Journal of Geoscience and Environment Protection
Vol.05 No.07(2017), Article ID:77796,44 pages
10.4236/gep.2017.57011
Sesame Husk as Adsorbent for Copper(II) Ions Removal from Aqueous Solution
Haitham Ahmed El-Araby1*, Abel Moneim Mohamed Ahmed Ibrahim2, Ahmed Hashem Mangood1, Adel A.-H. Abdel-Rahman1
1Chemistry Department, Faculty of Science, Menoufia University, Shebin El-Kom, Egypt
2Chemistry Department, Faculty of Science, Alexandria University, Alexandria, Egypt

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: May 28, 2017; Accepted: July 18, 2017; Published: July 21, 2017
ABSTRACT
In this study, the adsorption behavior of copper(II) ions from aqueous solutions onto sesame husk (SH) was investigated. The effect of different parameters such as pH, contact time, adsorbent dosage, adsorbate concentration, temperature and agitation speed was studied. Thermodynamic parameters, equilibrium isotherms and kinetic data have been evaluated. The functional groups and surface morphology of SH adsorbent were characterized by FTIR and SEM. Adsorption equilibrium isotherms were expressed by Langmuir, Freundlich and Dubinin-Radushkevich (D-R) adsorption models and it was found that Langmuir adsorption model fits the experimental data better than Freundlich and D-R models. The adsorption can be best described by the pseudo second-order kinetic model.
Keywords:
Copper(II) Ions Adsorption, Kinetics, Thermodynamic Parameters

1. Introduction
Serious environmental pollution arises as a result of the industrial activities and technology development due to waste streams of heavy metals from several industries which are poured into rivers. This occurs in many industries including mining process, smelting, metal plating, pigment, battery manufacturing processes, metallurgical industries [1] [2] [3] , tannery and fabrication [4] .
Various treatment methods and traditional technologies are used to remove heavy metals from wastewater, for instance, chemical oxidation/reduction [5] [6] , electrodialysis [7] , ultrafiltration [8] [9] , solvent extraction [10] , ion-ex- change, evaporation, membrane filtration [11] [12] [13] [14] , chemical precipitation [15] , reverse osmosis [16] and coagulation [17] . However, there are some disadvantages accompany with these technologies such as high cost, sensitive operating conditions and possibility of secondary sludge production [18] [19] which suffocate their generalization in industries.
Adsorption is considered one of the important methods used for the removal of heavy metals [20] [21] [22] [23] . Comparing to the other purification and separation methods, adsorption-as a wastewater treatment process-has demonstrated its efficiency and economic feasibility and has gained importance in industrial applications [24] [25] , as elimination of heavy metal cations from aqueous solution by selecting the suitable adsorbents under optimum operation conditions [26] .
One of the advantages of natural adsorbents is that, they are easily applicable because of their adaption to several conditions as pH, temperature, pressure and agitation. Besides, these materials are cheap and highly efficient [27] . So, development of alternative adsorbent materials featuring high availability, high adsorption capacities and feasibility is required [27] . In addition, the cost of these biosorbent materials is negligible compared with the cost of activated carbon or ion-exchange resins [28] .
Recently, agricultural by-products have been widely studied for sequestering of metals from water including peat [29] , wood [30] , pine bark [31] , banana pith [32] , rice bran, soybean and cottonseed hulls [33] , peanut shells [34] , hazelnut shell [35] , rice husk [36] , sawdust [37] , wool [38] , orange peel and compost [39] and leaves [40] . Most of this work has approved that natural products could be perfect adsorbents for heavy metals [26] .
Most metals in the fourth period of periodic table are carcinogenic. This carcinogenicity is assumed to be related to the electronic structure of transition and inner transitional metals [41] . Since copper is an essential metal in a number of enzymes for all forms of life, problems take place when it is deficient or in excess [26] . Copper is considered one of the most widely used heavy metal and the cupric ion, Cu(II), is the most prevalent species found in the environment and copper in this form is toxic to many living organisms [3] [42] [43] .
Human is exposed to abnormally high levels of toxic metals in drink and food due to some practices such as cooking in copper-lined or copper-glazed pots and using copper pipes to supply water [44] . Drainage discharge, fertilizer industries, mining wastes, plating baths, paints and pigments, etc. are different sources of copper waste [12] .
Although copper in trace amounts is essential to living organisms, excessive copper in water may cause harmful effects as neurotoxicity, jaundice, diarrhea, respiratory difficulties, liver and kidney failure, and even death [43] [45] [46] . Intake of excessively large portions of copper by man leads to severe mucosal irritation and corrosion, widespread capillary damage, hepatic and renal damage, central nervous system irritation followed by depression, gastrointestinal irritation [12] [47] .
One of the epidemiological evidences, a high incidence of cancer among coppersmiths, suggests a primary carcinogenic role for copper [41] . The allowable limit of copper in drinking water is 1.3 and 2.0 mg/L according to U.S. Environmental Protection Agency (EPA) and WHO respectively [48] .
Among the several methods described in literature, adsorption is the most effective method that has been successfully applied in the purification and recovery of Cu(II) ions from liquid waste because of its high efficiency and easy handling [49] .
Agricultural materials especially those containing cellulose present potential metal biosorption capacity. Set of functional groups are exist in the basic components of the agricultural waste materials biomass such as extractives, hemicellulose, lignin, lipids, proteins, simple sugars, water hydrocarbons, starch. The functional groups facilitate metal complexation which helps for the heavy metals elimination [18] [50] [51] [52] .
Agricultural waste materials seem to be viable option for heavy metal remediation for many reasons; 1) they are available in abundance, 2) are economic and ecofriendly, 3) more efficient, 4) they have unique chemical composition, 5) renewable and finally 6) low in cost. These materials are used in the removal of metal ions either in their natural form or after some physical or chemical modification [18] .
For centuries, sesame seeds have been plowed particularly in Asia and Africa. In 2009, the world production of sesame seed was around 4 million tons and the major production areas were Asia (nearly 2.5 million tons) and Africa (nearly 1.3 million tons), with a reported percentage of 62.6% and 33.1% of the total world production [53] . Sesame seeds present a considerably higher content of polyphenolic compounds [54] . Husks are used as feed or fuel, but they have relatively high contents of phenolics in some cases, usually higher than their contents in seeds [55] and phenolic compounds can act as metal chelators [56] . Sćiban et al. [57] conducted an experiment on the efficiency of sawdust as an adsorbent in the removal of Cu(II) ion. It was found that, sawdust contains various organic compounds (lignin, cellulose and hemicellulose) with polyphenolic groups that could bind heavy metal ions through different mechanisms [58] . From all these literature review, it is expected that sesame husk (SH) could be used as a promising adsorbent for removal of copper(II) from simulated wastewater (aqueous solution).
In this article, sesame husk (SH) was prepared and used as a novel adsorbent for copper(II) removal from aqueous solution. The resulting SH was characterized by SEM and FT-IR. Also, adsorption isotherms and kinetics were investigated in order to evaluate the maximum adsorption capacity and the optimum adsorption conditions.
2. Materials and Methods
2.1. Instrumentation
Weighing was done using Mettler Toledo microbalance (with 0.1 mg readability). The experimental values of pH were measured by pH Meter instrument model: Jenway 3510 (pH accuracy: ±0.003%, temperature resolution: 0.1˚C and temperature accuracy: ±0.5˚C). Before every measurement, the pH meter was calibrated with three standard buffer solutions (pH 4.0, pH 7.0 and pH 10.0 at 25˚C) supplied by Jenway. The adsorption experiments were carried out by mechanically shaking and BlueSpin Magnetic Hotplate Stirrer instrument model: BlueSpin MS7-H550-Pro was used (speed resolution: ±1 rpm, control accuracy of work plate ±1˚C [<100˚C] and the temperature is kept constant using external temperature sensor with accuracy of ±0.2˚C). The adsorption experiments were performed in a batch system with a 100 mL stoppered pyrex glass flask. The Cu(II) solution was filtered through Whatman filter paper (0.45 µm pore size) and heavy metal concentration of the initial and adsorbed solutions was measured by atomic absorption spectrometry (iCE 3300 AAS, Thermo Fisher Scientific Inc., UK) with air/acetylene flame. Quantification of the metals was based upon calibration curves of standard solutions of copper ion. Scanning electron microscopy (SEM), (JSM-5300, Jeol Ltd., Japan) was used to examine the surface morphology and to image the SH adsorbent before and after adsorption at a magnification of 2000× with an acceleration voltage of 20 kV. The Fourier transform-infrared spectroscopy (Spectrum BX FT-IR, PerkinElmer Inc., USA) study was conducted using a spectral frequency range of 4400 - 350 cm−1. Brunauer-Emmett-Teller (BET) surface area was determined using (Gas sorption analyzer, Quantachrome Ltd., UK). Elemental analysis (C, H and N) was carried out using the Elemental Analyzer (EA 1108 CHNS-O, Fisons Ltd., UK).
2.2. Sesame Husk Adsorbent
The adsorbent used in the present study was sesame husk (SH) which is brought from local company for manufacturing of Tahini in Alexandria, Egypt after the stage of separating sesame from its husk using sesame dehulling water tank. Sesame husk (SH) was grinded using herb grinder (speed: 25,000 rpm) to increase the surface area until fine powder is gained and then was sieved through a range of sieves, and only the particles that passed through a 0.25 mm mesh were used in our study. The sieves were shaken for around 15 min. After sieving, the separated particles of adsorbent was washed several times with Milli-Q water to remove any particles adhering to the surface and any water-soluble particles, then was oven-dried at 70˚C for 2 h and the cycle of drying, cooling, dessicating and weighing was repeated until a constant weight is obtained. SH was chosen for the adsorption tests without any pre-treatment and was stored in dark polyester container for any further use. A summary of the properties of sesame husk adsorbent are listed in Table 1.
2.3. Experimental Procedure
Aqueous solutions of copper were prepared from copper sulphate pentahydrate (CuSO4・5H2O) obtained from (Merck). Stock solutions of 1000 mg/L for Cu(II) were prepared, by dissolving desired amount of (CuSO4・5H2O) in 500 mL of distilled water. After, different concentrations of solutions were prepared by appropriate dilution of the stock solution. Before mixing these solutions with the
Table 1. Physico-chemical characterization of the sesame husk.
adsorbent, test solutions with pH values ranging from 2 to 6 (to permit a determination of the optimum pH for adsorption) by dropwise addition of 0.1N sodium hydroxide, NaOH (BDH) or 0.1N hydrochloric acid, HCl (BDH). All the chemical compounds used to prepare the reagent solutions were of Analar grade. After selecting the optimal pH, only one pH value was tested in all subsequent adsorption tests.
All the adsorption measurements were repeated three times to confirm reproducibility; hence, the reported value of metal ion adsorbed is the average of three measurements. Blanks containing no Cu(II) were used for each series of experiments. The adsorption capacity of SH was evaluated using the following expression [59] :
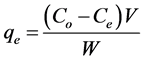 (1)
(1)
where qe (mg/g) is the adsorbed heavy metal (copper) amount per unit mass of the adsorbed sesame husk (SH), Co (mg/L) is the initial concentration of heavy metal solution and Ce (mg/L) is the concentration of the heavy metal in the aqueous phase at equilibrium, V (L) the volume of the aqueous phase and W (g) is the amount of SH adsorbent used.
The percent removal (% R) of Cu(II) ions was calculated by using following Equation [60] :
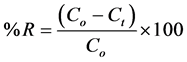 (2)
(2)
where Co and Ct (mg/L) are the Cu(II) initial concentration and the concentration at a given time t (min.) respectively.
3. Results and Discussion
3.1. Effect of pH
Varying the pH values is an important parameter in the adsorption process by influencing the surface charge of adsorbent, the degree of ionization and speciation of the adsorbate [13] . Therefore, studying the effect of pH on the removal efficiency of Cu(II) cations was performed by contacting 1.0 g of sesame husk adsorbent with 100 mL of 30 mg/L concentration of Cu(II) ion solution for 60 minutes with changing the solution pH range from 2 to 6, at 298 K and the sample was stirred using a magnetic stirrer at agitation speed 300 round per minute (rpm). As displayed in Figure 1, there is a noticed severe increase in the copper removal at equilibrium from 58.02% to 95.13% occurred when the pH values of the solutions changed from 2 to 6. Rising pH value above pH 6 causes precipitation and hampers the adsorption where copper(II) ions will form the insoluble Cu(OH)2 precipitate [61] [62] , so pH 6 was chosen as optimum pH for Cu(II) adsorption. The decrease of removal efficiency at low pH is due to:
1) The existence of higher concentration of hydronium ions in the solution which compete with the Cu(II) ions for the binding sites of adsorbent [63] .
2) At low pH, the sesame husk surface is positively charged due to protonation which is obvious at low pH values due to the presence of high concentration of H+ ions in the solution. Thereafter, electrostatic repulsion between the positively charged adsorbent surface and the metal ions in solution is engendered and the adsorption of Cu(II) becomes more unfavorable [64] [65] .
3) The Cl− species in solution which come from adjusting the solution pH value using HCl, lead to decreasing of the free Cu(II) ions and increasing in the formation of the chloro complex CuCl+. The molecular size of this complex is larger than that of the free Cu(II) and is affect inversely on the adsorption, resulting decrease in copper uptake but this effect is very limited [13] .
However, Increasing the pH leads to diminishing the hydronium ion concentration, the adsorbent surface became deprotonated and subsequently, increasing of Cu(II) uptake [66] .
Pandey et al. [67] studied the effect of Zeolite NaX as an adsorbent on removal of Cu(II) ions from aqueous stream and it was found that uptake capacity of
Figure 1. Effect of pH on the % removal of Cu(II) ions onto sesame husk (T: 298 K, 300 rpm, Co: 30 mg/L and 1.0 g of adsorbent).
copper(II) ions was maximum at pH 6. The same results were observed by Hossain et al. [61] and Oo et al. [62] .
3.2. Effect of Contact Time
One of the most critical parameters for successful adsorption process is determining the percentage removal of metal ions by changing the contact time of the aqueous solution with the adsorbent. To study the effect of contact time, the experiment conducted with initial copper concentration (Co: 30 mg/L) at optimum pH 6, with a dose of 1.0 g of SH/100 ml Cu(II) solution at 298 K and rotation speed of 300 rpm with contact time of 1, 3, 5, 7, 10, 15, 20, 30, 45 and 60 minutes. The effect of contact time on the percentage of Cu(II) elimination by SH adsorbent is shown in Figure 2. A two-stage manner was observed: firstly a highly fast stage, followed by a second slow stage of adsorption. The initial increase in the percentage adsorption of metal ions is attributed to existence of a large number of active sites on the SH surface that were swiftly occupied by a significant amount of copper ions. The second stage of low sorption rate until saturation process occurred due to two reasons: 1) the adsorbent pores become saturated at the initial stage of adsorption where the metal ions are adsorbed. Thus, a few numbers of ions are attached on the surface due to slower diffusion of solute into the interior of the adsorbent [68] , 2) The binding sites were shortly become limited and the remaining unoccupied surface sites are hard to be occupied by copper ions due to the arising of repulsive forces between the copper on the solid surface and the residual copper in the liquid phase [69] [70] [71] . Only 10 - 15 minutes equilibration period was needed for SH adsorbent to achieve equilibrium; no considerable further metal adsorption was noticed up to 60 minutes (Figure 2). However, a contact time of 30 min was set for the subsequent batch experiments. The elimination percentage of copper ions onto SH reached
Figure 2. Effect of contact time on the Cu(II) ions removal efficiency onto sesame husk at different initial concentrations (T: 298 K, 300 rpm, pH: 6 and 1.0 g of adsorbent for 60 min).
95.13% after 15 minutes of contact time. The lower contact time to reach equilibrium observed in this study indicates that the adsorption process is quite fast.
3.3. Effect of Adsorbent Dosage
The effect of adsorbent dosage on Cu(II) ions percentage removal and adsorption capacity was investigated using different doses: 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and 1.4 g in 100 mL of 30 mg/L Cu(II) by keeping other variables constant (at optimal pH of 6, temperature: 298 K, agitation speed: 300 rpm, contact time: 30 min. and Co: 30 mg/L) as shown in Figure 3. Results show that with the increase in adsorbent dose from 0.2 to 1.0 g, the percentage removal of copper ions rose from 63.50% to 95.13% then it decreases to be 90.27% with the consequence increase in dose up to 1.4 g respectively. This rising in the heavy metal percentage removal with increasing the adsorbent dosage is plausible because of the increase in adsorbent surface area and the availability of more exchangeable binding sites on the surface which are ready for metal ion uptake [72] [73] [74] [75] [76] . Decreasing the adsorption efficiency with further increase in dose above 1.0 g could be interpreted as a result of a partial overlapping or aggregation of adsorbent active sites as a result of overcrowding of adsorbent particles [77] [78] [79] , which results in interaction of active site with adsorbent atoms rather than adsorbate and thus, the total adsorption area decreases [80] [81] .
However, the copper(II) ions uptake capacity or amount of metal adsorbed (q, mg/g) decreases gradually upon increasing the dosage as shown in Figure 3. Adsorption capacity decreased from 8.839 mg/g at 0.2 g to 1.940 mg/g at 1.4 g. This occurs due to the fact that some adsorbent active sites stay unsaturated during the adsorption process. Hence, the number of unsaturated active sites available for sorption increases with increasing the adsorbent dose which can be ascribed to an insufficiency of metal ions in solution compared to the available
Figure 3. Effect of sesame husk dose on the % removal and adsorption capacity at equilibrium of Cu(II) ions (T: 298 K, 300 rpm, pH: 6 and Co: 30 mg/L for 30 min).
binding sites [82] [83] [84] [85] . Also, the decrease of qe with increasing the sorbent mass is attributed the decrease of the adsorbent total surface area and the increase in diffusion path length due to aggregation of adsorbent particles [86] [87] [88] . Furthermore upon increasing the adsorbent dosage, the binding sites of the adsorbent are shielded from metal where elevated dosage could impose a screening effect of the dense outer layer of the cells [89] [90] .
3.4. Effect of Adsorbate Concentration
The experiment was carried out with variable initial copper ion concentrations (30, 40, 50, 70 and 100 mg/L) and constant temperature (298 K), pH (6.0), contact time (30 min.), adsorbent dosage (0.2 g) and shaking speed (300 rpm). It can be observed that the copper(II) ions removal rate decreases with the increase of the initial concentration. The percent adsorption (%) is given in Figure 4(a) which shows that the percentage of Cu sorption on sesame husk decreased from 62.53% to 21.01% as the initial Cu(II) concentration increased from 30 mg/L to 100 mg/L respectively. This is because at low concentration the sorbent have enough active sites which could be easily occupied by metal ions since the ratio of available adsorption binding sites to the initial number of Cu(II) metal ions is large. Whereas at higher concentration, there is no more active sites to be occupied and the ratio of available adsorption active sites become fewer. That’s why; Cu ions are left unadsorbed in solution and the percentage removal of Cu(II) ions which depends upon the initial concentration, decreases [91] . This result is found matching with recent studies by Bhatti et al. [92] , Azouaou et al. [93] and Yao et al. [94] where the percent of metal adsorption decreases with increasing the concentration of adsorbate.
As seen in Figure 4(b), the adsorbed amount of metal ions per unit mass of adsorbent (qe) increases as the initial concentration of the adsorbate solution increases until it reaches maximum then decreases where qe increased from 8.704 mg/g at Cu(II) ions concentration of 30 mg/L to be highest value of 12.94 mg/g at 70 mg/L of Cu(II) followed by decreasing to be 10.35 mg/g at 100 mg/L. This can be interpreted as following; the increasing in qe value from initial concentration of 30 mg/L to 70 mg/L took place for two reasons: 1) with a solution of low concentration, the ratio between the number of metal ions to the number of available adsorption sites is small and subsequently the fractional adsorption becomes independent on initial concentration. However, at high concentration the available sites of adsorption becomes fewer and hence the adsorption of metal ions is dependent upon initial concentration. Thus, increasing the initial concentration of copper metal solution causes further increasing in qe value [88] [95] . 2) Higher concentration gradient can act as a driving force to overcome resistance to mass transfer of metal ions between the aqueous phase and the solid phase resulting in higher probability of collision between Cu(II) ions and the active sites [96] [97] . However, further increasing of the copper ions concentration from 70 mg/L to 100 mg/L leads to decreasing in qe value as observed where at a high certain concentration (70 mg/L), the active adsorption sites became saturated [93] [98] ; after which, qe decreases.


Figure 4. (a) Effect of initial concentration on the % removal of Cu(II) ions onto sesame husk (T: 298 K, 300 rpm, pH: 6 and 0.2 g of adsorbent for 30 min). (b) Effect of initial concentration on the adsorption capacity at equilibrium of Cu(II) ions onto sesame husk (T: 298 K, 300 rpm, pH: 6 and 0.2 g of adsorbent for 30 min).
Zhang and Wang [99] studied the effect of the initial concentration of Ni(II) ions on the adsorption capacity (qe) of lignocellulose/montmorillonite nanocomposite and the trend obtained of adsorption capacity was the same as the current study. qe first increased with increasing initial Ni(II) concentration until it reached maximum at 0.0032 mol/L then decreased until 0.0036 mol/L.
Similarly, Ang et al. [100] determined the copper(II) adsorption effect by the neem leaf powder (NLP). Results showed that the adsorption capacity is increased with increasing the initial copper(II) ions concentration until it reaches maximum then decreased due to saturation of active groups. This is complied with the results obtained from the present study.
3.5. Effect of Temperature
The adsorption of Cu(II) ions on sesame husk (SH) was examined for the initial solution concentration of 30 mg/L at 298, 303, 308 and 313 K. As presented in Figure 5(a) and Figure 5(b), upon rising the temperature from 298 to 313 K, the adsorption removal percentage decreased from 95.33% to 83.13% respectively. In the same manner, adsorption capacity of copper(II) metal ions onto SH decreased from 2.862 mg/g at 298 K to be 2.508 mg/g at 313 K. When the temperature is increased, degradation of the adsorbent and alteration of active functional groups take place which will vary the surface chemistry of sorbent and the


Figure 5. (a) Effect of temperature on the % removal of Cu(II) ions onto sesame husk (300 rpm, pH: 6, Co: 30 mg/L and 1.0 g of adsorbent for 30 min). (b) Effect of temperature on the adsorption capacity at equilibrium of Cu(II) ions onto sesame husk (300 rpm, pH: 6, Co: 30 mg/L and 1.0 g of adsorbent for 30 min).
number of active functional groups available for adsorption of heavy metal ions decreases. Besides, bonds are ruptured at higher temperature so desorption is favored [100] . Additionally on increasing the temperature, the thickness of the boundary layer decreases because the metal ions tend increasingly to flee from the biomass surface to the solution phase which limits the adsorption capacity [101] [102] . Moreover, at higher temperatures the surface activity of the biomass decreases [103] [104] , even there is a possibility of damaging the surface active sites which reduces the sorption ability of the materials [105] . Eventually, the loss in the adsorption capacity is caused by the change in the texture of the sorbent and as a result of the material deterioration [106] .
Alpat et al. [107] studied the effect of temperature on the biosorption capacity (qt) of Ni(II) on Circinella sp. and found that the increase in temperatures from 40˚C to 60˚C decreases the qt value. This decrease was ascribed to that, the biosorbent surface has been deactivated or some of biosorbent’s active sites were destroyed.
Similar results were obtained by Aksu and İşoğlu [108] who reported that the equilibrium uptake capacity of copper(II) ions using dried sugar beet pulp of sorbent decreased from 24.6 to 12.3 mg/g with increasing temperature from 25 to 45˚C.
3.6. Effect of Agitation Speed
The effect of agitation speed on adsorption of copper was studied over the range 100 - 500 rpm for 30 minutes with 100 ml solution containing 30 mg/L copper metal ions and 1.0 g of sesame husk. Figure 6 refers that the percent removal of adsorption increased from 87.81 to be maximum of 95.33% upon increasing the agitation speed from 100 rpm to 300 rpm. This agitation speed (300 rpm) was
Figure 6. Effect of agitation speed on the % removal of Cu(II) ions onto sesame husk (T: 298 K, pH: 6, Co: 30 mg/L and 1.0 g of adsorbent for 30 min.).
chosen as an optimum speed to be applied for other experiments. The Low speed cannot distribute the particles properly in the metal solution but accumulated [71] . This will bury some of the binding active sites of the adsorbent layer and not all of the adsorbent active sites can adsorb the metal ions. That’s why, the agitation rate should be sufficient enough to assure that all the surface binding sites are already available for metal uptake [109] . However, increasing the agitation speed beyond 300 rpm will affect negatively on the Cu(II) ions percent removal where it decreased from 95.33% at 300 rpm to 90.39% at 500 rpm. This is attributed to an increase in desorption tendency of adsorbate ions [109] . In addition, the high speed spreads vigorously the adsorbent particles in the solution and does not permit a sufficient time for adsorbent to bind with copper ions [110] .
3.7. Thermodynamic Parameters
There is a possibility to determine the thermodynamic parameters for the adsorption reaction by considering the equilibrium constants under the several experimental conditions. These parameters could be calculated by the following Van’t Hoff equation [109] [111] [112] :
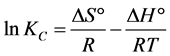 (3)
(3)
where R is universal gas constant (8.314 J/(mol・K)), T (K) is the absolute temperature in kelvin and Kc is the linear adsorption distribution coefficient defined as: Kc = Co/Ce in which Co and Ce (mg/L) are the initial adsorbate concentrations and adsorbate concentrations remained in the liquid phase at equilibrium respectively, ΔG° is the free energy of adsorption, ΔH° (kJ/mol) is the enthalpy change and ΔS° (J/(mol・K)) is the entropy change.
By plotting a graph between lnKc and 1/T as shown in Figure 7, a straight line
Figure 7. Effect of temperature on the thermodynamic behavior of adsorption of Cu(II) ions onto sesame husk (300 rpm, pH: 6, Co: 30 mg/L and 1.0 g of adsorbent for 30 min).
is obtained from which ΔH° and ΔS° values were estimated from slope and intercept respectively.
There is a direct relation between the change in Gibbs free energy upon adsorption ΔG° (kJ/mol) and both of the entropy change (ΔS°) and heat of adsorption (ΔH°) which can be calculated by the equation [13] :
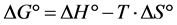 (4)
(4)
The thermodynamic parameters are displayed in Table 2. The negative value of ΔH° implies that the adsorption phenomenon is exothermic in nature. The sign of ΔS° indicates whether the adsorption reaction is an associative or dissociative mechanism. As shown in Table 2, since ΔS° has a negative value so an associative mechanism is involved during the adsorption process. Adsorption leads to order through the formation of an activated complex between adsorbate and adsorbent [109] . Also, ΔS° sign plays an important role in reflecting whether the order of the adsorbate at the solid/solution interface during the adsorption process becomes less random (ΔS° < 0) or more random (ΔS° > 0) [113] . Moreover, negative ΔS° value involves decreasing in the degree of freedom of Cu(II) ions in the solution.
The negative values of ΔG° indicate that the adsorption process is spontaneous However, the ΔG° value changed from negative to positive value on increasing the temperature which means that the adsorption reaction is non-feas- ible and non-spontaneous at higher temperature indicating that the spontaneous nature of adsorption is inversely proportional to the temperature [114] .
3.8. Characterization of Sesame Husk
3.8.1. Fourier Transform-Infrared Spectroscopic Analysis (FT-IR)
Examination the characteristic functional groups that makes the adsorption possible was done by Fourier Transform-Infrared Spectroscopic Analysis (FT- IR). The FT-IR spectra of sesame husk before and after adsorption of Cu(II) ions are shown in Figure 8. Different types of functional groups were detected in the adsorbent where the infrared spectrum displayed a large number of adsorption peaks.
The broad band peak at 3455.00 cm−1 was assigned to the stretching vibration of hydroxyl groups (O-H) of hydrogen bonded alcohols, phenols on the surface of sesame husk [115] [116] and −NH stretching [84] [117] but after adsorption it was slightly shifted to higher frequency at 3455.60 cm−1. The peaks at 2926.62 and 2856.38 cm−1 were due to the stretching vibration of CH3 and CH2 groups
Table 2. Thermodynamic parameters for the adsorption of Cu(II) onto sesame husk.
Figure 8. FTIR Spectra of sesame husk (a) before adsorption and (b) after adsorption of Cu(II) ions.
respectively [118] and these bands were changed to 2926.12 and 2855.80 cm−1 correspondingly. The two distinctive bands at 2366.42 and 2338.16 cm−1 are from the carbon dioxide (CO2) in air, they were shifted to 2361.87 and 2338.14 cm−1 respectively [119] [120] . The strong band that appeared at 1710.81 cm−1 is corresponding to the stretching C=O of ketone [121] [122] [123] and its intensity was decreased a bit to be 1710.58 cm−1. The band at 1621.11 cm−1 could be attributed to stretching vibration of CO from carboxylic acid in the presence of intermolecular hydrogen bonding [124] . The band at 1460.88 cm−1 is characteristic to the scissoring vibrations of −CH2 functional groups in lipids [125] [126] [127] . The band at 1316.25 cm−1 is ascribed to −CH2 wagging vibration in cellulose and hemicelluloses [128] . The band at 1157.61 cm−1 can be ascribed to the vibration of C-O-C in polysaccharides (cellulose and hemicelluloses) [129] [130] [131] [132] . The band at 1102.87 cm−1 can be indicative of O-H associated with cellulose and hemicelluloses [133] [134] . The peak at 1058.38 cm−1 presented the C?O stretching vibration in the lignin structure [135] [136] and the shifting from 1058.38 to 1060.02 cm−1 clearly showed the interaction of copper(II) with oxygen lone pair [136] . It can be noticed that, the characteristic peaks intensities were increased from 1621.11, 1460.88, 1316.25, 1157.61, 1102.87 and 1058.38 cm−1 to 1625.70, 1462.09, 1317.76, 1161.37, 1104.25 and 1060.02 cm−1 respectively. The band at 956.48 cm?1 is correlating to = C-H indicating the presence of alkenes [137] and after adsorption it was varied to be 956.25 cm−1. The peak at 886.77 cm−1 is representative for the out-of-plane C-H bending motions in terminal methylene groups [138] which was shifted to 890.83 cm−1. The peaks at 780.14 and 667.22 cm−1 refer to C-H out-of-plane bending (alkenes) and O-H out-of-plane bending (alcohols and phenols) respectively [139] [140] . The peak shown at 515.57 cm−1 is from O-C-O in plane bending [141] . These peaks around 780.14 cm−1 shifted to 781.05 cm−1, 667.22 cm−1 shifted to 670.44 cm−1 and 515.57 cm−1 changed to 516.00 cm−1.
After the sesame husk was loaded with copper, it was observed that there are differences in the intensities or the locations of the absorbance peaks. This shift in wave number corresponds to a change in bonding energy of the functional groups such as hydroxyl, amine group, carbonyl and carboxyl groups, alkenes groups or oxygen lone pair. This FT-IR result indicates that these functional groups in the sesame husk participated in the adsorption process and binding of copper ions [84] [115] [142] [143] [144] .
3.8.2. Scanning Electron Microscopy
Scanning electron microscopy (SEM) was used to analyze the surface morphology and fundamental physical properties of the adsorbent. The scanning electron micrographs before and after adsorption were shown in Figure 9. Before adsorption, The morphology of sesame husk (SH) adsorbent exhibited a rough and irregular surface with a distinguished dark spots of pores and cavities, implying that there was a possibility for Cu(II) metal ions to be trapped and adsorbed onto the surface. After adsorption, SEM image clearly showed that the pores were completely filled and the SH surface was more regular and relatively smoother with several agglomeration, indicating that copper metal ions have been attached to the surface.
Figure 9. SEM images of sesame husk (a) before adsorption and (b) after adsorption of Cu(II) ions.
From the characterization of the adsorbent, there is a high affinity between SH and Cu(II) ions due to the rough surface and functional groups of sesame husk which adsorb copper metal cations from solution.
3.9. Adsorption Isotherm Study
The adsorption isotherm is a primary tool for understanding the surface nature of the adsorbent. However, selecting the right suitable adsorption equation for different concentration ranges presents a clear picture of the surface.
The adsorption isotherm is significant to describe the following: 1) distribution of adsorbate molecules between the liquid and solid phase at equilibrium, 2) the manner of interaction between adsorbate and adsorbent and 3) the adsorption type and its features. For determining the adsorption system, the data were fitted for applying different models [145] such as Langmuir, Freundlich and Dubinin-Radushkevich (D-R) isotherms.
3.9.1. Langmuir Isotherm
The Langmuir adsorption isotherm assumes that adsorption takes place at totally homogenous adsorption surface [13] [64] [146] [147] . Further assumption is that the maximum adsorption corresponds to a saturated monolayer of adsorbate molecules on adsorbent surface and there is no significant interaction among adsorbed species. Thus, the adsorption energy is constant and there is no transmigration of adsorbate in the plane of the adsorbent surface [148] [149] [150] [151] .
The equilibrium data for metal cations have been correlated with the Langmuir isotherm over the concentration range of copper metal ions from 30 to 100 mg/L at 298 K. The Langmuir isotherm represented by the following equation [152] :

qe (mg/g) is the equilibrium adsorption capacity of ions on the adsorbent, Ce (mg/L) is the equilibrium ion concentration in solution, qmax (mg/g) is the maximum capacity of the adsorbent , which represents monolayer coverage of adsorbent with adsorbate, b (L/mg) is the Langmuir adsorption constant. qmax and b are Langmuir constants related to adsorption efficiency and energy of adsorption respectively [149] . As shown in Figure 10, the linear plot of Ce/qe ver-
sus Ce suggests the applicability of the Langmuir isotherm with a slope of 

tion data with R2 value larger than 0.97.
The adsorption isotherms of Cu(II) exhibit Langmuir behavior, which indicates a monolayer adsorption and the applicability of adsorption process can be
Figure 10. The linearized Langmuir adsorption isotherm for Cu(II) ions adsorption by sesame husk (T: 298 K, pH: 6, 300 rpm and 1.0 g of adsorbent for 30 min).
identified by dimensionless constant separation factor (RL) [153] which is shown below:

where Ci is the initial concentration of Cu(II). The RL value indicates whether the adsorption is: Unfavorable: RL > 1; Linear: RL = 1; Favorable: 0 < RL < 1; Irreversible: RL = 0. As presented in Table 3, RL values were 0.008, 0.005, 0.004, 0.003 and 0.002 for initial concentration of 30, 40, 50, 70 and 100 mg/L respectively. Since the RL values are found to be in the range between 0 and 1, this indicates that the adsorption of Cu(II) metal cations onto the adsorbent (sesame husk) is favorable [13] [88] [154] [155] .
3.9.2. Freundlich Isotherm
The Freundlich isotherm gives the relationship between equilibrium liquid and solid phase capacity consisting of heterogeneous surface of the adsorbent or surface supporting sites of diverse affinities [151] [156] [157] and this isotherm is applicable to multilayer sorption [64] . It states that the ratio of the amount of solute adsorbed onto a given mass of adsorbent to the concentration of the solute in the solution is not constant at different concentrations [158] .
The logarithmic form of Freundlich [159] is represented by the following Equation:

Equilibrium capacity qe and Ce are defined as above while Kf is the Freundlich adsorption constant representing the adsorption capacity, n is the empirical parameter relating the adsorption intensity of the solid adsorbent which varies with the heterogeneity of material. The magnitude of n gives a measure of the favorability of adsorption. If the value of n between 1 and 10 (1/n is lower than 1), this represents that the surface of the adsorbent was heterogeneous and adsorption occurred easily [64] [151] [157] [160] [161] .
Table 3. Parameters of Langmuir, Freundlich and Dubinin-Radushkevich (D-R) isotherms for the adsorption of Cu(II) ions onto sesame husk.
When logqe is plotted against logCe, a straight line with a slope 1/n and intercept logKf as obtained in Figure 11. In this study, the Freundlich plots yielded values for the coefficients Kf and n were 6.933 and 8.210 respectively as appears in Table 3. This n value indicates that the adsorption intensity is favorable [162] [163] .
Figure 11. Freundlich adsorption isotherm for Cu(II) ions adsorption by sesame husk (T: 298 K, pH: 6, 300 rpm and 1.0 g of adsorbent for 30 min).
3.9.3. Dubinin-Radushkevich (D-R) Isotherm
The Dubinin-Radushkevich (D-R) isotherm [164] explains multilayer formation in microporous solids. The Dubinin-Kaganer-Radushkevich (DKR) equation more general than the Langmuir isotherm since it does not assume a homogeneous surface or constant adsorption potential whereas it has been widely used to explain energetic heterogeneity of solid surfaces at low coverage. It was applied in order to distinguish between physical and chemical adsorptions [88] [147] [165] . The D-R equation has the following form:

Xm (mg/g) is the theoretical monolayer saturation capacity, β (mol2/kJ2) is the activity coefficient related to the mean free energy of adsorption per mole of the adsorbate when it is transferred from infinity in the solution to the surface of the solid and ε (J/mol) is Polanyi potential which is equal to:

where R is gas constant (R = 8.314 J/(mol・K)) and T is temperature (K).
The mean free energy E (kJ/mol) is calculated using the relationship [166] [167] [168] :

The plot of lnqe against ε2 for metal ions adsorption on sesame husk is shown in Figure 12. The slope yields β (mol2/kJ2) and the intercept yields the adsorption capacity Xm (mg/g). From the results in Table 3, the difference of qmax derived from Langmuir and Xm derived from D-R model may be attributed to the different definition of maximum capacity in the two models. In Langmuir model qmax represents the maximum adsorption of metal ions at monolayer coverage, but in D-R model Xm represents the maximum adsorption of metal ions at the total specific micropores volume of the adsorbent [88] [169] .
The magnitude of E is used for estimating the type of adsorption mechanism. If the magnitude of E is between 8 and 16 kJ/mol, it is indicated that the adsorption process is chemical adsorption, while for value of E < 8 kJ/mol; the adsorption process is physical in nature. The E value for Cu(II) on the sesame husk is 0.316 kJ/mol. The value of E is below 8 kJ/mol which indicates that physical adsorption is involved in the adsorption process [88] [170] [171] [172] [173] .
Figure 12. D-R isotherm adsorption plot for Cu(II) ions onto sesame husk (T: 298 K, pH: 6, 300 rpm and 1.0 g of adsorbent for 30 min).
As seen in Table 3, the value of maximum adsorption capacity (Xm) obtained from D-R model is more than the value of adsorption capacity (qmax) obtained from the Langmuir isotherm. On the other side, the correlation coefficient value of the linear plot of the Langmuir isotherm (R2 = 0.9740) was more satisfactory than those obtained by Freundlich and D-R models (R2 = 0.4197 and 0.6046 respectively) which means that Langmuir isotherm model fits quite well with the experimental data and is very suitable for describing the adsorption equilibrium of Cu(II) on SH. The maximum adsorption capacity (qmax) of copper ions on SH was 10.83 mg/g at 298 K. The complying of results with the Langmuir isotherm may be due to the homogeneous distribution of active sites on SH surface, since the Langmuir equation supposes that the surface is homogeneous.
This is in line with results reported by Cozmuta et al. [174] who studied the adsorption of lead metal ions on Na-clinoptilolite and by Li et al. [175] who studied the adsorption of Cu(II) ions on amino-functionalized magnetic nanoparticles.
3.9.4. Comparison of Maximum Adsorption Capacity of Different Adsorbents for Cu(II) Ions
Various adsorbents were investigated for Cu(II) ions removal. The adsorption capacities of such sorbents are given in Table 4. It is obvious that, the adsorption capacity of sesame husk towards Cu(II) is comparable or even better than 39 of
Table 4. Comparison of maximum adsorption capacity (qmax) of different reported adsorbents for Cu(II) ions.
43 other reported adsorbents, referring the promising future for sesame husk utilization in copper ions removal from aqueous solutions.
3.10. Adsorption Kinetics
Several adsorption kinetic models such as pseudo first-order, pseudo second- order and intra-particle diffusion models had been used to understand the characteristics and mechanism of adsorption [209] and the rate-limiting step during adsorption process [88] .
3.10.1. Pseudo First-Order Model
The adsorption rate constant suggested by Lagergren [210] and Ho [211] by applying first-order reaction kinetic is given by Equation (11):

where k1 is the adsorption rate constant for the first order adsorption, qt (mg/g) is the amount of Cu(II) metal adsorbed at time t and qe (mg/g) is the amount of heavy metal adsorbed at saturation.
The integration of the Equation (11) gives the following expression:

where C1 is the integration constant for first-order reaction kinetic.
If it is supposed that q = 0 at t = 0, then the pseudo first-order kinetic model is expressed by:

where qe and qt (mg/g) are the amounts of Cu(II) ions adsorbed onto sesame husk at equilibrium and at time t, respectively and k1 (min−1) is the rate constant of pseudo first-order kinetic model.
The straight line plots of 
Figure 13. Pseudo first-order plot for the adsorption of Cu(II) ions onto sesame husk at different initial concentrations (T: 298 K, 300 rpm, pH: 6 and 1.0 g of adsorbent).
value (qe,calc.) and the experimental value (qe,exp.) were not in agreement with each other referring a poor pseudo first-order kinetic model fit. Moreover, this kinetic model does not fit well to the whole range of contact time. It could only be applied over the initial stage of the adsorption process (1 - 7 minutes) [88] [212] [213] .
3.10.2. Pseudo Second-Order
The pseudo second-order model [214] [215] based on equilibrium adsorption is evaluated using the relationship:

where k2 is the rate constant of pseudo second-order kinetic model (g/(mg. min)). The straight line plots of t/qt against t (Figure 14) were used to determine the k2 and qe,calc.. This model was more likely to predict the behavior over whole range of contact time. According to results in Table 5, the R2 value for the pseudo second-order kinetic model was very close or even equal to unity. Moreover, the calculated equilibrium adsorption capacity values qe,calc. were very close to the experimental qe,exp. values indicating that the adsorption process of copper ions onto SH obeys pseudo second-order model kinetics at all initial Cu(II) concentrations.
In general, the adsorption process on a porous adsorbent will have four main stages. These stages involve: 1) the movement of the adsorbate from the bulk solution to the exterior film surrounding the adsorbent particle (bulk solution transport), 2) the transport of adsorbate across the external liquid film to the external surface sites on the adsorbent particle (film diffusion transport), 3) Migration of adsorbate within the pores of the adsorbent by intra-particle diffusion (pore diffusion) and finally 4) adsorption of adsorbate at internal surface sites [216] [217] .
Figure 14. Pseudo second-order plot for the adsorption of Cu(II) ions onto sesame husk at different initial concentrations (T: 298 K, 300 rpm, pH: 6 and 1.0 g of adsorbent).
Table 5. Comparison of kinetic parameters for the adsorption of Cu(II) ions onto sesame husk at different concentration (T: 298 K, speed: 300 rpm, pH: 6 and 1.0 g of adsorbent).
3.10.3. Intra-Particle Diffusion
The description of the adsorption process in a well-stirred batch adsorption system which occurs on a porous adsorbent is rebated by applying [218] intra-par- ticle diffusion model. The formation of this model is as follows:

where qt (mg/g) is the amount of Cu(II) ions adsorbed onto sesame husk at time t, and kp (mg/(g・min1/2)) is the intra-particle diffusion rate constant. The straight line plots of qt against t1/2 were used to determine the intra-particle diffusion rate (kp, slope), correlation coefficient R2 and the intra-particle diffusion constant mg/g (C, intercept) related to the thickness of the boundary layer: the larger the intercept, the greater the boundary layer effect [219] as shown in Table 5.
Based on Figure 15, the plot showed multi-linearity correlation which reveals that more than one step occurred during the adsorption process. Step “one” is the diffusion through the solution to the external surface of the adsorbent (the initial linear portions) which is also known as external mass transfer or film diffusion [220] , whereas step “two” corresponds to the intra-particle diffusion into the porous structure of the adsorbent [74] [221] . The later horizontal portions of the plot were slow and controlled by equilibrium diffusion mechanism [222] .
If Weber-Morris plot (qt against t1/2) is linear, so intra-particle diffusion occurs. And if the line passes through the origin, then the intra-particle diffusion is the only rate-limiting step [223] . In the current study, the plots of the intra-par- ticle diffusion model did not pass through the origin indicating that the intra-particle diffusion is not the sole limiting-step but the film diffusion also played an important role in adsorption. This is in coincidence with the fact that the adsorption processes followed pseudo second-order model [224] .
Figure 15. Intra-particle diffusion fit for the adsorption of Cu(II) ions onto sesame husk at different initial concentrations (T: 298 K, 300 rpm, pH: 6 and 1.0 g of adsorbent).
3.10.4. Test of Kinetic Validity:
To check quantitatively the validity of the used kinetic models in this study, a normalized standard deviation Δqe (%) is calculated by the following equation [225] [226] :

where n is the number of data points, qe,exp. (mg/g) is the observed experimental value, qe,calc. (mg/g) is the calculated qe from the models and the calculated results are listed in Table 5. It was found that the pseudo second-order kinetic model yielded the lowest Δqe (%) values. This agrees with the earlier obtained R2 values and confirms that the adsorption of Cu(II) ions onto sesame husk can be best described by the pseudo second-order kinetic model; the values of correlation coefficients (R2) obtained for the linear plots from the pseudo second-order model are greater than those obtained for the pseudo first-order and intra-par- ticle diffusion models under all conditions studied. Also, the values of Δqe (%) obtained for the pseudo second-order model were lower than those obtained from the pseudo first-order and intra-particle diffusion model under all conditions studied. Therefore, the adsorption of Cu(II) ions onto sesame husk adsorbent can be best described by the pseudo second-order kinetic model due to the higher correlation coefficient and a good agreement between the experimental and the calculated qe value.
3.11. Type of Adsorption
The type of adsorption can be estimated from four parameters ΔH°, activation energy Ea, the mean free energy 
The negative value of ΔH° for adsorption of Cu ions implies that the adsorption phenomenon is exothermic in nature [109] . According to literature [227] [228] [229] , if the enthalpy change value ΔH° of adsorbent is higher than 40 kJ/mol, the process is chemisorption which includes strong electrostatic chemical bonding between metal ions and adsorbent surface and when enthalpy change value is less than 20 kJ/mol, it indicates the adsorption is physical in nature. In this study, the ΔH° value for adsorption was −68.48 kJ/mol for Cu which reveal that the chemical adsorption (chemisorption) is involved for adsorption of Cu ions on SH adsorbent.
The magnitude of activation energy gives an idea about the type of sorption. Low activation energies Ea (5 - 40 kJ/mol) are characteristics for physisorption, while higher activation energies (40 - 800 kJ/mol) suggest chemisorption [230] [231] . The obtained Ea value (112.37 kJ/mol) indicates that the adsorption between Cu metal ions and adsorbent corresponds to chemisorption.
The magnitude of E is used for estimating the type of adsorption mechanism. If the magnitude of E is between 8 and 16 kJ/mol, it is indicated that the adsorption process is chemical adsorption, while for value of E < 8 kJ/mol; the adsorption process is physical in nature (physisorption). The E value for Cu(II) on the sesame husk was 0.316 kJ/mol. The value of E is below 8 kJ/mol which refer that; physical adsorption is involved in the adsorption process on the adsorbent surface [88] [170] [171] [172] [173] .
The trend of adsorption upon increasing the temperature can refer to the type of adsorption mechanism. In physical adsorption (physisorption), the force of attraction existing between adsorbate and adsorbent are Vander Waal’s forces (the force of attraction between the adsorbate and adsorbent are weak), therefore this type of adsorption can be easily reversed by heating or by decreasing the pressure which is called desorption. While in chemical adsorption (chemisorption), the adsorbate is chemically bound to the adsorbent and as a result, the forces between the two become 10 - 100 times greater than in physisorption [232] [233] . In the present study, the adsorption decreased upon rising temperature; this reversible behavior indicates that “physical adsorption occurred”.
These results suggested that the adsorption of copper(II) ions onto SH involves chemisorption as well as physisorption mechanism. From literature [149] [234] [235] , both physical and chemical adsorption may occur on the surface at the same time; a layer of molecules may be physically adsorbed on top of an underlying chemisorbed layer.
4. Conclusion
The present study highlighted the ability of sesame husk (SH) to adsorb Cu(II) from aqueous solutions. Experimental data are in alignment with Langmuir adsorption isotherm and the adsorption process complies well with pseudo second-order kinetic model. The adsorptive capacity of SH for copper ions is comparable or even better than many other natural adsorbents. The data obtained were used to calculate thermodynamic parameters such as ΔG°, ΔS° and ΔH°. Intra-particle diffusion is not the sole rate-controlling step. The investigation shows that sesame husk is as a widely available, natural, novel, easy prepared, fast, cheap and efficient adsorbent for the removal of Cu(II) from aqueous solutions (95.13% removal of 30 mg/L Cu(II) ions solution with small dose of SH (1.0 g/100ml) within 30 min. only) and thus this adsorbent would exhibit a promising technique for industrial wastewater cleanup.
Cite this paper
El-Araby, H.A., Ibrahim, A.M.M.A., Mangood, A.H. and Abdel-Rahman, A.A.-H. (2017) Sesame Husk as Adsorbent for Copper(II) Ions Re- moval from Aqueous Solution. Journal of Geoscience and Environment Protection, 5, 109-152. https://doi.org/10.4236/gep.2017.57011
References
- 1. de Luna, M.D., Flores, E.D., Cenia, M.C. and Lu, M.C. (2015) Removal of Copper Ions from Aqueous Solution by Adlai Shell (Coix lacryma-jobi L.) Adsorbents. Bioresource Technology, 192, 841-844.
https://doi.org/10.1016/j.biortech.2015.06.018 - 2. Vafakhah, S., Bahrololoom, M.E., Bazarganlari, R. and Saeedikhani, M. (2014) Removal of Copper Ions from Electroplating Effluent Solutions with Native Corn Cob And Corn Stalk and Chemically Modified Corn Stalk. Journal of Environmental Chemical Engineering, 2, 356-361.
https://doi.org/10.1016/j.jece.2014.01.005 - 3. Lee, H.W., Cho, H.J., Yim, J.H., Kim, J.M., Jeon, J.K., Sohn, J.M., Yoo, K.S., Kim, S.S. and Park, Y.K. (2011) Removal of Cu(II)-Ion over Amine-Functionalized Mesoporous Silica Materials. Journal of Industrial and Engineering Chemistry, 17, 504-509.
https://doi.org/10.1016/j.jiec.2010.09.022 - 4. Nilanjana, D. (2010) Recovery of Precious Metals through Biosorption—A Review. Hydrometallurgy, 103, 180-189.
https://doi.org/10.1016/j.hydromet.2010.03.016 - 5. Lina, R.V. and Nilanjana, D. (2015) Application of Nano-Biocomposites for Remediation of Heavy Metals from Aqueous Environment: An Overview. International Journal of ChemTech Research, 8, 566-571.
- 6. Feng, Y.J., Yang, L.S., Liu, J.F. and Logan, B.E. (2016) Electrochemical Technologies for Wastewater Treatment and Resource Reclamation. Environmental Science: Water Research & Technology, 2, 800-831.
- 7. Marder, L., Sulzbach, G.O., Bernardes, A.M. and Ferreira, J.Z. (2003) Removal of Cadmium and Cyanide from Aqueous Solutions through Electrodialysis. Journal of the Brazilian Chemical Society, 14, 610-615.
https://doi.org/10.1590/S0103-50532003000400018 - 8. Canizares, P., de Lucas, A., Pérez, á. and Camarillo, R. (2005) Effect of Polymer Nature and Hydrodynamic Conditions on a Process of Polymer Enhanced Ultrafiltration. Journal of Membrane Science, 253, 149-163.
https://doi.org/10.1016/j.memsci.2004.12.042 - 9. Trivunac, K. and Stevanovic, S. (2006) Removal of Heavy Metal Ions from Water by Complexation-Assisted Ultrafiltration. Chemosphere, 64, 486-491.
https://doi.org/10.1016/j.chemosphere.2005.11.073 - 10. Kumano, M., Yabutani, T., Motonaka, J., Mishima, Y. and Mishima, Y. (2006) Recovery and Extraction of Heavy Metal Ions Using Ionic Liquid as Green Solvent. International Journal of Modern Physics B, 20, 4051-4056.
https://doi.org/10.1142/S0217979206040842 - 11. Verma, V.K., Tewari, S. and Rai, J.P.N. (2008) Ion Exchange during Heavy Metal Bio-Sorption from Aqueous Solution by Dried Biomass of Macrophytes. Bioresource Technology, 99, 1932-1938.
https://doi.org/10.1016/j.biortech.2007.03.042 - 12. Ajmal, M., Khan, A.H., Ahmad, S. and Ahmad, A. (1998) Role of Sawdust in the Removal of Copper (II) from Industrial Wastes. Water Research, 32, 3085-3091.
https://doi.org/10.1016/S0043-1354(98)00067-0 - 13. Yao, Z.Y., Qi, J.H. and Wang, L.H. (2010) Equilibrium, Kinetic and Thermodynamic Studies on the Biosorption of Cu(II) onto Chestnut Shell. Journal of Hazardous Materials, 174, 137-143.
https://doi.org/10.1016/j.jhazmat.2009.09.027 - 14. Wang, X.S. and Qin, Y. (2005) Equilibrium Sorption Isotherms for Cu2+ on Rice Bran. Process Biochemistry, 40, 677-680.
https://doi.org/10.1016/j.procbio.2004.01.043 - 15. Mauchauffée, S. and Meux, E. (2007) Use of Sodium Decanoate for Selective Precipitation of Metals Contained in Industrial Wastewater. Chemosphere, 69, 763-768.
https://doi.org/10.1016/j.chemosphere.2007.05.006 - 16. Mohsen-Nia, M., Montazeri, P. and Modarress, H. (2007) Removal of Cu2+ and Ni2+ from Wastewater with a Chelating Agent and Reverse Osmosis Processes. Desalination, 217, 276-281.
https://doi.org/10.1016/j.desal.2006.01.043 - 17. El Samrani, A.G., Lartiges, B.S. and Villiéras, F. (2008) Chemical Coagulation of Combined Sewer Overflow: Heavy Metal Removal and Treatment Optimization. Water Research, 42, 951-960.
https://doi.org/10.1016/j.watres.2007.09.009 - 18. Sud, D., Mahajan, G. and Kaur, M.P. (2008) Agricultural Waste Material as Potential Adsorbent for Sequestering Heavy Metal Ions from Aqueous Solutions—A Review. Bioresource Technology, 99, 6017-6027.
https://doi.org/10.1016/j.biortech.2007.11.064 - 19. Ahluwalia, S.S. and Goyal, D. (2005) Removal of Heavy Metals by Waste Tea Leaves from Aqueous Solution. Engineering in Life Sciences, 5, 158-162.
https://doi.org/10.1002/elsc.200420066 - 20. Meena, A.K., Kadirvelu, K., Mishra, G.K., Rajagopal, C. and Nagar, P.N. (2008) Adsorptive Removal of Heavy Metals from Aqueous Solution by Treated Sawdust (Acacia arabica). Journal of Hazardous Materials, 150, 604-611.
https://doi.org/10.1016/j.jhazmat.2007.05.030 - 21. Gupta, V.K. and Ali, I. (2006) Adsorbents for Water Treatment: Development of Low-Cost Alternatives to Carbon, Encyclopedia of Surface and Colloid Science. 2nd Edition, Taylor and Francis, New York, 149-184.
- 22. Manohar, V.D.M., Noeline, B.F. and Anirudhan, T.S. (2006) Adsorption Performance of Alpillared Bentonite Clay for the Removal of Cobalt (II) from Aqueous Phase. Applied Clay Science, 31, 194-206.
https://doi.org/10.1016/j.clay.2005.08.008 - 23. Krishnan, K.A. and Anirudhan, T.S. (2008) Kinetic and Equilibrium Modelling of Cobalt (II) Adsorption onto Bagasse Pith Based Sulphurised Activated Carbon. Chemical Engineering Journal, 137, 257-264.
https://doi.org/10.1016/j.cej.2007.04.029 - 24. McKay, G. (1996) Use of Adsorbents for the Removal of Pollutants from Wastewaters. CRC Press Inc., Tokyo.
- 25. Girgis, B.S. and Ihsak, M.F. (1999) Activated Carbon from Cotton Stalks by Impregnation with Phosphoric Acid. Materials Letters, 39, 107-114.
https://doi.org/10.1016/S0167-577X(98)00225-0 - 26. Demirbas, O., Karadag, A., Alkan, M. and Dogan, M. (2008) Removal of Copper Ions from Aqueous Solutions by Hazelnut Shell. Journal of Hazardous Materials, 153, 677-684.
https://doi.org/10.1016/j.jhazmat.2007.09.012 - 27. Demirbas, A. (2008) Heavy Metals Adsorption onto Agro-Based Waste Materials: A Review. Journal of Hazardous Materials, 157, 220-229.
https://doi.org/10.1016/j.jhazmat.2008.01.024 - 28. Farahani, M. and Behbahaninia, A. (2014) Using Natural Adsorbents to Reduce Polycyclic Aromatic Hydrocarbons Contamination of Oily Wastewater. Journal of Biomedical and Environmental Sciences, 5, 274-281.
- 29. Ho, Y.S. and McKay, G. (2000) The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Research, 34, 735-742.
- 30. Poots, V.J.P., McKay, G. and Healy, J.J. (1978) Removal of Basic Dye from Effluent Using Wood as an Adsorbent. Journal of Water Pollution Control Federation, 50, 926-935.
- 31. Al-Asheh, S. and Duvnjak, Z. (1997) Sorption of c Admium and Other Heavy Metals by Pine Bark. Journal of Hazardous Materials, 56, 35-51.
https://doi.org/10.1016/S0304-3894(97)00040-X - 32. Low, K.S., Lee, C.K. and Leo, A.C. (1995) Removal of Metals from Electroplating Wastes Using Banana Pith. Bioresource Technology, 51, 227-231.
https://doi.org/10.1016/0960-8524(94)00123-I - 33. Marshall, W.E. and Johns, M.M. (1996) Agricultural By-Products as Metal Adsorbents: Sorption Properties and Resistance to Mechanical Abrasion. Journal of Chemical Technology & Biotechnology, 66, 192-198.
https://doi.org/10.1002/(SICI)1097-4660(199606)66:2%3C192::AID-JCTB489%3E3.0.CO;2-C - 34. Wafwoyo, W., Seo, C.W. and Marshall, W.E. (1999) Utilization of Peanut Shells as Adsorbents for Selected Metals. Journal of Chemical Technology & Biotechnology, 74, 1117-1121.
https://doi.org/10.1002/(SICI)1097-4660(199911)74:11%3C1117::AID-JCTB151%3E3.0.CO;2-R - 35. Cimino, G., Passerini, A. and Toscano, G. (2000) Removal of Toxic Cations and Cr(VI) from Aqueous Solution by Hazelnut Shell. Water Research, 34, 2955-2962.
https://doi.org/10.1016/S0043-1354(00)00048-8 - 36. Mishra, S.P., Tiwari, D. and Dubey, R.S. (1997) The Uptake Behavior of Rice (Jaya) Husk in the Removal of Zn(II) Ions: A Radiotracer Study. Applied Radiation and Isotopes, 48, 877-882.
https://doi.org/10.1016/S0969-8043(97)00340-0 - 37. Yu, B., Zhang, Y., Shukla, A., Shukla, S.S. and Dorris, K.L. (2001) The Removal of Heavy Metals from Aqueous Solutions by Sawdust Adsorption: Removal of Lead and Comparison of Its Adsorption with Copper. Journal of Hazardous Materials, 84, 83-94.
https://doi.org/10.1016/S0304-3894(01)00198-4 - 38. Balkose, D. and Baltacioglu, H. (1992) Adsorption of Heavy Metal Cations from Aqueous Solutions by Wool Fibers. Journal of Chemical Technology & Biotechnology, 54, 393-397.
https://doi.org/10.1002/jctb.280540414 - 39. Azab, M.S. and Peterson, P.J. (1989) The Removal of Cadmium from Water by the Use of Biological Sorbents. Water Science and Technology, 21, 1705-1706.
- 40. Zaggout, F.R. (2001) Removal of Copper from Water by Decaying Tamrix Gallica Leaves. Asian Journal of Chemistry, 13, 639-650.
- 41. Luckey, T.D. and Venugopal, B. (1977) Metal Toxicity in Mammals. Plenum Press, New York.
- 42. Chang, S.H., Teng, T.T. and Ismail, N. (2010) Extraction of Cu (II) from Aqueous Solutions by Vegetable Oil-Based Organic Solvents. Journal of Hazardous Materials, 181, 868-872.
https://doi.org/10.1016/j.jhazmat.2010.05.093 - 43. Guijuan, J.I., Weiwei, B.A.O., Guimei, G.A.O., Baichao, A.N. and Shucai, G.A.N. (2012) Removal of Cu (II) from Aqueous Solution Using a Novel Crosslinked Alumina-Chitosan Hybrid Adsorbent. Chinese Journal of Chemical Engineering, 20, 641-648.
https://doi.org/10.1016/S1004-9541(11)60229-2 - 44. Manahan, S.E. (1991) Environmental Chemistry. 5th Edition, Lewis Publishers, Chelsea.
- 45. Larous, S. and Meniai, A.H. (2012) Removal of Copper (II) from Aqueous Solution by Agricultural By-Products-Sawdust. Energy Procedia, 18, 915-923.
https://doi.org/10.1016/j.egypro.2012.05.106 - 46. Reza, B.L., Hamid, R.Z. and Mohammad, E.B. (2014) Removal of Cu(II) Ions from Aqueous Solutions by Low-Cost Natural Hydroxyapatite/Chitosan Composite: Equilibrium, Kinetic and Thermodynamic Studies. Journal of the Taiwan Institute of Chemical Engineers, 45, 1642-1648.
https://doi.org/10.1016/j.jtice.2013.11.009 - 47. World Health Organization and United Nations Environment Programme (1995) Health Risks from Marine Pollution in the Mediterranean. Part VII. Evaluation of Health Risks from Chemically-Contaminated Seafood. World Health Organization Regional Office for Europe, Copenhagen.
- 48. The Council of the European Communities (1982) Directive 82/176/EEC—On Pollution Caused by Certain Dangerous Substances Discharged into the Aquatic Environment of the Community. European Union, Brussels.
- 49. Kurniawan, T.A., Chan, G.Y., Lo, W.H. and Babel, S. (2006) Comparisons of Low-Cost Adsorbents for Treating Wastewaters Laden with Heavy Metals. Science of the Total Environment, 366, 409-426.
https://doi.org/10.1016/j.scitotenv.2005.10.001 - 50. Bailey, S.E., Olin, T.J., Bricka, R.M. and Adrian, D.D. (1999) A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Research, 33, 2469-2479.
https://doi.org/10.1016/S0043-1354(98)00475-8 - 51. Hashem, A., Abdel-Halim, E.S., El-Tahlawy, K.F. and Hebeish, A. (2005) Enhancement of Adsorption of Co (II) and Ni (II) Ions onto Peanut Hulls though Esterification Using Citric Acid. Adsorption Science and Technology, 23, 367-380.
https://doi.org/10.1260/026361705774355478 - 52. Hashem, A., Akasha, R.A., Ghith, A. and Hussein, D.A. (2007) Adsorbent Based on Agricultural Wastes for Heavy Metal and Dye Removal: A Review. Energy Education Science and Technology, 19, 69-86.
- 53. Food and Agriculture Organization of the United Nations (2011) FAO Statistics Division 2011, Sesame Seed. Food and Agriculture Organization of the United Nations, New York.
- 54. Manal, A.M.H. (2013) Studies on Egyptian Sesame Seeds (Sesamum indicum L.) and Its Products. 3. Effect of Roasting Process on Gross Chemical Composition, Functional Properties, Antioxidative Components and Some Minerals of Defatted Sesame Seeds Meal (Sesamum indicum L.). World Journal of Dairy & Food Sciences, 8, 51-57.
- 55. Stefan, S. and Jan, P. (2005) Potential Application of Oilseeds as Sources of Antioxidants for Food Lipids—A Review. Czech Journal of Food, 23, 93-102.
- 56. Michalak, A. (2006) Phenolic Compounds and Their Antioxidant Activity in Plants Growing under Heavy Metal Stress. Polish Journal of Environmental Studies, 15, 523-530.
- 57. Sciban, M., Klasnja, M. and Skrbic, B. (2006) Modified Softwood Sawdust as Adsorbent of Heavy Metal Ions from Water. Journal of Hazardous Materials, 136, 266-271.
https://doi.org/10.1016/j.jhazmat.2005.12.009 - 58. Ojedokun, A.T. and Olugbenga, S.B. (2015) An Overview of Low Cost Adsorbents for Copper (II) Ions Removal. Journal of Biotechnology & Biomaterials, 5, 175-177.
- 59. Alyüz, B. and Veli, S. (2009) Kinetics and Equilibrium Studies for the Removal of Nickel and Zinc from Aqueous Solutions by Ion Exchange Resins. Journal of Hazardous Materials, 167, 482-488.
https://doi.org/10.1016/j.jhazmat.2009.01.006 - 60. Ramakrishna, G. and Susmita, M. (2010) Process Optimization of Adsorption of Cr (VI) on Activated Carbons Prepared from Plant Precursors by a Two-Level Full Factorial Design. Chemical Engineering Journal, 160, 99-107.
https://doi.org/10.1016/j.cej.2010.03.015 - 61. Hossain, M.A., Ngo, H.H., Guo, W.S. and Setiadi, T. (2012. Adsorption and Desorption of Copper (II) Ions onto Garden Grass. Bioresource Technology, 121, 386-395.
https://doi.org/10.1016/j.biortech.2012.06.119 - 62. Oo, C., Osman, H., Fatinathan, S. and Zin, M. (2013) The Uptake of Copper (II) Ions by Chelating Schiff Base Derived from 4-Aminoantipyrine and 2-Methoxybenzaldehyde. International Journal of Non-Ferrous Metals, 2, 1-9.
https://doi.org/10.4236/ijnm.2013.21001 - 63. Leandro, V.A.G. and Laurent, F.G. (2009) Adsorption of Cu (II), Cd (II) and Pb (II) from Aqueous Single Metal Solutions by Succinylated Twice-Mercerized Sugarcane Bagasse Functionalized with Triethylenetetramine. Water Research, 43, 4479-4488.
https://doi.org/10.1016/j.watres.2009.07.017 - 64. Meena, A.K., Kiran, C.R. and Mishra, G.K. (2010) Removal of Heavy Metal Ions from Aqueous Solutions Using Chemically (Na2S) Treated Granular Activated Carbon as an Adsorbent. Journal of Scientific and Industrial Research, 69, 449-453.
- 65. Zendelska, A., Golomeova, M., Blazev, K., Krstev, B., Golomeov, B. and Krstev, A. (2015) Adsorption of Copper Ions from Aqueous Solutions on Natural Zeolite. Environment Protection Engineering, 41, 17-36.
- 66. Chuan, F.Z., Yu, R., Fang, F.W., Hong, L. and Yu, Y.C. (2016) Adsorption of Cu (II) from Aqueous Solutions by Ammoniated Cotton Fiber. Journal of Engineered Fibers and Fabrics, 11, 9-18.
- 67. Pandey, P., Sambi, S.S., Sharma, S.K. and Singh, S. (2009) Batch Adsorption Studies for the Removal of Cu (II) Ions by ZeoliteNaX from Aqueous Stream. Proceedings of the World Congress on Engineering and Computer Science, San Francisco, 20-22 October 2009, 122-127.
- 68. Belay, K. and Hayelom, A. (2014) Removal of Methyl Orange from Aqueous Solutions Using Thermally Treated Egg Shell (Locally Available and Low Cost Biosorbent). Chemistry of Materials, 6, 31-39.
- 69. Srivastava, V.C., Mall, I.D. and Mishra, I.M. (2006) Equilibrium Modelling of Single and Binary Adsorption of Cadmium and Nickel onto Baggase Fly Ash. Journal of Chemical & Engineering, 117, 79-91.
https://doi.org/10.1016/j.cej.2005.11.021 - 70. Achak, M., Hafidi, A., Ouazzani, N., Sayadic, S. and Mandi, L. (2009) Low Cost Biosorbent Banana Peel for the Removal of Phenolic Compounds from Olive Mill Wastewater: Kinetic and Equilibrium Studies. Journal of Hazardous Materials, 166, 117-125.
https://doi.org/10.1016/j.jhazmat.2008.11.036 - 71. Hossain, M.A., Ngo, H.H., Guo, W.S. and Nguyen, T.V. (2012) Removal of Copper from Water by Adsorption onto Banana Peel as Bioadsorbent. International Journal of Geotechnique, Construction Materials and Environment, 2, 227-234.
https://doi.org/10.21660/2012.4.3c - 72. Esposito, A., Pagnanelli, F., Lodi, A., Solisio, C. and Vegliò, F. (2001) Biosorption of Heavy Metals by Sphaerotilus natans: An Equilibrium Study at Different pH and Biomass Concentrations. Hydrometallurgy, 60, 129-141.
https://doi.org/10.1016/S0304-386X(00)00195-X - 73. Babel, S. and Kurniawan, T.A. (2004) Cr (VI) Removal from Synthetic Wastewater Using Coconut Shell Charcoal and Commercial Activated Carbon Modified with Oxidizing Agents and/or Chitosan. Chemosphere, 54, 951-967.
https://doi.org/10.1016/j.chemosphere.2003.10.001 - 74. Mall, D.I., Srivastava, V.C. and Agarwal, N.K. (2006) Removal of Orange-G and Methyl Violet Dyes by Adsorption onto Bagasse Fly Ash—Kinetic Study and Equilibrium Isotherm Analyses. Dyes and Pigments, 69, 210-223.
https://doi.org/10.1016/j.dyepig.2005.03.013 - 75. Ola, A. (2007) Kinetic and Isotherm Studies of Copper (II) Removal from Wastewater Using Various Adsorbents. Egyptian Journal of Aquatic Research, 33, 125-143.
- 76. Vijayaraghavan, K., Teo, T.T., Balasubramanian, R. and Joshi, U.M. (2009) Application of Sargassum Biomass to Remove Heavy Metal Ions from Synthetic Multi-Metal Solutions and Urban Storm Water Runoff. Journal of Hazardous Materials, 164, 1019-1023.
https://doi.org/10.1016/j.jhazmat.2008.08.105 - 77. Garg, V.K., Gupta, R., Yadav, A.B. and Kumar, R. (2003) Dye Removal from Aqueous Solution by Adsorption on Treated Sawdust. Bioresource Technology, 89, 121-124.
https://doi.org/10.1016/S0960-8524(03)00058-0 - 78. Karthikeyan, S., Balasubramanian, R. and Yer, C.S.P. (2007) Evaluation of the Marine Algae Ulva fasciata and Sargassum for the Biosorption of Cu (II) from Aqueous Solutions. Bioresource Technology, 98, 452-455.
https://doi.org/10.1016/j.biortech.2006.01.010 - 79. Auphedeous, Y.D., Boansi, A.O. and Pedavoah, M.M. (2015) Reduction of Fluorine in Water Using Clay Mixed with Hydroxyapatite. International Journal of Clothing Science and Technology, 5, 45-55.
- 80. Annadurai, G., Juang, R.S. and Lee, D.J. (2003) Adsorption of Heavy Metals from Water Using Banana and Orange Peels. Water Science and Technology, 47, 185-190.
- 81. Ameh, P.O. and Odoh, R. (2012) Iraqi Palm-Date as Adsorbent for Removal of Pb (II) and Ni (II) Ions from Aqueous Solution. International Journal of Modern Chemistry, 4, 11-18.
- 82. Fourest, E. and Roux, J.C. (1992) Heavy Metal Biosorption by Fungal Mycelial By-Products: Mechanisms and Influence of pH. Applied Microbiology and Biotechnology, 37, 399-403.
https://doi.org/10.1007/BF00211001 - 83. Babalola, J.O., Overah, L.C., Adesola, B., Vincent, O.O. and Olatunde, A. (2011) Kinetic, Equilibrium and Thermodynamic Studies on the Biosorption of Cd (II) from Aqueous Solutions by the Leaf Biomass of Calotropis procera ‘Sodom Apple’. Journal of Applied Sciences and Environmental Management, 15, 607-615.
- 84. El-Hassouni, H., Abdellaoui, D. and Bengueddour, R. (2013) Kinetic and Isotherm Studies of Cu (II) Removal From Aqueous Solution Using Gigartina acicularis Biomass. Environmental Earth Sciences, 3, 44-52.
- 85. El-Hassouni, H., Abdellaoui, D., El-Hani, S. and Bengueddour, R. (2014) Biosorption of Cadmium (II) and Copper (II) from Aqueous Solution Using Red Alga (Osmundea pinnatifida) Biomass. Journal of Materials and Environmental Science, 5, 967-974.
- 86. Unuabonah, E.I., Adebowale, K.O., Olu-Owolabi, B.I., Yang, L.Z. and Kong, L.X. (2008) Adsorption of Pb (II) and Cd (II) from Aqueous Solutions onto Sodium Tetraborate-Modified Kaolinite Clay: Equilibrium and Thermodynamic Studies. Hydrometallurgy, 93, 1-9.
https://doi.org/10.1016/j.hydromet.2008.02.009 - 87. Olu-Owolabi, B.I., Diagboya, P.N. and Ebaddan, W.C. (2012) Mechanism of Pb2+ Removal from Aqueous Solution Using a Non Living Moss Biomass. Chemical Engineering Journal, 195-196, 270-275.
https://doi.org/10.1016/j.cej.2012.05.004 - 88. Taha, A.A., Ahmed, A.M., Abdel-Rahman, H.H., Abouzeid, F.M. and AbdelMaksoud, M.O. (2017) Removal of Nickel Ions by Adsorption on Nano Bentonite: Equilibrium, Kinetic and Thermodynamics. Journal of Dispersion Science and Technology, 38, 757-767.
https://doi.org/10.1080/01932691.2016.1194211 - 89. Pons, M.P. and Fusté, C.M. (1993) Uranium Uptake by Immobilized Cells of Pseudomonas Strain EPS 5028. Applied Microbiology and Biotechnology, 39, 661-665.
https://doi.org/10.1007/BF00205071 - 90. Tumin, N.D., Chuah, A.L., Zawani, Z. and Abdul-Rashid, S. (2008) Adsorption of Copper from Aqueous Solution by Elais guineensis Kernel Activated Carbon. Journal of Engineering Science & Technology, 3, 180-189.
- 91. Yu, L.J., Shukla, S.S., Dorris, K.L., Shukla, A. and Margrave, J.L. (2003) Adsorption of Chromium from Aqueous Solutions by Maple Sawdust. Journal of Hazardous Materials, 100, 53-63.
https://doi.org/10.1016/S0304-3894(03)00008-6 - 92. Bhatti, I., Qureshi, K., Kazi, R.A. and Ansari, A.K. (2008) Preparation and Characterisation of Chemically Activated Almond Shells by Optimization of Adsorption of Chromium VI from Aqueous Solutions. International Journal of Chemical and Biomolecular Engineering, 1, 150-155.
- 93. Azouaou, N., Belmedani, M., Mokaddem, H. and Sadaoui, Z. (2013) Adsorption of Lead from Aqueous Solution onto Untreated Orange Barks. Chemical Engineering Transactions, 32, 55-60.
- 94. Yao, S., Sun, S., Wang, S. and Shi, Z. (2016) Adsorptive Removal of Lead Ion from Aqueous Solution by Activated Carbon/Iron Oxide Magnetic Composite. Indian Journal of Chemical Technology, 23, 146-152.
- 95. Najim, T.S., Elais N.J. and Dawood, A.A. (2009) Adsorption of Copper and Iron Using Low Cost Material as Adsorbent. Journal of Chemistry, 6, 161-168.
- 96. Barka, N., Abdennouri, M., Makhfouk, M.E. and Qourzal, S. (2013) Biosorption Characteristics of Cadmium and Lead onto Eco-Friendly Dried Cactus (Opuntia ficus indica) Cladodes. Journal of Environmental Chemical Engineering, 1, 144-149.
https://doi.org/10.1016/j.jece.2013.04.008 - 97. Pandey, P.K., Sharma, S.K. and Sambi, S.S. (2010) Kinetics and Equilibrium Study of Chromium Adsorption on ZeoliteNaX. International Journal of Environmental Science and Technology, 7, 395-404.
https://doi.org/10.1007/BF03326149 - 98. Martínez, M., Miralles, N., Hidalgo, S., Fiol, N., Villaescusa, I. and Poch, J. (2006) Removal of Lead and Cadmium from Aqueous Solution Using Grape Stalk Waste. Journal of Hazardous Materials, 133, 203-211.
https://doi.org/10.1016/j.jhazmat.2005.10.030 - 99. Zhang, X. and Wang, X. (2015) Adsorption and Desorption of Nickel (II) Ions from Aqueous Solution by a Lignocellulose/Montmorillonite Nanocomposite. PLoS One, 10, e0117077.
https://doi.org/10.1371/journal.pone.0117077 - 100. Ang, X.W., Sethu, V.S., Andresen, J.M. and Sivakumar, M. (2013) Copper (II) Ion Removal from Aqueous Solutions Using Biosorption Technology: Thermodynamic and SEM-EDX Studies. Clean Technologies and Environmental Policy, 15, 401-407.
https://doi.org/10.1007/s10098-012-0523-0 - 101. Aksu, Z. and Kutsal, T.A. (1991) A Bioseparation Process for Removing Pb (II) Ions from Wastewater by Using C. Vulgaris. Journal of Chemical Technology & Biotechnology, 52, 108-118.
- 102. Jnr, M.H. and Spiff, A.I. (2005) Effects of Temperature on the Sorption of Pb2+ and Cd2+ from Aqueous Solution by Caladium Bicolor (Wild Cocoyam) Biomass. Electronic Journal of Biotechnology, 8, 162-169.
https://doi.org/10.2225/vol8-issue2-fulltext-4 - 103. Zhou, J.L. and Banks, C.J. (1993) Mechanism of Humic Acid Color Removal from Natural Waters by Fungal Biomass Biosorption. Chemosphere, 27, 607-620.
https://doi.org/10.1016/0045-6535(93)90096-N - 104. Karthik, V., Velan, M., Kumar, M.D. and Renganathan, S. (2009) Removal of Brill Red 5B from an Aqueous Solution Using Cicca acida Biomass. Canadian Journal of Chemistry, 87, 554-561.
https://doi.org/10.1002/cjce.20188 - 105. Shafqat, F., Bhatti, H.N., Hanif, M.A. and Zubair, A. (2008) Kinetic and Equilibrium Studies of Cr (III) and Cr (VI) Sorption from Aqueous Solution Using Rosa gruss an Teplitz (Red Rose) Waste Biomass. Journal of the Chilean Chemical Society, 53, 1667-1672.
https://doi.org/10.4067/S0717-97072008000400006 - 106. Areco, M.M. and Afonso, M.S. (2010) Copper, Zinc, Cadmium and Lead Biosorption by Gymnogongrus torulosus. Thermodynamics and Kinetics Studies. Colloids and Surfaces B: Biointerfaces, 81, 620-628.
https://doi.org/10.1016/j.colsurfb.2010.08.014 - 107. Alpat, S., Alpat, S.K., Cadirci, B.H., Ozbayrak, O. and Yasa, I. (2010) Effects of Biosorption Parameter: Kinetics, Isotherm and Thermodynamics for Ni (II) Biosorption from Aqueous Solution by Circinella. Electronic Journal of Biotechnology, 13, 1-19.
- 108. Aksu, Z. and Isoglu, I.A. (2005) Removal of Copper (II) Ions from Aqueous Solution by Biosorption onto Agricultural Waste Sugar Beet Pulp. Process Biochemistry, 40, 3031-3044.
https://doi.org/10.1016/j.procbio.2005.02.004 - 109. Aregawi, B.H. and Mengistie, A.A. (2013) Removal of Ni (II) from Aqueous Solution Using Leaf, Bark and Seed of Moringa stenopetala Adsorbents. Bulletin of the Chemical Society of Ethiopia, 27, 35-47.
- 110. Anwar, J., Shafique, U., Zaman, W., Salman, M., Dar, A. and Anwar, S. (2010) Removal of Pb (II) and Cd (II) from Water by Adsorption on Peels of Banana. Bioresource Technology, 101, 1752-1755.
https://doi.org/10.1016/j.biortech.2009.10.021 - 111. Wang, Y., Wang, X., Wang, X., Liu, M., Wu, Z., Yang, L., Xia, S. and Zhao, J. (2013) Adsorption of Pb (II) from Aqueous Solution to Ni-Doped Bamboo Charcoal. Journal of Industrial and Engineering Chemistry, 19, 353-359.
https://doi.org/10.1016/j.jiec.2012.08.024 - 112. Njoku, V.O., Islam, M.A., Asifd, M. and Hameed, B.H. (2014) Preparation of Mesoporous Activated Carbon from Coconut Frond for the Adsorption of Carbofuran Insecticide. Journal of Analytical and Applied Pyrolysis, 110, 172-180.
https://doi.org/10.1016/j.jaap.2014.08.020 - 113. Tran, H.N., You, S.J. and Chao, H.P. (2016) Thermodynamic Parameters of Cadmium Adsorption onto Orange Peel Calculated from Various Methods: A Comparison Study. Journal of Environmental Chemical Engineering, 4, 2671-2682.
https://doi.org/10.1016/j.jece.2016.05.009 - 114. Bulut, Y. and Tez, Z. (2007) Adsorption Studies on Ground Shells of Hazelnut and Almond. Journal of Hazardous Materials, 149, 35-41.
https://doi.org/10.1016/j.jhazmat.2007.03.044 - 115. Liu, L., Liu, J., Li, H., Zhang, H., Liu, J. and Zhang, H. (2012) Equilibrium, Kinetic, and Thermodynamic Studies of Lead (II) Biosorption on Sesame Leaf. BioResources, 7, 3555-3572.
- 116. Pednekar, P.A. and Raman, B. (2013) The FT-IR Spectrometric Studies of Vibrational Bands of Semecarpus anacardiumlinn. F. Leaf, Stem Powder and Extracts. Asian Journal of Pharmaceutical and Clinical Research, 6, 159-198.
- 117. Thatte, C.S., Rathnam, M.V. and Pise, A.C. (2014) Chitosan-Based Schiff Base-Metal Complexes (Mn, Cu, Co) as Heterogeneous, New Catalysts for the β-Isophorone Oxidation. Journal of Chemical Sciences, 126, 727-737.
https://doi.org/10.1007/s12039-014-0601-4 - 118. Kannan, S. (2014) FT-IR and EDS Analysis of the Seaweeds Sargassum wightii (Brown Algae) and Gracilaria corticata (Red Algae). International Journal of Current Microbiology and Applied Sciences, 3, 341-351.
- 119. Sanati, M. and Andersson, A. (1993) DRIFT Study of the Oxidation and the Ammoxidation of Toluene over a TiO2, (B)-Supported Vanadia Catalyst. Journal of Molecular Catalysis A: Chemical, 81, 51-62.
https://doi.org/10.1016/0304-5102(93)80022-M - 120. Chen, Y., Zou, C., Mastalerz, M., Hu, S., Gasaway, C. and Tao, X. (2015) Applications of Micro-Fourier Transform Infrared Spectroscopy (FTIR) in the Geological Sciences—A Review. International Journal of Molecular Sciences, 16, 30223-30250.
https://doi.org/10.3390/ijms161226227 - 121. Sharma, B.K. (2005) Infrared Spectroscopy. In: Instrumental Methods of Chemical Analysis, Goel Publishing House, Krishna Prakashan Media Ltd., Meerut, 193-333.
- 122. Ravishankara, D.K. and Chandrashekara, P.G. (2012) Synthesis of Some Novel Benzimidazole Derivatives and Its Biological Evaluation. European Journal of Chemistry, 3, 359-362.
https://doi.org/10.5155/eurjchem.3.3.359-362.607 - 123. Hussein, F.H., Abbas, A.F. and Hussein, M.A. (2016) Synthesis and Characterization of Some New Heterocyclic Compounds Derived from 1,3-Bis(4-Nitrophenyl) Prop-2-en-1-One. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 7, 1837-1841.
- 124. Han, R., Zhang, L., Song, C., Zhang, M., Zhu, H. and Zhang, L. (2010) Characterization of Modified Wheat Straw, Kinetic and Equilibrium Study about Copper Ion and Methylene Blue Adsorption in Batch Mode. Carbohydrate Polymers, 79, 1140-1149.
https://doi.org/10.1016/j.carbpol.2009.10.054 - 125. Harder, P., Grunze, M. and Dahint, R. (1998) Molecular Conformation in Oligo (Ethylene Glycol)-Terminated Self-Assembled Monolayers on Gold and Silver Surfaces Determines Their Ability to Resist Protein Adsorption. Journal of Physical Chemistry B, 102, 426-436.
https://doi.org/10.1021/jp972635z - 126. Gunasekaran, S., Natarajan, R.K. and Kala, A. (2007) FT-IR Spectra and Mechanical Strength Analysis of Some Selected Rubber Derivatives. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy, 68, 323-330.
https://doi.org/10.1016/j.saa.2006.11.039 - 127. Sharma, M., Crosbie, J.C., Puskar, L. and Rogers, P.A.W. (2013) Microbeam-Irradiated Tumour Tissue Possesses a Different Infrared Absorbance Profile Compared to Broad Beam and Sham-Irradiated Tissue. International Journal of Radiation Biology, 89, 79-87.
https://doi.org/10.3109/09553002.2012.721052 - 128. Ferrer, A., Alciaturi, C., Faneite, A. and Ríos, J. (2016) Analyses of Biomass Fibers by XRD. In: Jr, S.V., Ed., FT-IR, and NIR. In Analytical Techniques and Methods for Biomass, Springer, Cham, 45-83.
https://doi.org/10.1007/978-3-319-41414-0_3 - 129. Pandey, K.K. and Pitman, A.J. (2004) Examination of the Lignin Content in S Softwood and a Hardwood Decayed by a Brown-Rot Fungus with the Acetyl Bromide Method and Fourier Transform Infrared Spectroscopy. Journal of Polymer Science Part A: Polymer Chemistry, 42, 2340-2346.
https://doi.org/10.1002/pola.20071 - 130. Smidt, E. and Meissl, K. (2007) The Applicability of Fourier Transform Infrared (FT-IR) Spectroscopy in Waste Management. Waste Management, 27, 268-276.
https://doi.org/10.1016/j.wasman.2006.01.016 - 131. Pandey, K.K., Hughes, M. and Vuorinen, T. (2010) Dimensional Stability, UV Resistance, and Static Mechanical Properties of Scots Pine Chemically Modified with Alkylene epoxides. BioResources, 5, 598-615.
- 132. Anastopoulos, I., Panagiotou, M., Ehaliotis, C., Tarantilis, P.A. and Massas, I. (2015) NaOH Pretreatment of Compost Derived from Olive Tree Pruning Waste Biomass Greatly Improves Biosorbent Characteristics for the Removal of Pb2+ and Ni2+ from Aqueous Solutions. International Society of Chemical Ecology, 31, 724-740.
https://doi.org/10.1080/02757540.2015.1095294 - 133. Via, B.K., Fasina, O. and Pan. H. (2011) Assessment of Pine Biomass Density through Mid-Infrared Spectroscopy and Multivariate Modeling. BioResources, 6, 807-822.
- 134. Ozgenc, O., Okan, O.T., Yildiz, U.C. and Deniz, I. (2013) Wood Surface Protection against Artificial Weathering with Vegetable Seed Oils. BioResources, 8, 6242-6262.
https://doi.org/10.15376/biores.8.4.6242-6262 - 135. Cheraghi, E., Ameri, E. and Moheb, A. (2015) Adsorption of Cadmium Ions from Aqueous Solutions Using Sesame as a Low-Cost Biosorbent: Kinetics and Equilibrium Studies. International Journal of Environmental Science and Technology, 12, 2579-2592.
https://doi.org/10.1007/s13762-015-0812-3 - 136. Cheraghi, E., Ameri, E. and Moheb, A. (2016) Continuous Biosorption of Cd (II) Ions from Aqueous Solutions by Sesame Waste: Thermodynamics and Fixed-Bed Column Studies. Desalination and Water Treatment, 57, 6936-6949.
https://doi.org/10.1080/19443994.2015.1012744 - 137. Janakiraman, N. and Johnson, M. (2015) Functional Groups of Tree Ferns (Cyathea) Using FT-IR: Chemotaxonomic Implications. Romanian Journal of Biophysics, 25, 131-141.
- 138. Tappert, R., Wolfe, A.P., McKellar, R.C., Tappert, M.C. and Muehlenbachs, K. (2011) Characterizing Modern and Fossil Gymnosperm Exudates Using Micro-Fourier Transform Infrared Spectroscopy. International Journal of Biological Sciences, 172, 120-138.
- 139. Pavia, D.L., Lampman, G.M., Kriz, G.S. and Vyvyan, J.R. (2009) Infrared Spectroscopy. In: Introduction to Spectroscopy, Cengage Learning, Washington DC, 15-30.
- 140. Aladdin, N.A., Jamal, J.A., Talip, N., Hamsani, N.A.M., Rahman, M.R.A., Sabandar, C.W., Muhammad, K., Husain, K. and Jalil, J. (2016) Comparative Study of Three Marantodes pumilum Varieties by Microscopy, Spectroscopy and Chromatography. Brazilian Journal of Pharmacognosy, 26, 1-14.
https://doi.org/10.1016/j.bjp.2015.10.002 - 141. Subhan, M.A., Sarker, P. and Ahmed, T. (2014) Analysis of Kidney and Gall Stones of Patients from North-East Bangladesh by FTIR. Journal of Scientific Research, 6, 553-561.
https://doi.org/10.3329/jsr.v6i3.16721 - 142. Ngah, W.S.W. and Hanafiah, M.A.K.M. (2008) Biosorption of Copper Ions from Dilute Aqueous Solutions on Base Treated Rubber (Hevea brasiliensis) Leaves Powder: Kinetics, Isotherm, and Biosorption Mechanisms. Journal of Environmental Sciences-China, 20, 1168-1176.
https://doi.org/10.1016/S1001-0742(08)62205-6 - 143. Chen, H. and Wang, A.Q. (2009) Adsorption Characteristics of Cu (II) from Aqueous Solution onto Poly (Acrylamide)/Attapulgite Composite. Journal of Hazardous Materials, 165, 223-231.
https://doi.org/10.1016/j.jhazmat.2008.09.097 - 144. Lasheen, M.R., Ammar, N.S. and Ibrahim, H.S. (2012) Adsorption/Desorption of Cd (II), Cu (II) and Pb (II) Using Chemically Modified Orange Peel: Equilibrium and Kinetic Studies. Solid State Sciences, 14, 202-210.
https://doi.org/10.1016/j.solidstatesciences.2011.11.029 - 145. Dagdelen, S., Acemioglu, B., Baran, E. and Kocer, O. (2014) Removal of Remozal Brilliant Blue R from Aqueous Solution by Pirina Pretreated with Nitric Acid and Commercial Activated Carbon. Water, Air, & Soil Pollution, 225, 1-15.
https://doi.org/10.1007/s11270-014-1899-8 - 146. Bilgili, A.S. (2006) Adsorption of 4-Chlorophenol from Aqueous Solutions by Xad-4 Resin: Isotherm, Kinetic, and Thermodynamic Analysis. Journal of Hazardous Materials, 137, 157-164.
https://doi.org/10.1016/j.jhazmat.2006.01.005 - 147. You, L., Lu, F., Song, L., Yin, Y. and Zhang, Q. (2015) Enhanced Decolorization of Aqueous Dye Solutions by a High Quality Copolymer Flocculant. Royal Society of Chemistry, 5, 64711-64723.
https://doi.org/10.1039/c5ra07662e - 148. Gaballah, I. and KIlbertus, G. (1998) Recovery of Heavy Metal Ions through Decontamination of Synthetic Solutions and Industrial Effluents Using Modified Barks. Journal of Geochemical Exploration, 62, 241-286.
https://doi.org/10.1016/S0375-6742(97)00068-X - 149. Hema, M. and Arivoli, S. (2008) Adsorption Kinetics and Thermodynamics of Malachite Green Dye unto Acid Activated Low Cost Carbon. Journal of Applied Sciences and Environmental Management, 12, 43-51.
- 150. Kannan, A. and Thambidurai, S. (2008) Comparative Studies on the Removal of Nickel (II) from Aqueous Solution by Using Carbon Derived from Palmyra Palm Fruit Seeds and Commercial Activated Carbon. International Journal of Science and Technology, 1, 93-107.
- 151. El-Haddad, M. (2016) Removal of Basic Fuchsin Dye from Water Using Mussel Shell Biomass Waste as an Adsorbent: Equilibrium, Kinetics, and Thermodynamics. Journal of Taibah University for Science, 10, 664-674.
https://doi.org/10.1016/j.jtusci.2015.08.007 - 152. Langmuir, I. (1916) The Constitution and Fundamental Properties of Solids and Liquids. Journal of the American Chemical Society, 38, 2221-2295.
https://doi.org/10.1021/ja02268a002 - 153. Hall, K.R., Eagleton, L.C., Acrivos, A. and Vermeulen, T. (1966) Pore and Solid-Diffusion Kinetics in Fixed-Bed Adsorption under Constant-Pattern Conditions. Industrial & Engineering Chemistry Research, 5, 212-223.
- 154. Karapinar, N. and Donat, R. (2009) Adsorption Behavior of Cu2+ and Cd2+ onto Natural Bentonite. Desalination, 249, 123-129.
https://doi.org/10.1016/j.desal.2008.12.046 - 155. Halnor, S., Farooqui, M. and Ubale, M. (2013) Removal of Copper (II) from Aqueous Solution and Waste Water by Prosopis juliflora Leaf Powder by Adsorption. International Journal of Application or Innovation in Engineering & Management, 2, 125-131.
http://www.ijaiem.org/Volume2Issue3/IJAIEM-2013-03-14-028.pdf - 156. Igwe, J.C. and Abia. A.A. (2007) Adsorption Kinetics and Intra-Particulate Diffusivities for Bioremediation of Co (II), Fe (II) and Cu (II) Ions from Waste Water Using Modified and Unmodified Maize Cob. International Journal of Physics-Science, 2, 119-127.
- 157. Gecgel, ü., Kocabiyik, B. and üner, O. (2015) Adsorptive Removal of Methylene Blue from Aqueous Solution by the Activated Carbon Obtained from the Fruit of Catalpa Bignonioides. Water, Air, & Soil Pollution, 226, 1-14.
https://doi.org/10.1007/s11270-015-2513-4 - 158. Li, Q., Yue, Q.Y., Sun, H.J., Su, Y. and Gao, B.Y. (2010) A Comparative Study on the Properties, Mechanisms and Process Designs for the Adsorption of Non-Ionic or Anionic Dyes onto Cationic-Polymer/Bentonite. Journal of Environmental Management, 91, 1601-1611.
https://doi.org/10.1016/j.jenvman.2010.03.001 - 159. Freundlich, H.M.F. (1906) Over the Adsorption in Solution. Journal of Physical Chemistry, 57, 385-470.
- 160. Friess, W. (1998) Collagen-Biomaterial for Drug Delivery. European Journal of Pharmaceutics and Biopharmaceutics, 45, 113-136.
https://doi.org/10.1016/S0939-6411(98)00017-4 - 161. Qiang, T., Bu, Q., Ren, L. and Wang, X. (2014) Adsorption Behaviors of Cr (III) on Carboxylated Collagen Fiber. Journal of Applied Polymer Science, 131, 1-6.
https://doi.org/10.1002/app.40285 - 162. Hasany, S.M., Saeed, M.M. and Ahmed, M. (2002) Sorption and Thermodynamic Behavior of Zinc (II)-Thiocyanate Complexes onto Polyurethane Foam from Acidic Solutions. Journal of Radioanalytical and Nuclear Chemistry, 252, 477-484.
https://doi.org/10.1023/A:1015890317697 - 163. Ngah, W.S.W. and Fatinathan, S. (2010) Adsorption Characterization of Pb (II) and Cu (II) Ions onto Chitosan-Tripolyphosphate Beads: Kinetic, Equilibrium and Thermodynamic Studies. Journal of Environmental Management, 91, 958-969.
https://doi.org/10.1016/j.jenvman.2009.12.003 - 164. Dubinin, M.M. and Radushkevich, L.V. (1947) The Equation of the Characteristic Curve of the Activated Charcoal. Proceedings of the Union of Soviet Socialist Republics Academy of Sciences, 55, 331-337.
- 165. Ozcan, A.S., Erdem, B. and Ozcan, A. (2005) Adsorption of Acid Blue 193 from Aqueous Solutions onto BTMA-Bentonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 266, 73-81.
https://doi.org/10.1016/j.colsurfa.2005.06.001 - 166. Hobson, J.P. (1969) Physical Adsorption Isotherms Extending from Ultrahigh Vacuum to Vapor Pressure. Journal of Physical Chemistry, 73, 2720-2727.
https://doi.org/10.1021/j100842a045 - 167. Hasany, S.M. and Chaudhary, M.H. (1996) Sorption Potential of Hare River Sand for the Removal of Antimony from Acidic Aqueous Solution. Applied Radiation and Isotopes, 47, 467-471.
https://doi.org/10.1016/0969-8043(95)00310-X - 168. Dubey, S.S. and Gupta, R.K. (2005) Removal Behavior of Babool Bark (Acacia Nilotica) for Submicro Concentrations of Hg2+ from Aqueous Solutions: A Radiotracer Study. Separation and Purification Technology, 41, 21-28.
https://doi.org/10.1016/j.seppur.2004.03.012 - 169. Koyuncu, H. and Kul, A.R. (2014) An Investigation of Cu (II) Adsorption by Native and Activated Bentonite: Kinetic, Equilibrium and Thermodynamic Study. The Journal of Environmental Chemical Engineering, 2, 1722-1730.
https://doi.org/10.1016/j.jece.2014.06.012 - 170. Kundu, S. and Gupta, A.K. (2006) Arsenic Adsorption onto Iron Oxide-Coated Cement (IOCC): Regression Analysis of Equilibrium Data with Several Isotherm Models and Their Optimization. Chemical Engineering Journal, 122, 93-106.
https://doi.org/10.1016/j.cej.2006.06.002 - 171. Dang, V.B.H., Doan, H.D., Dang-Vu, T. and Lohi, A. (2009) Equilibrium and Kinetics of Biosorption of Cadmium (II) and Copper (II) Ions by Wheat Straw. Bioresource Technology, 100, 211-219.
https://doi.org/10.1016/j.biortech.2008.05.031 - 172. Farooq, U., Kozinski, J.A., Khan, M.A. and Athar, M. (2010) Biosorption of Heavy Metal Ions Using Wheat Based Biosorbents—A Review of the Recent Literature. Bioresource Technology, 101, 5043-5053.
https://doi.org/10.1016/j.biortech.2010.02.030 - 173. Abasi, C.Y., Abia, A.A. and Igwe, J.C. (2011) Adsorption of Iron (III), Lead (II) and Cadmium (II) Ions by Unmodified Raphia Palm (Raphia hookeri) Fruit Endocarp. Environmental Research, 5, 104-113.
https://doi.org/10.3923/erj.2011.104.113 - 174. Cozmuta, L.M., Cozmuta, A.M., Peter, A., Nicula, C., Nsimba, E.B. and Tutu, H. (2012) The Influence of pH on the Adsorption of Lead by Na-Clinoptilolite: Kinetic and Equilibrium Studies. Water SA, 38, 269-278.
https://doi.org/10.4314/wsa.v38i2.13 - 175. Li, H., Xiao, D., He, H., Lin, R. and Zuo, P. (2013) Adsorption Behavior and Adsorption Mechanism of Cu (II) Ions on Amino-Functionalized Magnetic Nanoparticles. Transactions of Nonferrous Metals Society of China, 23, 2657-2665.
https://doi.org/10.1016/S1003-6326(13)62782-X - 176. Pehlivan, E., Cetin, S. and Yanik, B.H. (2006) Equilibrium Studies for the Sorption of Zinc and Copper from Aqueous Solutions Using Sugar Beet Pulp and Fly Ash. Journal of Hazardous Materials, 135, 193-199.
https://doi.org/10.1016/j.jhazmat.2005.11.049 - 177. Villaescusa, I., Fiol, N., Martínez, M., Miralles, N., Poch, J. and Serarols, J. (2004) Removal of Copper and Nickel Ions from Aqueous Solutions by Grape Stalks Wastes. Water Research, 38, 992-1002.
https://doi.org/10.1016/j.watres.2003.10.040 - 178. El-Ashtoukhy, E.S.Z., Amin, N.K. and Abdelwahab, O. (2008) Removal of Lead (II) and Copper (II) from Aqueous Solution Using Pomegranate Peel as a New Adsorbent. Desalination, 223, 162-173.
https://doi.org/10.1016/j.desal.2007.01.206 - 179. Onundi, Y.B., Mamun, A.A., Al-Khatib, M.F. and Ahmed Y.M. (2010) Adsorption of Copper, Nickel and Lead Ions from Synthetic Semiconductor Industrial Wastewater by Shell Activated Carbon. Environmental Science & Technology, 7, 751-758.
https://doi.org/10.1007/bf03326184 - 180. Karabulut, S., Karabakan, A., Denizli, A. and Yürüm, Y. (2000) Batch Removal of Copper (II) and Zinc (II) from Aqueous Solutions with Low-Rank Turkish Coals. Separation and Purification Technology, 18, 177-184.
https://doi.org/10.1016/S1383-5866(99)00067-2 - 181. Yu, B., Zhang, Y., Shukla, A., Shukla, S.S. and Dorris, K.L. (2000) The Removal of Heavy Metal from Aqueous Solutions by Sawdust Adsorption—Removal of Copper. Journal of Hazardous Materials, 80, 33-42.
https://doi.org/10.1016/S0304-3894(00)00278-8 - 182. Gupta, V.K. and Ali, I. (2000) Utilization of Bagasse Fly Ash (a Sugar Industry Waste) for the Removal of Copper and Zinc from Wastewater. Separation and Purification Technology, 18, 131-140.
https://doi.org/10.1016/S1383-5866(99)00058-1 - 183. Kalavathy, M.H. and Miranda, L.R. (2010) Comparison of Copper Adsorption from Aqueous Solution Using Modified and Unmodified Hevea brasiliensis Saw Dust. Desalination, 255, 165-174.
https://doi.org/10.1016/j.desal.2009.12.028 - 184. Meunier, N., Laroulandie, J., Blais, J.F. and Tyagi, R.D. (2003) Cocoa Shells for Heavy Metal Removal from Acidic Solutions. Bioresource Technology, 90, 255-263.
https://doi.org/10.1016/S0960-8524(03)00129-9 - 185. Habib, A., Islam, N., Islam, A. and Shafiqul-Alam, A.M. (2007) Removal of Copper from Aqueous Solution Using Orange Peel, Sawdust and Bagasse. Pakistan Journal of Analytical & Environmental Chemistry, 8, 21-25.
- 186. Aydin, H., Bulut, Y. and Yerlikaya, C. (2008) Removal of Copper (II) from Aqueous Solution by Adsorption onto Low-Cost Adsorbents. Journal of Environmental Management, 87, 37-45.
https://doi.org/10.1016/j.jenvman.2007.01.005 - 187. Sciban, M. and Klasnja, M. (2004) Wood Sawdust and Wood Originate Materials as Adsorbents for Heavy Metal Ions. European Journal of Wood and Wood Products, 62, 69-73.
https://doi.org/10.1007/s00107-003-0449-7 - 188. Altun, T. and Pehlivan, E. (2007) Removal of Copper (II) Ions from Aqueous Solutions by Walnut-, Hazelnut- and Almond-Shells. CLEAN-Soil, Air, Water, 35, 601-606.
https://doi.org/10.1002/clen.200700046 - 189. Putra, W.P., Kamari, A., Yusoff, S.N.M., Ishak, C.F., Mohamed, A., Hashim, N. and Isa, I.M. (2014) Biosorption of Cu (II), Pb (II) and Zn (II) Ions from Aqueous Solutions Using Selected Waste Materials: Adsorption and Characterisation Studies. American Journal of Applied Sciences, 4, 25-35.
- 190. Ahamed, A.J. and Begum, A.S. (2012) Adsorption of Copper from Aqueous Solution Using Low-Cost Adsorbent. Archives of Applied Science Research, 4, 1532-1539.
- 191. Shukla, S.R. and Pai, R.S. (2005) Adsorption of Cu (II), Ni (II) and Zn (II) on Modified Jute Fibres. Bioresource Technology, 96, 1430-1438.
https://doi.org/10.1016/j.biortech.2004.12.010 - 192. Shukla, S.R. and Pai, R.S. (2005) Adsorption of Cu (II), Ni (II) and Zn (II) on Dye Loaded Groundnut Shells and Sawdust. Separation and Purification Technology, 43, 1-8.
https://doi.org/10.1016/j.seppur.2004.09.003 - 193. Kalavathy, M.H., Karthikeyan, T., Rajgopal, S. and Miranda, L.R. (2005) Kinetic and Isotherm Studies of Cu (II) Adsorption onto H3PO4-Activated Rubber Wood Sawdust. Journal of Colloid and Interface Science, 292, 354-362.
https://doi.org/10.1016/j.jcis.2005.05.087 - 194. Paulino, A.G., Cunha, A.J.D., Alfaya, R.V.D.S. and Alfaya, A.A.D.S. (2014) Chemically Modified Natural Cotton Fiber: A Low-Cost Biosorbent for the Removal of the Cu (II), Zn (II), Cd (II), and Pb (II) from Natural Water. Desalination and Water Treatment, 52, 4223-4233.
https://doi.org/10.1080/19443994.2013.804451 - 195. Cao, X., Ma, L.Q., Rhue, D.R. and Appel, C.S. (2004) Mechanisms of Lead, Copper, and Zinc Retention by Phosphate Rock. Environmental Pollution, 131, 435-444.
https://doi.org/10.1016/j.envpol.2004.03.003 - 196. Reddad, Z., Gerente, C., Andres, Y. and Cloirec, P. (2002) Adsorption of Several Metal Ions onto a Low-Cost Biosorbent: Kinetic and Equilibrium Studies. Environmental Science & Technology, 36, 2067-2073.
https://doi.org/10.1021/es0102989 - 197. Basci, N., Kocadagistan, E. and Kocadagistan, B. (2004) Biosorption of Copper (II) from Aqueous Solutions by Wheat Shell. Desalination, 164, 135-140.
https://doi.org/10.1016/S0011-9164(04)00172-9 - 198. Brown, P., Jefcoat, I.A., Parrish, D., Gill, S. and Graham, E. (2000) Evaluation of the Adsorptive Capacity of Peanut Hull Pellets for Heavy Metals in Solution. Advances in Environmental Research, 4, 19-29.
https://doi.org/10.1016/S1093-0191(00)00004-6 - 199. Cay, S., Uyanik, A. and Ozasik, A. (2004) Single and Binary Component Adsorption of Copper (II) and Cadmium (II) from Aqueous Solutions Using Tea-Industry Waste. Separation & Purification Technology, 38, 273-280.
https://doi.org/10.1016/j.seppur.2003.12.003 - 200. Ngah, W.S.W. and Hanafiah, M.A.K.M. (2008) Adsorption of Copper on Rubber (Hevea brasiliensis) Leaf Powder: Kinetic, Equilibrium and Thermodynamic Studies. Biochemical Engineering Journal, 39, 521-530.
https://doi.org/10.1016/j.bej.2007.11.006 - 201. Shukla, P.M. and Shukla, S.R. (2013) Biosorption of Cu (II), Pb (II), Ni (II), and Fe (II) on Alkali Treated Coir Fibers. Separation Science and Technology, 48, 421-428.
https://doi.org/10.1080/01496395.2012.691933 - 202. Al-Asheh, S., Banat, F., Al-Omari, R. and Duvnjak, Z. (2000) Predictions of Binary Sorption Isotherms for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data. Chemosphere, 41, 659-665.
https://doi.org/10.1016/S0045-6535(99)00497-X - 203. Rahman, M.S. and Islam, M.R. (2009) Effects of pH on Isotherms Modeling for Cu (II) Ions Adsorption Using Maple Wood Sawdust. Chemical Engineering Journal, 149, 273-280.
https://doi.org/10.1016/j.cej.2008.11.029 - 204. Lee, S.H. and Yang, J.W. (1997) Removal of Copper in Aqueous Solution by Apple Wastes. Separation Science and Technology, 32, 1371-1387.
https://doi.org/10.1080/01496399708000966 - 205. Sekomo, C.B., Rousseau, D.P.L. and Lens, P.N.L. (2012) Use of Gisenyi Volcanic Rock for Adsorptive Removal of Cd (II), Cu (II), Pb (II), and Zn (II) from Wastewater. Water, Air, & Soil Pollution, 223, 533-547.
https://doi.org/10.1007/s11270-011-0880-z - 206. Sulaiman, O., Amini, M.H.M., Rafatullah, M., Hashim, R. and Ahmad, A. (2010) Adsorption Equilibrium and Thermodynamic Studies of Copper (II) Ions from Aqueous Solutions by Oil Palm Leaves. International Journal of Chemical Reactor Engineering, 8, A108.
https://doi.org/10.2202/1542-6580.2350 - 207. Ozsoy, H.D. and Kumbur, H. (2006) Adsorption of Cu (II) Ions on Cotton Boll. Journal of Hazardous Materials, 136, 911-916.
https://doi.org/10.1016/j.jhazmat.2006.01.035 - 208. Ho, Y.S. (2003) Removal of Copper Ions from Aqueous Solution by Tree Fern. Water Research, 37, 2323-2330.
https://doi.org/10.1016/S0043-1354(03)00002-2 - 209. Veli, S. and Alyüz, B. (2007) Adsorption of Copper and Zinc from Aqueous Solutions by Using Natural Clay. Journal of Hazardous Materials, 149, 226-233.
https://doi.org/10.1016/j.jhazmat.2007.04.109 - 210. Lagergren, S. (1898) About the Theory of So-Called Adsorption of Soluble Substance. Kungliga Svenska Vetenskapsakademiens. Handlingar, 24, 1-39.
- 211. Ho, Y.S. (2004) Citation Review of Lagergren Kinetic Rate Equation on Adsorption Reaction. Scientometrics, 59, 171-177.
https://doi.org/10.1023/B:SCIE.0000013305.99473.cf - 212. ünlü, N. and Ersoz, M. (2006) Adsorption Characteristics of Heavy Metal Ions onto a Low Cost Biopolymeric Sorbent from Aqueous Solutions. Journal of Hazardous Materials, 136, 272-280.
https://doi.org/10.1016/j.jhazmat.2005.12.013 - 213. Crini, G. and Badot, P.M. (2008) Application of Chitosan, a Natural Aminopolysaccharide, for Dye Removal from Aqueous Solutions by Adsorption Processes Using Batch Studies: A Review of Recent Literature. Progress in Polymer Science, 33, 399-447.
https://doi.org/10.1016/j.progpolymsci.2007.11.001 - 214. Ho, Y.S. and McKay, G. (1998) Sorption of Dye from Aqueous Solution by Peat. Chemical Engineering Journal, 70, 115-124.
https://doi.org/10.1016/S0923-0467(98)00076-1 - 215. Ho, Y.S. and McKay, G. (1999) Pseudo-Second Order Model for Sorption Processes. Process Biochemistry, 34, 451-465.
https://doi.org/10.1016/S0032-9592(98)00112-5 - 216. McKay, G. (2001) Solution to the Homogeneous Surface Diffusion Model for Batch Adsorption Systems Using Orthogonal Collocation. Chemical Engineering Journal, 81, 213-221.
https://doi.org/10.1016/S1385-8947(00)00191-1 - 217. Akretche, D.E. (2011) Metals Removal from Industrial Effluents. In: Coca-Prados, J. and Gutierrez-Cervello, G., Eds., Water Purification and Management, Springer Science-Business Media BV, Algiers, 95-117.
https://doi.org/10.1007/978-90-481-9775-0_3 - 218. Weber, W.J. and Morris, J.C. (1963) Kinetics of Adsorption on Carbon from Solutions. Journal of the Sanitary Engineering Division, American Society of Civil Engineers, 89, 31-60.
- 219. Tan, I.A.W. and Hameed, B. (2010) Adsorption Isotherms, Kinetics, Thermodynamics and Desorption Studies of Basic Dye on Activated Carbon Derived from Oil Palm Empty Fruit Bunch. Journal of Applied Sciences, 10, 2565-2571.
https://doi.org/10.3923/jas.2010.2565.2571 - 220. Walker, G.M., Hansen, L., Hanna, J.A. and Allen, S.J. (2003) Kinetics of a Reactive Dye Adsorption onto Dolomitic Sorbents. Water Research, 37, 2081-2089.
https://doi.org/10.1016/S0043-1354(02)00540-7 - 221. Abdel-Ghani, N.T., El-Chaghaby, G.A. and Zahran, E.M. (2015) Pentachlorophenol (PCP) Adsorption from Aqueous Solution by Activated Carbons Prepared from Corn Wastes. International Journal of Environmental Science and Technology, 12, 211-222.
https://doi.org/10.1007/s13762-013-0447-1 - 222. Diagboya, P.N., Olu-Owolabi, B.I. and Adebowale, K.O. (2014) Microscale Scavenging of Pentachlorophenol in Water Using Amine and Tripolyphosphate-Grafted SBA-15 Silica: Batch and Modeling Studies. Journal of Environmental Management, 146, 42-49.
https://doi.org/10.1016/j.jenvman.2014.04.038 - 223. Suteu, D. and Malutan, T. (2013) Industrial Cellolignin Waste as Adsorbent for Methylene Blue Dye from Aqueous Solutions. Bioresource, 8, 427-446.
- 224. Nethaji, S., Sivasamy, A. and Mandal, A.B. (2013) Adsorption Isotherms, Kinetics and Mechanism for the Adsorption of Cationic and Anionic Dyes onto Carbonaceous Particles Prepared from Juglans regia Shell Biomass. International Journal of Environmental Science and Technology, 10, 231-242.
https://doi.org/10.1007/s13762-012-0112-0 - 225. Song, S.T., Saman, N., Johari, K. and Mat, H. (2013) Removal of Hg (II) from Aqueous Solution by Adsorption Using Rawand Chemically Modified Rice Straw as Novel Adsorbents. Industrial & Engineering Chemistry Research, 52, 13092-13101.
https://doi.org/10.1021/ie400605a - 226. Li, K., Cao, J., Li, H., Liu, J., Lu, M. and Tang, D. (2016) Nitrogen Functionalized Hierarchical Microporous/Mesoporous Carbon with a High Surface Area and Controllable Nitrogen Content for Enhanced Lead (II) Adsorption. Royal Society of Chemistry, 6, 92186-92196.
- 227. Uslu, G. and Tanyol, M. (2006) Equilibrium and Thermodynamic Parameters of Single and Binary Mixture Biosorption of Lead (II) and Copper (II) Ions onto Pseudomonas Putida: Effect of Temperature. Journal of Hazardous Materials, 135, 87-93.
https://doi.org/10.1016/j.jhazmat.2005.11.029 - 228. Khan, M.A., Kim, S., Rao, R.A.K., Abou-Shanaba, R.A.I., Bhatnagar, A., Song, H., and Jeon, B.H. (2010) Adsorption Studies of Dichloromethane on Some Commercially Available GACs: Effect of Kinetics, Thermodynamics and Competitive Ions. Journal of Hazardous Materials, 178, 963-972.
https://doi.org/10.1016/j.jhazmat.2010.02.032 - 229. Gorzin, F. and Bahri-Rasht, A.M.M. (2017) Adsorption of Cr (VI) from Aqueous Solution by Adsorbent Prepared from Paper Mill Sludge: Kinetics and Thermodynamics Studies. Adsorption Science and Technology, 1-21.
https://doi.org/10.1177/0263617416686976 - 230. Nollet, H., Roels, M., Lutgen, P., Van, M.P. and Verstraete, W. (2003) Removal of PCBs from Wastewater Using Fly Ash. Chemosphere, 53, 655-665.
https://doi.org/10.1016/S0045-6535(03)00517-4 - 231. Xiong, C. (2010) Adsorption of Cadmium (II) by Chitin. Journal of the Chemical Society of Pakistan, 32, 429-435.
- 232. Ruthven, D.M. (1984) Principles of Adsorption and Adsorption Processes. John Wiley and Sons, New York.
- 233. Srivastava, N.C. and Eames, I.M. (1998) A Review of Adsorbents and Adsorbates in Solid-Vapour Adsorption Heat Pump Systems. Applied Thermal Engineering, 18, 707-714.
https://doi.org/10.1016/S1359-4311(97)00106-3 - 234. Denizli, A., Say, R. and Arica, Y. (2000) Removal of Heavy Metal Ions from Aquatic Solutions by Membrane Chromatography. Separation and Purification Technology, 21, 181-190.
https://doi.org/10.1016/S1383-5866(00)00203-3 - 235. Al-Anber, M.A. (2011) Thermodynamics Approach in the Adsorption of Heavy Metals. In: Moreno-Pirajan, J.C., Ed., Thermodynamics-Interaction Studies-Solids, Liquids and Gases, InTech, Rijeka, 737-764.
















