives a gigantic underground refinery system and also a salt factory. It is suggested that the result of these processes is that ScriW is rejuvenating the world’s ocean waters, as all of the ocean water circulates into the porous oceanic crust and out again in cycles of less than a million years. In summary, we suggest that ScriW participates in and is partly responsible for: 1) Ocean water rejuvenation and formation; 2) Fundamental geological processes, such as volcanism, earthquakes, and metamorphism (including serpentinization); 3) Solid salt production, accumulation, transportation, and (salt) dome formation; 4) The initiation and driving of mud, serpentine, and asphalt volcanoes; 5) Dissolution of organic matter and petroleum, including transportation and phase separation (fractionation), when passing into the subcritical domain of (liquid) water.
Keywords:Supercritical Seawater; Hydrothermal Salt Model; Rifting Sediment Basins; Salt Precipitation from Supercritical Seawater; Petroleum Migration; Volcanism; Mud Volcanoes
1. Introduction
One of the last century’s greatest discoveries was done in the late 1970s [1] with the direct observation of deep-sea active hydrothermal venting: “The typical basaltic terrain at the ridge axis is bleak indeed. Monotonous fields of brown pillows are cut by faults and fissures. One must examine several square meters to find a single organism. Yet here was an oasis. Reefs of mussels and fields of giant clams were bathed in the shimmering water, along with crabs, anemones and large pink fish. The remaining five hours of ‘bottom time’ passed in something close to frenzy” [2]. Because the water depth at this location was only 2500 m, the water inside the observed hydrothermal chimney was likely boiling as it exited the chimney as the pressure is not high enough for supercritical seawater [3]. As the cited narration indicates, it was immediately realized that deep-sea “phase separation” had profound and unexpected side-effects, including the fuelling of chemosynthetic life forms [4,5].
However, what was not realized at that time was that a few hundred metres away from the observers, was perhaps the most lethal fluid imaginable—supercritical water (ScriW). In this brief article, we will discuss some of the properties of ScriW, its occurrences and some of its geological effects and consequences. To us, it seems as if this clearly under-explored water phase has a central role in primary geological and thermodynamic, hydrological processes, not only on our planet, but also on others. It has newly been determined that mafic arc magmas contain about 4 wt% water [6]. Because water in the mantle is a supercritical fluid, it influences many geological processes, including earthquakes and volcanic eruptions [7].
2. Supercritical Water (ScriW)
The critical point (CP) for distilled water is 374.15˚C and 221.2 bar. Beyond this point, the physical and chemical properties of water change fundamentally; e.g. the dielectric constant declines from the normal water value of 80 to only 2 at CP [8]. Similarly, the ionic dissociation constant declines from the normal water value of 10−14 down to 10−23 at CP, and the density of water attains a value of ~0.3 g/cm3 [8,9]. This strongly influences the inter-molecular behavior of water, and Raman spectra of deuterated water in the supercritical region show only remnants of hydrogen bonding [10,11]. Consequently, the supercritical water behaves essentially as a non-polar low-density fluid, with solvation properties resembling those of low-polarity organic fluids; e.g., it is fully miscible with petroleum and unable to dissolve common sea salts [8,9].
However, the physico-chemical properties of ScriW with its high diffusivity, allows it to permeate into nanoscale voids, fissures, and cracks, and alter the surrounding rocks. These properties of ScriW are important when studying high temperature hydrothermal systems and the hydrothermal alteration of rocks. However, it should be noted that theoretical calculations of the dielectric constant of water at high T and P were just recently achieved [12]. This lead to the formation of a new numerical “Deep Earth Water” (DEW) model [7], on which further exciting results will undoubtedly be based.
2.1. Supercritical Brines
When salt is added to pure water, one more degree of freedom is added to the system, with the CP changing along a line connecting the CP of the two pure components (salt and water). Depending on the local salinity, supercritical conditions in seawater and brines will be reached at pressures and temperatures that differ significantly from those of pure water. The onset of critical behaviour in normal seawater with salinity 3.5 wt% occurs at around 300 bars and 407˚C [3]. If boiling takes place, a higher fluid column (steam and water) is necessary in order to reach a sufficient hydrostatic pressure to attain the CP. Some experimental studies of supercritical brines have been reported in the literature; e.g., Tester et al. [8], who examined the phase behavior of synthetic brines below and above their CP. The researchers observed the process through a sapphire window in the pressure chamber, and when the brines were passing into the supercritical region at a temperature of 407˚C and 298 bar pressure (the CP of seawater), they could see a “cloud” formed by the onset of “shock crystallization” of NaCl and Na2SO4 [8,13,14]. The sudden phase transition occurred as the solubility of the salts declined to nearzero over a temperature range of only a few degrees C. The resulting solids were found to consist of amorphous microscopic particles of sizes between 10 and 100 µm. The reason for the observed shock crystallization is discussed below.
2.2. Supercritical Brines in Nature
Natural occurrences of supercritical water and associated processes are well hidden from direct observation, deep below the Earth surface and inside the deep sea hot vents [15]. In the ocean, the critical pressure for seawater corresponds to a depth of ~2940 m [16]. Given that the world average ocean depth is more than 3600 m, there is a great potential for the formation of ScriW in hot and fractured oceanic crust. Scientific deep sea drilling of oceanic crust has also shown regional porosities of up to 25% [17,18], and consequently, a large potential for the circulation of huge volumes of fluids. At these depths, the exposure of circulating seawater to strong heat sources; e.g. intrusive basalts of typically 800˚C - 1200˚C, will potentially cause the water to become supercritical [14]. A fundamental knowledge of the behavior of water and salt at various temperatures and pressures encountered in the Earth’s crust must be the starting point for understanding the natural processes associated with supercritical water.
The ability of water to dissolve salts is due to the highly dipolar nature of the water molecules, allowing the water molecules to form shell-like structures around the dissociated salt ions. These shells screen the ions’ charges and significantly attenuate their Coulomb interactions with each other. The dipolar moment of water and its orientational selectivity is directly reflected in the strength of the hydrogen bonded network. At 5˚S on the Mid-Atlantic Ridge sampling of fluids occurring above the critical TP point (CP) was achieved [16].
2.3. Occurrence of ScriW on Earth
It is estimated that about 60% of Earth’s surface is covered by oceanic crust, which has thicknesses in the order of zero to 10 km. Oceanic crustal plates are highly dynamic, and are known to move at speeds of up to 4 cm yr−1. The oceanic crust is highly fractured, especially along the extensive long transform shear faults, like the well-known Mendocino and Murray transforms in the eastern Pacific Ocean. Because of the high regional porosity of the oceanic crust (mentioned above) it represents a huge reservoir of brine, located directly on top of the mantle, which has a minimum temperature of 1100˚C. The volume of brine residing in the world’s oceanic crust is of the same magnitude as that of the entire ocean. It has been calculated that all oceanic water circulates in and out of the oceanic crust at least once per million years [18]. This means that the ocean water becomes exposed to the critical or near critical conditions once every million years. The consequence of such an exposure is undoubtedly that the seawater becomes rejuvenated, both with regard to its salt content and interaction with the rocks of the oceanic crust. Organic components dispersed in the seawater will also be filtered off, or dissolved in the super critical domain of the crust. The natural domain for ScriW on Earth is deep in the sub-surface and inside deep-sea hot vents [15,19], well hidden from direct observation.
We here define the Potential Critical Point (PCP) as being the depth at which the pressure is equivalent to the critical point for the liquid residing in the crust or at the particular water depth (Figure 1). As mentioned previously, the PCP for normal seawater will be at 2940 m [16]. This means that the water has the potential to become supercritical if in contact with a sufficiently strong heat-source, e.g., a magma chamber or a volcanic sill, with temperatures higher than 407˚C [16].
One of the most important aspects of ScriW is associated with water that dissipates out of subduction slabs and lowers the melting point of the surrounding rocks. This leads to the formation of flowing magma and volcanism in subduction zones and back arc regions. Therefore, volcanism is a direct result of ScriW formation at depth.
At several locations in the deep ocean, very dense brines have been documented. These are called “hypersaline brine lakes” or brine pools [21,22]. As the concentration of salt increases, the PCP will change accordingly and move deeper (towards higher pressure). Consequently, it is concluded that every location on Earth has its own PCP depth, also on land. In March, 2010, the scientific “Icealand Deep Drilling Project” succeeded in performing a brief flow test of transparent superheated steam at 410˚C (see www.iddp.is). They drilled near the Krafla geothermal power plant and extinct volcano, where they located magma at 2104 metres below surface, at a temperature of ~900˚C. In their next scientific drilling (well IDDP-2), they are planning to generate electric power at a downhole temperature of 430˚C - 550˚C, a downhole pressure of 230 - 260 bar, at a rate of 0.67 m3/s. They expect to produce ~50 mWe, compared to only one tenth of this amount (~5 mWe) for an equivalent conventional “dry-steam” geothermal system [23].
Mid-Ocean Spreading Ridges and Subduction Zones
A mid-ocean ridge constitutes the boundary between two tectonic plates. The spreading ridges are characterized by an uplifted seafloor that results from convection currents of magma rising in the mantle along a linear weakness in the oceanic crust. These magma currents emerge as lava on the seafloor, creating new crust upon cooling. Black smokers are commonly associated with the mid-ocean ridges, building up mounds of sulphides and other minerals accumulating from the hydrothermal activity. In the Red Sea, however, the hydrothermal systems of new oceanic crust typically occupy depressions, known as “deeps”. Wherever such systems are active at ocean depths near 2900 m, ScriW will either be exposed to or resides close to the seawater column (Figure 1).
There are several types of subduction zones, including those where serpentinization occurs at depth, westward of the Mariana trough, where serpentinite mud volcanoes occur [24]. Thus, we can also say that ScriW causes
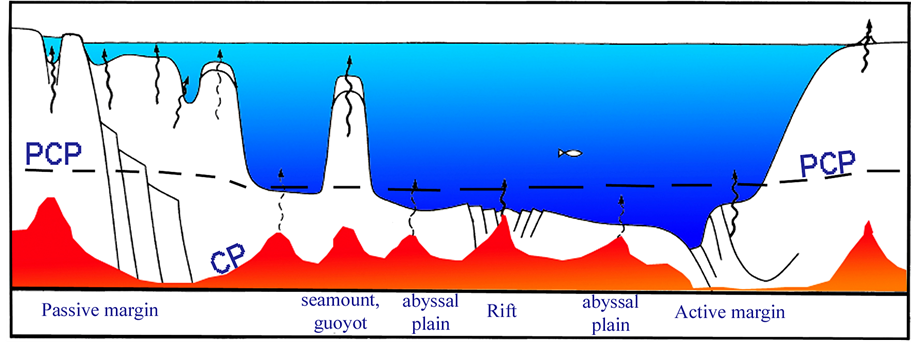
Figure 1. Sketch illustrating that although there is a potential to produce supercritical water (ScriW) due to pressure, the heat source may be located much deeper, which means that the critical point of any water present, will be at its critical point, CP, only according to the local heatflow. Blue is water, red indicates above water CP-temperature conditions. “PCP” indicates the potential critical point for water, according to pressure only. Arrows indicate known occurrences of upward fluid flow. Based on Hovland and Judd [20].
massive production of clay type particles. In subduction zones, the PCP will be deflected downwards, because of cooling by the down-going oceanic plate (Figure 1).
3. Hydrothermal “Outsalting”
The sub-sea salt accumulations of the Red Sea are several km thick [25]. Ever since the discovery of these enormous accumulations of sea salts, their mode of formation has represented a challenging puzzle to geologists. The original theory of evaporation was developed on the basis of observed salt lakes during the early 1900’s and later modified during the 1970’s and -80’s, into the “top-down” “evaporite” theory [26-28]. Briefly, the solar evaporite theory suggests that deep salt water basins with restricted communication to the open ocean dried out and filled up several times, causing thick accumulations of salts.
This “evaporite” explanation may be realistic for salt thicknesses some tens of metres, however, the accumulation of salt thicknesses of several thousand metres, as found in the Mediterranean, the Red Sea, and other places world-wide, is difficult to explain with the evaporite theory [29,30]. It is especially difficult to explain how large volumes of salt have accumulated in sedimentary basins such as in South-Western Canada, which contains volumes of salts approaching those of the entire world oceans [31].
Lowell and Rona [32] have already suggested that the magma chamber in a hydrothermal cell may be insulated from the near surface area by a high density brine: “The formation of a brine layer at the base of a hydrothermal system may act as a thermal conductive barrier between the overlying hydrothermal circulation and the magma body and be a salinity source for saline vent fluids” [32].
3.1. The Hydrothermal Salt Model
As mentioned previously, a key to understanding the subsurface accumulation of large salt bodies is linked to how brines behave in the subsurface when pressurized and heated beyond their critical point. Above 570˚C, a nearly pure vapor phase coexists with solid salt, and convection is driven exclusively by the temperature gradient [33]. The inability of ScriW to dissolve common sea salts results in the accumulation of salt particles in the conduits whenever brines move into the supercritical domain.
By combining numerical molecular modeling with basin modeling, it was possible to simulate how large amounts of solid salts could be produced and accumulated hydrothermally by supercritical “out-salting” [14]. The hydrothermal salt model [14] demonstrates how large masses of halite (and other sea salts) can accumulate by precipitating from supercritical seawater at places where seawater is exposed to high heat flow in the subsurface. An important aspect of the model is that it allows for the formation of vertical “piercement” salt structures (“salt domes”) as first suggested by Lewis and Holness [34]. These vertical salt structures may also be conduits of hot fluids, including brines, and can therefore act as giant hydrothermal chimneys, that not only transport inorganic minerals, but also organic molecules, including long-chain hydrocarbons [35]. Salt may also precipitate in subduction zones, when porous rocks containing saline porewater are exposed to sufficiently high temperature and pressure.
A conceptual digital model of a hydrothermal system in the Atlantis II Deep of the Red Sea was constructed [14]. It includes a shallow magma-chamber that causes a sufficiently high heat-flow to drive a hydrothermal convection cell of seawater through a package of sedimentary rocks (Figure 2). Based on the physical mechanisms that govern the supercritical behavior of seawater and results of laboratory tests on salt crystals at high TPconditions [36], a numerical simulation was performed for the hydrothermal flow. The simulation model results in the accumulation of salt that precipitates along the flow lines within the supercritical region (Figure 2). In the zone closest to the magma-chamber, temperatures will be beyond 800˚C which is close to the melting point of halite (Figure 2). According to these simulation results, the supercritical water vapor will rise vertically in the central reflux zone, and the flow will have highest velocity immediately above the magma chamber due to the buoyancy forces [14].
Tectonically active (rifting) environments with high sedimentation rates, combined with hot brine pools surrounding the hydrothermal centers, such as in the Red Sea, may offer the necessary protection to submarine hydrothermally precipitated salts. The Red Sea is also characterized by a relatively high sedimentation rate and has near tropical climatic conditions with high surface evaporation, which also helps to keep the general salinity high.
3.2. Hot Brine Pools
Along the centre of the 2000 km long, narrow Red Sea, there are about 25 local “deeps”. These active spreading centres [39] are hosting hydrothermal circulation, and the deepest troughs contain hot brines. According to Lowell and Germanovich [40], the first evidence of hydrothermal activity at a submerged seafloor spreading centre
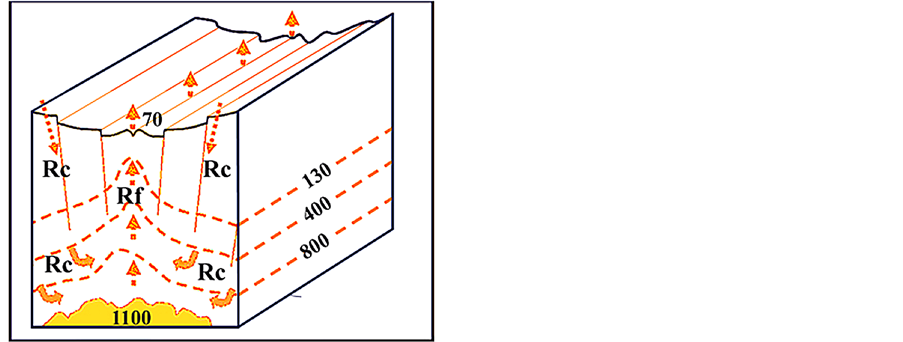
Figure 2. A conceptual drawing (not to scale), illustrating the hydrothermal salt model in the sub-surface. It is based on the inferred sub-surface conditions below the Atlantis II Deep in the Red Sea [37,38]. Arrows indicate the forced convection of water, vapour, and particles, driven by the strong heat-flow gradient [14]. MC = magma chamber. All numbers are temperatures in degree C. The depth to seafloor in the Atlantis II Deep is 2100 m, and the sub-surface depth to top of magma chamber is inferred to be 1.5 - 2 km. Based on Hovland et al. [14].
appeared as an anomaly in temperature and salinity in hydrographic data. Subsequent sampling of the water column and seafloor also revealed that mid-water acoustic reflections were produced by the impedance contrast caused by hydrothermal brines containing suspended metal sulphides [40].
Three pools of hot brines are found within the Atlantis II Deep [41], and sediments underlying these brines are enriched in heavy metals. The Atlantis II Deep is defined by the 2000 m isobath. It is 14 km long, about 5 km wide, and contains approximately 5 km3 of density-stratified brines, which are actively accumulating through the discharge of hydrothermal solutions [21]. The temperatures of the hydrothermal fluids that enter the Deep are variably estimated to range from approximately 210˚C to as high as ~450˚C. These hot brines have high concentrations of Fe, Mn, Zn, Cu, Pb, Co, Ba, Li, and Si which precipitate as layers of sulphides and silicates [21]. This indicates seawater contact with the mantle (peridotite), also suggesting that some of the circulating seawater may participate in serpentinization reactions.
4. Mud Volcanoes
The process of mud volcanism remains one of the most enigmatic unresolved active processes in modern marine and terrestrial sedimentary geology. Over-pressure caused by rapid sedimentation and the escape of formation water from low permeable sediments during compaction is suggested to be one of the driving forces behind mud volcanism [42]. Also, the expulsion of water sourced by the conversion of smectite to illite in clay-rich sediments is proposed as a contributor. But mud volcanism is also recognised as an active process on Mars [43] where such processes are unlikely to occur. It is therefore important to find out more on the nature of the driving mechanisms. The idea that mud volcanoes may represent surface manifestation of phase separation of water at depth was first discussed some years ago [13], see Figure 3. Since then, new data has appeared in the geologic literature, which tends to support that idea.
Mud volcanoes occur in terrestrial and submarine sedimentary basins. Where mud volcanoes occur close to each other, their mud flows may coalesce to cover large areas of the land surface (e.g. in Azerbaijan [44]) or on the seabed (e.g. Gelendzhik mud plateau on the Mediterranean Ridge [45]). The main characteristics of mud volcanoes are summarized as follows: They are vertical structures in sedimentary basins, which continuously produce a multi-component mixture consisting of mud (“mud gel”), water (low and high salinity), and hydrocarbons (liquid and gaseous). They normally have two modus operandi: quiescent and violent, where the quiescent modus may last tens of years, and be more-or-less in a steady state situation [46], but sometimes interrupted
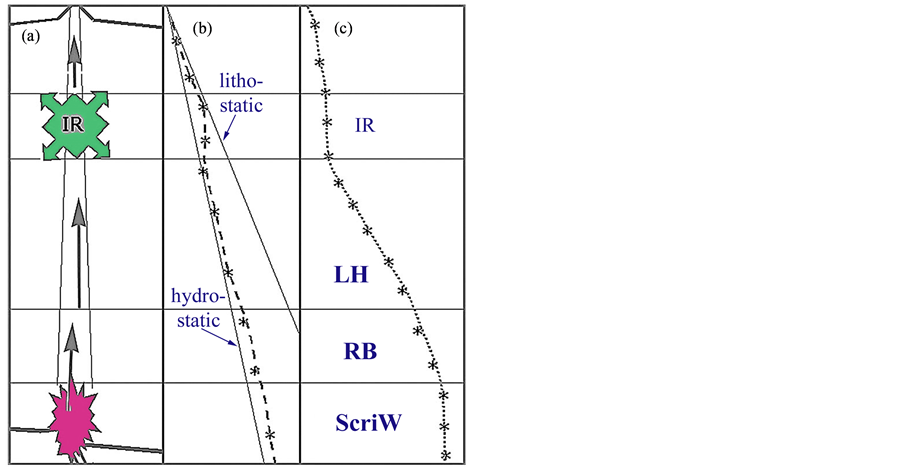
Figure 3. The concept of mud volcanism representing the surface manifestation of point-source phase separation of H2O at depth (modified from [13]). The sketch is based on mud volcano studies in Azerbaijan (including Feyzullayev et al. [50]; Planke et al. [51]), and shows, (a) The inferred section through a ca. 20 km deep mud volcano, which is rooted in a supercritical water (ScriW) zone at a basement fault (shown as a dark star-shape); (b) Inferred pressure profile inside the confined mud volcano conduit. Lower straight line: hydrostatic pressure gradient; upper line: lithostatic pressure gradient; (c) Inferred temperature profile inside the conduit. IR = intermediate (gas) reservoir, LH = latent heat (adiabatic, expansion) zone, RB = retrograde boiling zone, ScriW = supercritical water zone.
by short bursts of violent eruptions, only lasting for hours or days [44,47]. They are normally of ambient temperature, but show both lower and much higher temperatures than the ambient surface sediment (pore-water) temperature. Some mud volcanoes have been in continuous activity for thousands, perhaps millions of years. Thus, Dimitrov [48] identified reports of 11 eruptive mud volcano events around the world only in the year 2001, eight of them in Azerbaijan, two in Barbados, and one in New Zealand [49].
Early publications on mud volcanoes [44,52-54] revealed that the material brought to surface may originate from depths of up to 20 km. In 1923, the Russian geologist Golubyatnicov, described mud volcanoes as natural survey “boreholes”—free of charge [48]. But, despite having been targeted for scientific studies by numerous researchers over the last century, there are still very few conceptual models that explain their formation. Hedberg [55] suggested that mud volcanoes are products of hydrocarbon (oil and gas) generation and over-pressure, at depth. Researchers have related positively to this proposed mechanism, even though it has been difficult to explain the formation of some of the products that are welling up together with hydrocarbons in most mud volcanoes. Rapid sedimentation, dewatering, and hydrofracturing of over-pressured sediments are also used to explain the features [55]. However, recently, a link to hydrothermal processes has been made, mainly based on temperature considerations and geochemical sampling results [35,46,56-58].
The most distinct morphological mud volcanoes are found in Trinidad [59], Venezuela [60], Colombia [61], Italy [62,63], Azerbaijan [44,59,64,65], the Makran coast of Iran and Pakistan [66-68], and on Java, Sumatra, and Borneo/Kalimantan [56]. Besides the Makran coast of Pakistan and parts of Trinidad, the central regions of Azerbaijan are the terrestrial areas with the highest density of mud volcanoes. In addition, there are mud volcanoes on the seafloor that are associated with gas hydrate formation [69].
4.1. Possible Triggers and Propulsion of Mud Volcanoes
All mud volcanoes are associated with gas venting, and from this fact it is evident that the generation of gas at depth is an important and necessary contributor to the lifting force inside the conduits. The gas may have two possible sources:
● The thermogenic formation and expulsion of gas from organic rich sediments in the sedimentary sequence;
● The production of gas by the serpentinization (peridotite hydration) process, in the deep crust/upper mantle at temperatures 200˚C to 500˚C [70].
If hydrocarbon formation was the main driver of mud volcanism, then mud volcanoes should perhaps occur frequently in hydrocarbon producing sedimentary basins. This is not the case, and this indicates that other processes must be present. It is also observed that the last eruption of Lokbatan mud volcano (in the Baku area, Azerbaijan) did not cause any significant pressure kick in the nearby oil reservoir (Pers. com. Guliyev, 2005). This observation indicates that the conduits of mud volcanoes do not necessarily communicate with nearby oil reservoirs; and that another gas source may exist for the mud volcanoes. This leads us to cautiously suggest that the main trigger and driving force for mud volcanoes are to be found in the hydrothermal processes originating from the underlying crust, and more specifically; from serpentinization processes that also produce gases (methane and carbon dioxide).
The mere fact that mud volcanoes contain vertical pipes (conduits) with a regular and stable flow of a mixture of water, gas, oil, and mud, is very special. In order to obtain such a flow the particles need to be of clay to silt size; e.g. finer than 10 micron, to avoid sorting inside the conduit. Furthermore, the water (dispersion agent) should be fresh, to avoid flocculation of the clay during transportation. Based on this, it is also inferred that sediments with saline formation water would not easily be eroded by migrating brine with similar salinity in the conduits of the mud volcano. This also puts restrictions on the possibility of producing mud from over-pressured sediments; in that the formation water that is to be expelled must be of low salinity to be able to transport fine particles in dispersion. Therefore, in our view, there must be alternative sources for the migrating water that are capable of eroding and transporting the mud that is seen venting out of mud volcanoes. Furthermore, for “cold mud volcanoes” a gas is needed for the last part of the transportation of mud, when the water vapour condenses to liquid water and loses its lifting capacity.
Because ScriW also behaves as a non-polar fluid, it is fully miscible with hydrocarbons, and is therefore capable of also bringing hydrocarbons upwards through the conduits until the critical point is reached. At the CP, a phase separation of water and hydrocarbons will occur, where the water will continue to migrate upwards as a sub-critical steam, and upon further cooling it will condense to distilled water. This fresh-water—driven by “gas-lift”—will easily erode silicate particles from the sidewalls of the conduit(s) and transport them in suspension up to the surface. It is observed that mud venting at the surface of mud volcanoes (forming gryphons and mud gel deposits) is always a fresh-water suspension [65]. There are often three types of vents associated with terrestrial mud volcanoes: fresh-water mud-producing structures, called “gryphons”; salt-water pools, called “salses”, and oil-dominated pools, forming crude oil ponds, often along the outer sides of major mud volcanic craters or calderas, e.g. at Dashgil, Azerbaijan [65].
4.2. The LUSI Mud Volcano, Indonesia
One of the most spectacular and perhaps frightful eruptions of mud, in modern times, took place at Sidoarjo, on the island of Java on May 29th, 2006. The eruption site was named LUSI, “Lumpur (mud)—Sidoarjo” [56]. During one year of eruption, the area covered by the terrestrial mud flows reached more than 6.3 km2. The eruption dynamics of LUSI and an attempt to identify possible causes were presented by Mazzini et al. [56]. During the initial phase of eruption, boiling mud containing ~60% water was ejected several tens of metres above the crater. An up to 50 m high plume of steam accompanied the mud and water. The flow rate escalated from 5000 to 120,000 m3/d during the first eleven weeks. “Then the erupted volume started to pulsate between almost zero and 120,000 m3/d in the period August 14 to September 10, whereas it increased dramatically following swarms of earthquakes in September, before reaching almost 180,000 m3/d in December 2006” [56].
Downhole measurements in a well drilled near the crater reached a temperature of 138˚C at 2667 m, which gives a high geothermal gradient of 42˚C/km, suspected to reflect proximity to a volcanic arc [56]. However, in the crater, the gradient is likely to be considerably steeper, as the mud is at least 100˚C, at surface and, therefore, probably much higher at 2667 m. Thus, with a 42˚C/km temperature gradient, the temperature at this depth should be an additional 212˚C (Table 1).
The expelled water shows the chloride content, 39% lower than for seawater. Also the concentration of other solutes, like SO4 and Mg were lower than in seawater. However, the following compounds are enriched: B, Ca, Li, Na, Sr, and Br. Judging from the combined information on biostratigraphy, clay mineralogy, and vitrinite results, Mazzini et al. [56] conclude that the main source of the erupted mud is between 1615 and 1828 m below surface. Although the origin of methane is traced to organic material, the high concentration of CO2 in the gas phase took the researchers by surprise [56].
However, a new analysis has now totally altered these conclusions of 2007. The new results suggest that the roots of LUSI mud volcano is actually part of a much deeper high heat-flow system associated with subsurface volcanic sills [57]. On the basis of all the compiled information, Mazzini et al. [57] conclude that the pulsating activity of LUSI and the high temperatures reflect a “quasi-hydrothermal” behaviour of the eruptive system: “These results are consistent with a scenario of deep seated (>4000 m) magmatic intrusions and hydrothermal fluids responsible for the enhanced heat that altered source rocks and/or gas reservoirs. The neighboring magmatic Arjuno complex and its fluid–pressure system combined with high seismic activity could have played a key role in the Lusi genesis and evolution” [57]. However, in this case with such high temperatures, the eruption is driven both by formation of ScriW, the condensation to steam, and expanding gases.
We find no reason to expect a termination of muds at a depth of 2667 m, although this is the depth reached by the nearby drilling campaign. From seismic information, it is shown that the Kajung limestone formation underlies this “mud source”. In our interpretation of LUSI, we suggest using a gradient as shown in Table1
Table 1. A table showing a constructed temperature with depth distribution for LUSI.
In this case (Table 1), the temperature would reach critical point at a depth of about 6 km below surface, with the potential of forming ScriW; depending on the salinity of the formation water at that depth. In this case, leakages of ScriW vapour from >6 km depth will heat up the confined conduit to maintain supercritical conditions up to nearly 3 km depth. Here, the ScriW will turn into (sub-critical) steam that will continue its flow upwards and erode and drain the non-consolidated sediments above the Kajung limestone formation. Condensation of steam will dilute the salty pore water from the non-consolidated sediments above the Kajung Formation. Thus, the lifting mechanism is a combined effect of steam and gases (methane and CO2) that lifts the mud up to the surface.
Poort et al. [71] also found very high heat-flow values (up to 13,000 mW/m2) in submarine mud volcanoes of the Sorokin Trough, Black Sea, whereas the heat-flow is between 35 and 75 mW/m2 in the surrounding area. This is extreme, and means that mud volcanoes may carry a lot of heat to the surface, even without influencing the general background heat-flow values. In this case there is a concave upwards temperature gradient. Further evidence for mud volcanism associated with extreme heatflow values have also been found in the western Nile delta, in the Mediterranean Sea. Thus, both the Giza and the North Alex mud volcanoes had temperature gradients of up to 4˚C per m in their upper portions. Whereas the background sediments in the area had a heat-flow of 30 mW/m2, Giza was measured to have a central heat-flow of 3.5 W/m2 and North Alex one of 16 W/m2, the highest measured heat-flow of a mud volcano, to date [72]. Elevated temperatures in mud volcanoes, combined with venting of brines and hydrocarbon bearing fluids have also been documented above some salt diapirs in the Gulf of Mexico, as described in Hornbach et al. [73].
4.3. The Chapopote Asphalt Volcano, Gulf of Mexico
In order to explain how liquefied asphalt could have been emplaced as a “lava flow” on the seafloor in the southern deep portion of the Gulf of Mexico [74], the development of ScriW below a salt diapir-associated “asphalt volcano”, the Chapopote structure, was inferred to have occurred [35]. In this case, we do not only suggest that the asphalt volcano is a manifestation of ScriW at depth, but also that the primary piercement structure, the salt dome, which harnesses it, has been formed partially by the aid of ScriW at depth.
4.4. What Are the Driving Mechanisms for Mudand Asphalt Volcanoes?
The main questions we have presented, here, are 1) can locally extreme heat-flows occurring at the basement of sedimentary basins, where there may be a juxtaposition of intersecting deep fractures and faults cause the formation of ScriW trigger and drive mud volcanism; and 2) is the role of gas lift inside the vertical transport conduits of mud volcanoes important for lifting the multi-component material of solids and liquids to the surface? Actually, the whole question may be simplified as one of sediment thickness below the mud volcano and the correct temperature gradient. To our knowledge, there exist no drillingor temperature logging-results that have been performed deeper than about 20 m into any mud volcano, to date. Therefore, in order to find realistic temperature gradients throughout the whole depth of mud volcano conduits, we need to check drilling records into such systems. The Ocean Drilling Program (ODP) performed a series of scientific drilling campaigns into “sedimented ridges”. One of the most remarkable temperature gradients measured by ODP in sediments, was from the “Dead Dog” site (Hole 858G) on the “Sedimented Ridges II”, ODP Leg 169, at Middle Valley, Juan de Fuca spreading ridge in the North-Eastern Pacific Ocean [18]. In this hole, the highest temperature (272˚C) was measured only 98 metres below seafloor (mbsf), i.e., the temperature gradient was 2.68˚C/m. From this depth to the bottom of the hole, at 206 mbsf, the temperature was more-or-less isothermal, i.e., it had a temperature gradient of 0˚C/km. Furthermore, a total of eight discrete temperature measurements in four holes within the “Bent Hill” area of the Middle Valley provided temperature gradients ranging from 1.24 to 2.18˚C/km. Future investigations on mud volcanoes should perhaps bear these possibilities in mind and pay attention to the true geochemical and mineral signatures contained in the gel muds of mud volcanoes and what these signatures might tell about the origin of the material.
5. Summary and Conclusions
Whereas Hovland et al. [14] justified how large accumulations of salt form in the Red Sea, as a direct consequence of ScriW forming in deep, hydrothermal systems, and the produced salt is transported upwards to the seafloor where it is protected by sediments and high concentration brines, we here refer to observations from the Dashgil mud volcano, Azerbaijan, together with observations from other terrestrial and submarine mud volcanoes to suggest that the driving mechanisms for offshore and terrestrial mud volcanoes are local extreme high heatflows, associated with deep seated ScriW, causing the formation of mud gel and gases at depth. The main driving forces for mud and asphalt volcanoes are thus inferred to be liquefaction of sediments (clay formation) by boiling and ScriW, hydro-fracturing and gas lift. Mud volcanoes are suggested to be located where there is a juxtaposition of intersecting deep fractures and faults with the mud volcanoes representing the end-member of buried local, long-lived hydrothermal system.
In summary, we suggest that ScriW participates in and is partly responsible for: 1) Ocean water rejuvenation and formation; 2) Fundamental geological processes, such as volcanism, earthquakes, and metamorphism (including serpentinization); 3) Solid salt production, accumulation, transportation, and (salt) dome formation; 4) The initiation and driving of mud, serpentine, and asphalt volcanoes; 5) Dissolution of organic matter and petroleum, including transportation and phase separation (fractionation), when passing into the subcritical domain of (liquid) water.
REFERENCES
- J. B. Corliss and R. D. Ballard, “Oases of Life in the Cold Abyss,” Natural Geographic Magazine, Vol. 152, No. 2, 1977, pp. 441-453.
- J. M. Edmond and K. Von Damm, “Hot Springs on the Ocean Floor” Scientific American, Vol. 248, No. 4, 1978, pp. 70-85.
- J. L. Bischoff and R. J. Rosenbauer, “Salinity Variations in Submarine Hydrothermal Systems by Layered DoubleDiffusive Convection,” Journal of Geology, Vol. 97, No. 5, 1989, pp. 613-623. http://dx.doi.org/10.1086/629338
- H. W. Jannasch, “Microbial Processes at Deep Sea Hydrothermal Vents,” In: P. A. Rona, K. Boström, L. Laubier and K. L. Smith Jr., Eds., Hydrothermal Processes at Seafloor Spreading Centers, Plenum Press, New York and London, 1983, pp. 677-709. http://dx.doi.org/10.1007/978-1-4899-0402-7_28
- J. F. Grassle, “Introduction to the Biology of Hydrothermal Vents,” In: P. A. Rona, K. Boström, L. Laubier and K. L. Smith Jr., Eds., Hydrothermal Processes at Seafloor Spreading Centers, Plenum Press, New York and London, 1983, pp. 665-675. http://dx.doi.org/10.1007/978-1-4899-0402-7_27
- T. Plank, K. A. Kelley, M. Zimmer, E. H. Hauri and P. J. Wallace, “Why Do Mafic Arc Magmas Contain ~4 wt% Water on Average?” Earth and Planetary Science Letters, Vol. 364, 2013, pp. 168-179. http://dx.doi.org/10.1016/j.epsl.2012.11.044
- D. A. Sverjensky, B. Harrison and A. Azzolini, “Deep Earth Water (DEW) Model for Predicting Aqueous Species Thermodynamic Properties to 6 GPa and 1200˚C: Preliminary Applications and the Need for Fundamental Data,” American Geophysical Union (AGU), Washington DC, 2013.
- J. Tester, H. R. Holgate, F. J. Armellini, P. A.Webley, W. R. Killilea, G. T. Hong and H. E. Berner, “Supercritical Water Oxidation Technology,” In: D. W. Tedder and F. G. Pohland, Eds., Emerging Technologies in Hazardous Waste Management III, American Chemical Society, Washington DC, 1993, pp. 35-76.
- M.-C. Bellissent-Funel, “Structure of Supercritical Water,” Journal of Molecular Liquids, Vol. 90, No. 1-3, 2001, pp. 313-322. http://dx.doi.org/10.1016/S0167-7322(01)00135-0
- E. U. Franck, “Properties of Water,” In: D. de G. Jones and R.W. Staehle, High Temperature, High Pressure Electrochemistry in Aqueous Solutions, National Association of Corrosion Engineers, Houston, 1976, pp. 109- 116.
- W. Kohl, H. A. Lindner and E. U. Franck, “Raman Spectra of Water to 400˚C and 3000 Bar,” Berichte der Bunsengesellschaft für Physikalische Chemie, Vol. 95, No. 12, 1991, pp. 1586. http://dx.doi.org/10.1002/bbpc.19910951202
- D. Pan, L. Spanu, B. Harrison, D. A. Sverjensky and G. Galli, “Dielectric Properties of Water under Extreme Conditions and Transport of Carbonates in the Deep Earth,” Proceedings of National Academy of Sciences (PNAS), Vol. 110, No. 17, 2013, pp. 6646-6650. http://dx.doi.org/10.1073/pnas.1221581110
- M. Hovland, C. Fichler, H. Rueslåtten and H. K. Johnsen, “Deep-rooted piercement structures in deep sedimentary basins—Manifestations of supercritical water generation at depth?” Journal of Geochemical Exploration, Vol. 89, No. 1-3, 2006, pp. 157-160. http://dx.doi.org/10.1016/j.gexplo.2005.11.056
- M. Hovland, H. Rueslåtten, H. K. Johnsen, B. Kvamme and T. Kutznetsova, “Salt Formation Associated with Sub-Surface Boiling and Supercritical Water,” Marine and Petroleum Geology, Vol. 23, No. 8, 2006, pp. 855- 869. http://dx.doi.org/10.1016/j.marpetgeo.2006.07.002
- K. L. Von Damm, M. D. Lilley, W. C. Shanks III., M. Bockington, A. M. Bray, K. M. O’Grady, E. Olson, A. Graham and G. Proskurowski, The Souepr Science Party, “Extraordinary Phase Separation and Segregation in Vent Fluids from the Southern East Pacific Rise,” Earth and Planetary Science Letters, Vol. 206, No. 3-4, 2003, pp. 365-378.
- A. Koschinsky, D. Garbe-Schönberg, S. Sander, K. Schmidt, H.-H. Gennerich and H. Strauss, “Hydrothermal Venting at Pressure-Temperature Conditions above the Critical Point of Seawater, 5˚S on the Mid-Atlantic Ridge,” Geology, Vol. 36, No. 8, 2008, pp. 615-618. http://dx.doi.org/10.1130/G24726A.1
- K. Becker, “Permeability measurements in Hole 896A and Implications for the Lateral Variability of Upper Crustal Permeability at Sites 504 and 896. Ocean Drilling Program,” Scientific Results, Vol. 148, 1999, pp. 353- 363.
- E. E. Davis and K. Becker, “Observations of Temperature and Pressure: Constraints on Ocean Crustal Hydrologic State, Properties, and Flow.” In: E. Davis and H. Elderfield, Eds., Hydrogeology of the Oceanic Lithosphere, Cambridge University Press, Cambridge, 2004, pp. 225- 271.
- A. Simakin and A. Ghassemi, “Salt Loaded Heat Pipes: Steady-State Operation and Related Heat and Mass Transport,” Earth and Planetary Science Letters, Vol. 215, No. 3-4, 2003, pp. 411-424. http://dx.doi.org/10.1016/S0012-821X(03)00428-X
- M. Hovland and A. G. Judd, “Seabed Pockmarks and Seepages. Impact on Geology, Biology and the Marine Environment,” Graham & Trotman Ltd., London, 1988, 293 p.
- F. Pirajno, “Hydrothermal Processes and Mineral Systems,” Springer, Berlin, 2009, 1250 p. http://dx.doi.org/10.1007/978-1-4020-8613-7
- M. C. Pierret, N. Clauer, D. Bosch, G. Blanc and C. France-Lanord, “Chemical and Isotopic (87Sr/86Sr, δ18O, δD) Constraints on the formation of Red Sea brines,” Geochimica et Cosmochimica Acta, Vol. 65, No. 8, 2001, pp. 1259-1275. http://dx.doi.org/10.1016/S0016-7037(00)00618-9
- G. O. Fridleifsson, “Drilling into Supercritical Geothermal Systems,” Iceland Deep Drilling Project, 2011. www.iddp.is
- M. J. Mottl, C. G. Wheat, P. Fryer, J. Gharib and J. B. Martin, “Chemistry of Springs across the Mariana Forearc Shows Progressive Devolatilization of the Subducting Plate,” Geochimica et Cosmochimica Acta, Vol. 68, No. 23, 2004, pp. 4915-4933. http://dx.doi.org/10.1016/j.gca.2004.05.037
- E. Savoyat, A. Shiferaw and T. Balcha, “Petroleum Exploration in the Ethiopian Red Sea,” Journal of Petroleum Geology, Vol. 12, No. 2, 1989, pp. 187-204. http://dx.doi.org/10.1111/j.1747-5457.1989.tb00232.x
- R. F. Schmalz, “Deep-Water Evaporite Deposition: A Genetic Model,” AAPG Bulletin, Vol. 53, No. 4, 1969, pp. 798-823.
- P. Stoffers and R. Kühn, “Red Sea Evaporites: A Petrographic and Geochemical Study,” In: R. B.Whitmarsh, et al., Leg 23, Deep Sea Drilling Project, Final Report, Texas A&M University, 1973, pp. 821-847.
- B. C. Schreiber and K. J. Hsü, “Evaporites,” In: G. D. Hobson, Ed., Developments in Petroleum Geology, Applied Science Ltd., London, 1980, pp. 87-138.
- B. C. Schreiber, S. Lugli and M. Babel, “Evaporites through Space and Time,” Special Publication, No. 285, Geological Society of London, pp. 1-13.
- J. K. Warren, “Evaporites: Sediments, Resources and Hydrocarbons,” Springer Verlag, 2010, 998 p.
- D. F. Hewitt, “Salt in Ontario,” Industrial Mineral Report No. 6. Ontario Department of Mines, Ontario, 1962, 40 p.
- R. P. Lowell and P. Rona, “Hydrothermal Activity,” In: R. C. Selley, L. R. M. Cocks and I. R. Plimer, Eds., Encyclopedia of Geology, Vol. 5, Elsevier, Amsterdam, 2005, pp. 362-372. http://dx.doi.org/10.1016/B0-12-369396-9/00126-X
- S. Geiger, T. Driesner, C. A. Heinrich and S. K. Matthäi, “On the Dynamics of NaCl-H2O Fluid Convection in the Earth’s Crust,” Journal of Geophysical Research, Vol. 110, No. B7, 2005, Article ID: B07101. http://dx.doi.org/10.1029/2004JB003362
- S. Lewis and M. Holness, “Equilibrium Halite-H2O Dihedral Angles: High Rock-Salt Permeability in the Shallow Crust?” Geology, Vol. 24, No. 5, 1996, pp. 432-434. http://dx.doi.org/10.1130/0091-7613(1996)024<0431:EHHODA>2.3.CO;2
- M. Hovland, I. MacDonald, H. Rueslåtten, H. K. Johnsen, T. Naehr and G. Bohrmann, “Chapopote Asphalt Volcano May Have been Generated by Supercritical Water,” Eos, Transactions American Geophysical Union, Vol. 86, No. 42, 2005, pp. 397-402.
- M. B. Holness and S. Lewis, “The Structure of HaliteBrine Interface Inferred from Pressure and Temperature Variations of Equilibrium Dihedral Angles in the Halite-H2O-CO2 System,” Geochimica et Cosmochimica Acta, Vol. 61, No. 4, 1997, pp. 795-804. http://dx.doi.org/10.1016/S0016-7037(96)00370-5
- C. Ramboz, E. Oudin and Y. Thisse, “Geyser-Type Discharge in Atlantis II Deep, Red Sea: Evidence of Boiling from Fluid Inclusions in Epigenetic Anhydrite,” Canadian Mineralogist, Vol. 26, 1988, pp. 765-786.
- R. A. Zierenberg and M. E. Holland, “Sedimented Ridges as a Laboratory for Exploring the Subsurface Biosphere,” In: W. S. D. Wilcock, E. F. DeLong, D. S. Kelley, J. A. Baross and S. C. Cary, Eds., The Subseafloor Biosphere at Mid-Ocean Ridges, Geophysical Monograph, Vol. 144, 2004, pp. 305-323.
- N. C. Mitchell, M. Ligi, V. Ferrante, E. Bonatti and E. Rutter, “Submarine Salt Flows in the Central Red Sea,” Geological Society of America Bulletin, Vol. 122, No. 5-6, 2010, pp. 701-713. http://dx.doi.org/10.1130/B26518.1
- R. P. Lowell and L. N. Germanovich, “Evolution of a Brine-Saturated Layer at the Base of a Ridge-Crest Hydrothermal System,” Journal of Geophysical Research, Vol. 102, No. B5, 1997, pp. 10245-10255. http://dx.doi.org/10.1029/97JB00264
- E. T. Degens and D. A. Ross, “Hot Brines and Recent Heavy Metal Deposits in the Red Sea,” Springer Verlag, New York, 1969, pp. 535-541. http://dx.doi.org/10.1007/978-3-662-28603-6
- A. J. Kopf, “Significance of Mud Volcanism,” Review of Geophysics, Vol. 40, No. 2, 2002, pp. 2-1-2-52. http://dx.doi.org/10.1029/2000RG000093
- D. Z. Oehler and C. C. Allen, “Evidence for Pervasive Mud Volcanism in Acidalia Planitia, Mars,” Icarus, Vol. 208, No. 2, 2010, pp. 636-657. http://dx.doi.org/10.1016/j.icarus.2010.03.031
- A. A. Yakubov, A. A. Ali-Zade and M. M. Zeinalov, “Mud Volcanoes of the Azerbaijan SSR: Atlas,” Academy of Sciences of Azerbaijan, Baku, 1971, 258 p.
- A. F. Limonov, J. M. Woodside, M. B. Cita and M. K. Ivanov, “The Mediterranean Ridge and Related Mud Diapirism: A Background,” Marine Geology, Vol. 132, No. 1-4, 1996, pp. 7-19. http://dx.doi.org/10.1016/0025-3227(96)00150-8
- N. Onterdonk, A. Mazzini, L. Shafer and H. Svensen, “Controls on the Geomorphic Expression and Evolution of Gryphons, Pools, and Caldera Features at Hydrothermal Seeps in the Salton Sea Geothermal Field, Southern California,” Geomorphology, Vol. 130, No. 3-4, 2011, pp. 327-342. http://dx.doi.org/10.1016/j.geomorph.2011.04.014
- I. S. Guliyev, “South-Caspian Depression—An Intensive Area of Hydrocarbon Fluid Formation and Migration,” In: Abstract Vol 7th International Conference Gas in Marine Sediments, Baku, 7-12 October 2002, Nafta Press, Baku, 2002, pp. 66-69.
- L. I. Dimitrov, “Mud Volcanoes—the Most Important Pathway for Degassing Deeply Buried Sediments,” Earth Science Review, Vol. 59, No. 1-4, 2002, pp. 49-76. http://dx.doi.org/10.1016/S0012-8252(02)00069-7
- A. G. Judd and M. Hovland, “Submarine Fluid Flow, the Impact on Geology, Biology, and the Marine Environment,” Cambridge University Press, Cambridge, 2007, 475 p. http://dx.doi.org/10.1017/CBO9780511535918
- A. A. Feyzullayev, I. S. Guliyev and M. F. Tagiyev, “Source Potential of the Mesozoic-Cenozoic rocks in the South Caspian Basin and Their Role in Forming the Oil Accumulations in the Lower Pliocene Reservoirs,” Petroleum Geoscience, Vol. 7, No. 4, 2001, pp. 409-417. http://dx.doi.org/10.1144/petgeo.7.4.409
- S. Planke, H. Svensen, M. Hovland, D. A. Banks and B. Jamtveit, “Mud and Fluid Migration in Active Mud Volcanoes in Azerbaijan,” Geo-Marine Letters, Vol. 23, No. 3-4, 2003, pp. 258-268. http://dx.doi.org/10.1007/s00367-003-0152-z
- G. S. Rogers, “Intrusive Origin of the Gulf Coast Salt Domes,” Economic Geology, Vol. 13, No. 6, 1918, pp. 447-485. http://dx.doi.org/10.2113/gsecongeo.13.6.447
- V. A. Sokolov, Z. A. Buniat-Zade, A. A. Goedekian and F. G. Dadashev, “The Origin of Gases of Mud Volcanoes and the Regularities of Their Powerful Eruptions,” In: Advances in Organic Geochemistry, Pergamon Press, New York, 1968, pp. 473-484.
- P. R. Williams, C. J. Pingram and D. B. Dow, “Mélange Production and the Importance of Shale Diapirism in Accretionary Terranes,” Nature, Vol. 309, 1984, pp. 145- 146. http://dx.doi.org/10.1038/309145a0
- H. D. Hedberg, “Relation of Methane Generation to Undercompacted Shales, Shale Diapirs and Mud Volcanoes,” American Association of Petroleum Geologists Bulletin, Vol. 58, No. 4, 1974, pp. 661-673.
- A. Mazzini, H. Svensen, G. G. Akhmanov, G. Aloisi, S. Planke, A. Malthe-Sørensen and B. Istadi, ”Triggering and Dynamic Evolution of the LUSI Mud Volcano, Indonesia,” Earth and Planetary Science Letters, Vol. 261, No. 3-4, 2007, pp. 375-388. http://dx.doi.org/10.1016/j.epsl.2007.07.001
- A. Mazzini, G. Etiope and H. Svensen, “A New Hydrothermal Scenario for the 2006 Lusi Eruption, Indonesia. Insight from Gas Geochemistry,” Earth and Planetary Science Letters, Vol. 317-318, 2012, pp. 305-318. http://dx.doi.org/10.1016/j.epsl.2011.11.016
- C. Hensen, M. Nuzzo, E. Hornibrook, L. M. Pinheiro, B. Bock, V. H. Magelhaes and W. Bruckmann, “Sources of Mud Volcano Fluids in the Gulf of Cadiz—Indications of Hydrothermal Imprint,” Geochimica et Cosmochimica Acta, Vol. 71, No. 5, 2007, pp. 1232-1248. http://dx.doi.org/10.1016/j.gca.2006.11.022
- H. G. Kugler, “Contribution to the Knowledge of Sedimentary Volcanism in Trinidad,” Journal of the Institute of Petroleum Technology, Trinidad, Vol. 19, 1933. pp. 743-760.
- G. E. Higgins and J. B. Saunders, “Report on 1964 Chatham Mud Island, Erin Bay, Trinidad, West Indies,” American Association of Petroleum Geologists Bulletin, Vol. 51, No. 1, 1967, pp. 55-64.
- A. Ganser, “Uber Schlamvulkane und Salzdome (On Mud Volcanoes and Salt Domes)” Vol. 15, Mitteliungen Geologishe Institut, Technische Hochschule, Universität Zurich, Zurich, 1960. 46 p.
- G. Chiodini, L. Marini and M. Russo, “Geochemical Evidence for the Existance of High-Temperature Hydrothermal Brines at Vesuvio Volcano, Italy,” Geochimica et Cosmochimica Acta, Vol. 65, No. 13, 2001, pp. 2129- 2147. http://dx.doi.org/10.1016/S0016-7037(01)00583-X
- G. Etiope and A. V. Milkov, “A New Estimate of Global Methane Flux from Onshore and Shallow Submarine Mud Volcanoes to the Atmosphere,” Environmental Geology, Vol. 46, No. 8, 2004, pp. 997-1002. http://dx.doi.org/10.1007/s00254-004-1085-1
- I. S. Guliyev, “A Review of Mud Volcanism,” Azerbaijan Academy of Sciences, Institute of Geology, Azerbaijan, 1992, 65 p.
- M. Hovland, A. Hill and D. Stokes, “The Structure and Geomorphology of the Dashgil Mud Volcano, Azerbaijan,” Geomorphology, Vol. 21, No. 1, 1997, pp. 1-15. http://dx.doi.org/10.1016/S0169-555X(97)00034-2
- J. V. Harrison, “Mud Volcanoes on the Makran Coast,” The Geographical Journal, Vol. 103, No. 4, 1944, pp. 180-181. http://dx.doi.org/10.2307/1789250
- R. E. Snead, “Active Mud Volcanoes of Baluchistan, West Pakistan,” Geographical Review, Vol. 54, No. 4, 1964, pp. 546-560. http://dx.doi.org/10.2307/212981
- G. Delisle, U. von Rad, H. Andruleit, C. H. von Daniels, A. R. Tabrez and A. Inam, “Active Mud Volcanoes onand Offshore Eastern Makran, Pakistan,” International Journal of Earth Sciences (Geologische Rundschau), Vol. 91, No. 1, 2002, pp. 93-110. http://dx.doi.org/10.1007/s005310100203
- U. Tinivella and M. Giustiniani, “An Overview of Mud Volcanoes Associated to Gas Hydrate System,” Intech Open Science, Updates in Volcanology—New Advances in Understanding Volcanic Systems, 2013, pp. 225-267. http://dx.doi.org/10.5772/51270
- J. L. Charlou, J. P. Donval, C. Konn, H. Ondréas and Y. Fouquet, “High Production and Fluxes of H2 and CH4 and Evidence of Abiotic Hydrocarbon Synthesis by Serpentinization in Ultramafic-Hosted Hydrothermal Systems on the Mid-Atlantic Ridge,” In: Rona, et al., Eds., Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, AGU Geophysical Monograph Series, Vol. 188, American Geophysical Union, Washington, 2010, pp. 265- 295.
- J. Poort, R. Kutas, A. Vassilev and J. Klerkx, “Heat Flow Characterization of the Dnepr Paleo-Delta and the Sorokin Trough, Northern Margin of the Black Sea (Abstract),” International Workshop on Methane in Sediments and Water Column of the Black Sea, Sevastopol, Ukraine, 17-22 May 2005, p. 43.
- T. Feseker, K. R. Brown, C. Blanchet, F. Scholz, M. Nuzzo, A. Reitz, M. Schmidt and C. Hensen, “Active Mud Volcanoes on the Upper Slope of the Western Nile Deep-Sea Fan—First Results from the P362/2 cruise of R/V Poseidon,” Geo-Marine Letters, Vol. 30, No. 3-4, 2010, pp. 169-186. http://dx.doi.org/10.1007/s00367-010-0192-0
- D. M. Saffer, C. Lee Van Dover and W. S. Holbrook, “Coupled Geophysical Constraints on Heat Flow and Fluid Flux at a Salt Diapir,” Geophysical Research Letters, Vol. 32, No. 24, 2005, Article ID: L24617. http://dx.doi.org/10.1029/2005GL024862
- I. R. MacDonald, G. Bohrmann, E. Escobar, F. Abegg, P. Blanchon, V. Blinova, W. Brückmann, M. Drews, A. Eisenhauer, X. Han, K. Heeschen, F. Meier, C. Mortera, T. Naehr, B. Orcutt, B. Bernard, J. Brooks and M. de Faragó, “Asphalt Volcanism and Chemosynthetic Life in the Campeche Knolls, Gulf of Mexico,” Science, Vol. 304, No. 5673, 2004, pp. 999-1002.


