Open Journal of Modern Neurosurgery
Vol.4 No.1(2014), Article ID:42062,4 pages DOI:10.4236/ojmn.2014.41010
Intramedullary Spinal Cord Glioma Following Microinjection of Glioblastoma Cell Line C6 in Rats
1Department of Neurosurgery, Abant Izzet Baysal University, Medical School, Bolu, Turkey
2Department of Pathology, Abant Izzet Baysal University, Medical School, Bolu, Turkey3Department of Histology and Embryology, Abant Izzet Baysal University, Medical School, Bolu, Turkey41st Neurosurgery Clinic in Bakirkoy Mental Diseases Hospital, Bakirkoy, Istanbul, TurkeyEmail: *dagistanyasar@hotmail.com
Copyright © 2014 Yasar Dagistan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Yasar Dagistan et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 4, 2013; revised January 4, 2014; accepted January 11, 2014
Keywords: Intramedullary Spinal Cord Glioma; Animal Model; C6 Glioblastoma Cell
ABSTRACT
Background: This paper describes the establishment of a rat intramedullary spinal cord tumor (IMSCT) model and histopathological characterization of the tumor model. Methods: Fourteen male Wistar rats were randomized into two groups. The rats in group 1 (control group, n = 7) received a 5 μl intramedullary injection of serum physiologic (SF). Those in group 2 (experimental group, n= 7) received a 5 μl intramedullary implantation of media containing 5 × 105 C6 glioma cells. The animals were sacrificed for histopathological examination at 21 days. Results: The control group showed normal functional and histopathological findings. The group 2 rats implanted with C6 glioblastoma cells developed hind-limb paraplegia. Pathological sections confirmed intramedullary C6 glioblastoma invading the spinal cord. Conclusions: A rat C6 IMSCT model was successfully established. This model may be useful in increasing understanding of intramedullary spinal cord gliomas in humans.
1. Introduction
Intramedullary spinal cord tumors (IMSCTs) are relatively rare neoplasms, accounting for only 2% - 4% of all central nervous system (CNS) tumors and 15% of all spinal cord tumors. Of these, approximately 70% are lowmalignancy lesions, such as low-grade astrocytomas and ependymomas [1-3]. High-grade astrocytomas occur less frequently. The ratio of highto low-grade astrocytomas has been reported to be 1:3 [2]. Glioblastoma multiforme (GBM) of the spine is rare, accounting for only 1.5% of all spinal cord tumors and 1% to 5% of all GBM cases [4]. These highly malignant lesions occur mainly in the cervicothoracic segments, have a slight tendency to occur in the first decades of life, and have a short clinical history before diagnosis [5]. From the surgical point of view, the optimum treatment of these tumors is controversial. With recent advances in neuroimaging equipment, e.g., magnetic resonance imaging, microsurgical techniques, e.g., intraoperative ultrasound, laser therapy, and intraoperative electrophysiological monitoring, and total resection of most tumors is possible [6].
The development of a novel model of IMSCTs using glial cell lines would facilitate biological and histopathological studies, as well as lowering costs and increasing the utility of the model. In previous studies aimed at generating a model of IMSCTs, tumor lines of intracranial glial tumors, such as 9L gliosarcoma and F98 glioma, were commonly used [7].
In this study, we present for the first time a novel rat model of IMSCTs using glioblastoma cell line C6 and discuss the methodology and histopathological features.
2. Methods
Glioma cell lines and culture condition: The rat glioblastoma cell line C6 was obtained from ATCC (RockvilleUSA), and serial passages were made in modified Eagle medium of Dulbecco, which contained 15% heat-inactivated fetal calf serum, 0.2 mM glutamine, 50 mg/ml neomycin, and 100 mg/ml streptomycin. Culture flasks were kept in an electronic incubator under a humidified atmosphere with 5% CO2 at 37˚C.
Animals and implantation procedure. The cells were harvested via 2 ml trypsin-EDTA solution C (Biological Industries, Israel) and centrifuged at 1 × 103 rpm for 3 minutes after the addition of 1.5 cc of F12 medium and the fetal calf serum mixture. After removing the supernatant, the pellet was resuspended with 2 cc of medium. The cell suspension was concentrated; so that 5 microliters of any injection volume contained 5 × 105 cells. It was then placed in a microcentrifuge tube and kept in a water-ice mixture environment during the whole implantation procedure, which always lasted less than 2 hours.
Fourteen male Wistar rats were randomized into two groups. The rats in group 1 (control group, n = 7) received a 5 μl intramedullary injection of serum physiologic (SF). The rats in group 2 (experimental group, n = 7) received a 5 μl intramedullary implantation of media containing 5 × 105 C6 glioma cells. The rats were sacrificed for histopathological examination at 21 days.
3. Surgical Technique
The rats were anesthetized with an intraperitoneal (i.p.) injection (0.4 - 0.6 ml) of a stock solution containing ketamine hydrochloride (50 mg/ml) (Ketalar®, Pfizer İla- çları Ltd. Sti., Istanbul), xylazine hydrochloride (10 mg/ ml, Rompun®, Bayer), and 14.25% ethanol in normal saline. The animals were placed on a sterile field, and their backs were shaved and prepared with a betadine solution. The spinous process of thoracic vertebrae 5 (T5) was identified, and a 2-cm longitudinal incision was made over the dorsal midthoracic region. The underlying fascia and the paravertebral muscles were retracted laterally, the spinous process of T5 was removed with rongeurs, and the ligamentum flavum was removed, exposing the intervertebral space. The cell suspension was injected through the dorsal intervertebral space with a 26-gauge Hamilton syringe (Hamilton Company, Reno, NV). The needle was advanced until the dorsal aspect of the vertebral body was felt and then retracted slightly (1 - 2 mm). The incisions were sutured closely in layers after the injection. After recovery, the rats were returned to their cages where the room temperature was 28˚C.
4. Histopathological Analysis
After sacrifice, the spinal column of each animal was exposed, and a segment encompassing all macroscopically visible tumor was excised en bloc, placed in 4% formalin, and embedded in paraffin. The spinal cord was sectioned in 5-μm slices for hematoxylin-eosin staining.
5. Results
In the histopathological examination of the control rats, there were no significant findings relating to tumors, and normal cytoarchitectures were observed in the spinal cord (Figures 1(A) and (B)).
The histopathological examination of the group 2 rats implanted with the C6 glioblastoma cells demonstrated intramedullary spinal cord tumors. Tumor tissue protruding from the skin was visible (Figure 2(A)). The tumors had highly cellular, well-defined lesions, with compression of the surrounding structures (Figure 2(B)). In the tumor tissue, cellularity changes were less frequently demonstrated, and tumor-containing myxoid areas were present (Figure 2(C)). The bone structure was found to be infiltrated by the tumor (Figure 2(D)). Within the tumors, glial tumor cells, ovoid and round nuclei, pink fibrillary cytoplasm, moderate pleomorphism, and high mitotic activity were observed (Figure 2(E)). In addition, the area of necrosis and the tumor cells surrounded palizadik were found (Figure 2(F)).
6. Discussion
As mentioned above, astrocytomas and ependymomas represent the most common intramedullary neoplasms. It is known that the intracranial to spinal ratios for astrocytomas and ependymomas are 10:1 and 3:1 to 20:1, respectively [8]. Treatment of IMSCTs includes resection, radiotherapy, or a combination of these approaches [9- 12]. Although the mechanisms by which IMSCTs produce neurological dysfunction are not precisely known, the effects of pressure and edema on axonal conduction probably play a key role. The clinical presentation of IMSCTs is determined in part by the location of the tumor [13]. Midline back pain is the most common initial symptom of spinal cord tumors. Leg weakness and sensory changes are the other symptoms that bring patients to diagnostic evaluation [14]. The improvement in function after surgical resection of the tumor is finding a
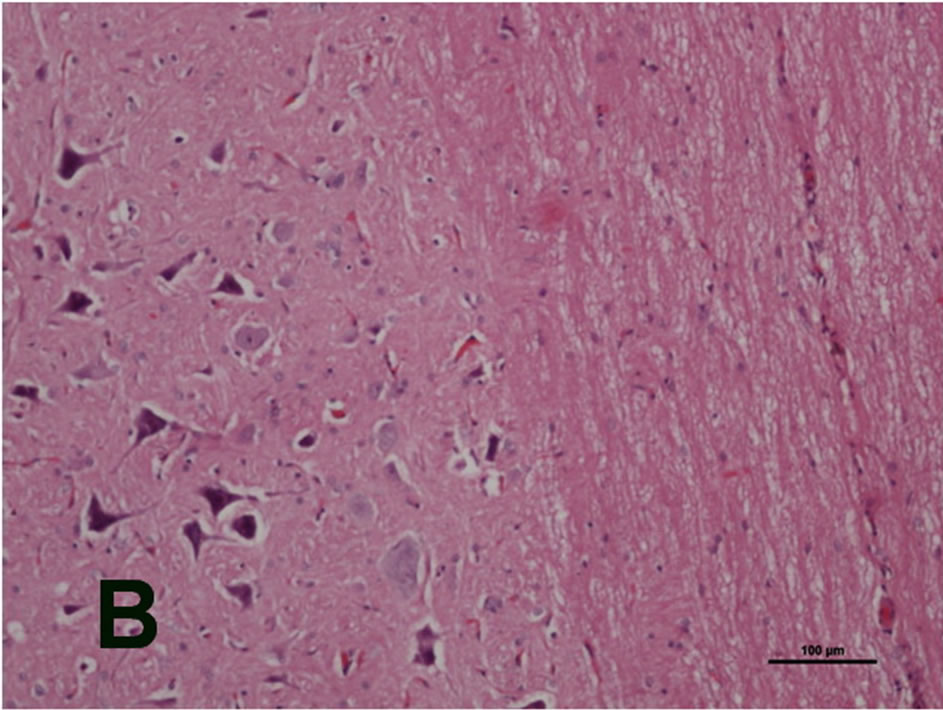
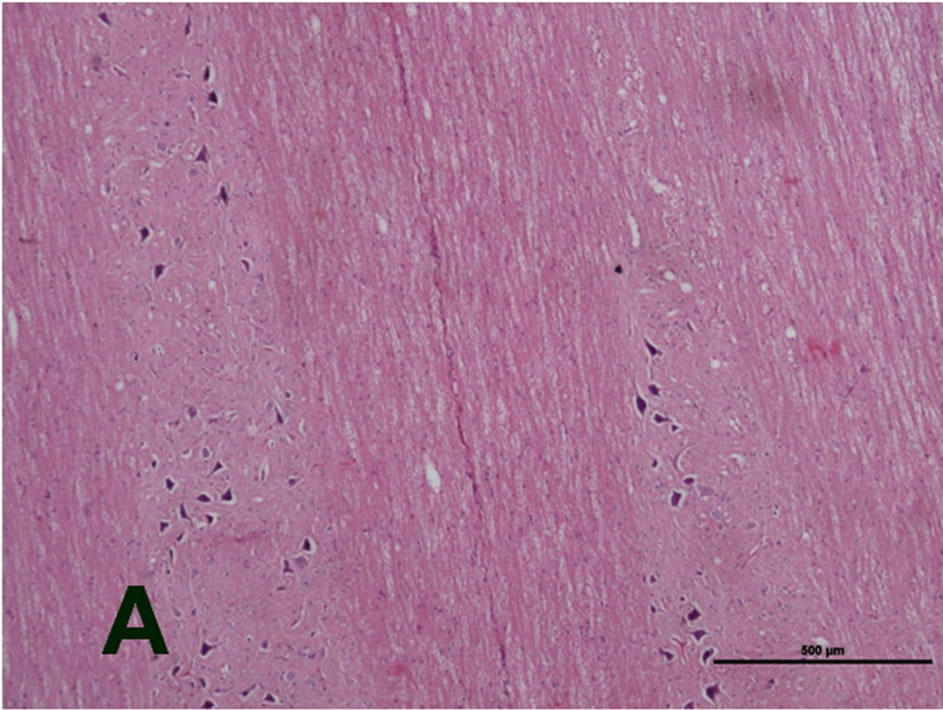
Figure 1. Microphotographs of a cross section (stained with HE) of the spinal cord of a control rat. No significant findings of tumor and normal cytoarchitectures were observed in the spinal cord ((A): H&E, original magnification ×4x. (B) H&E, original magnification ×10).
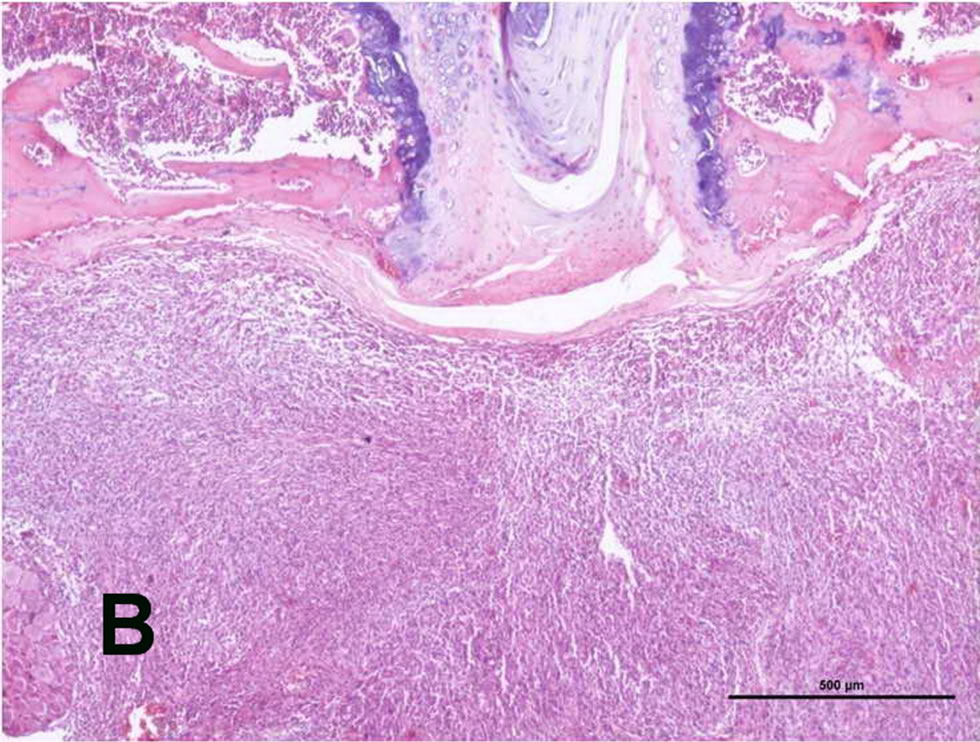

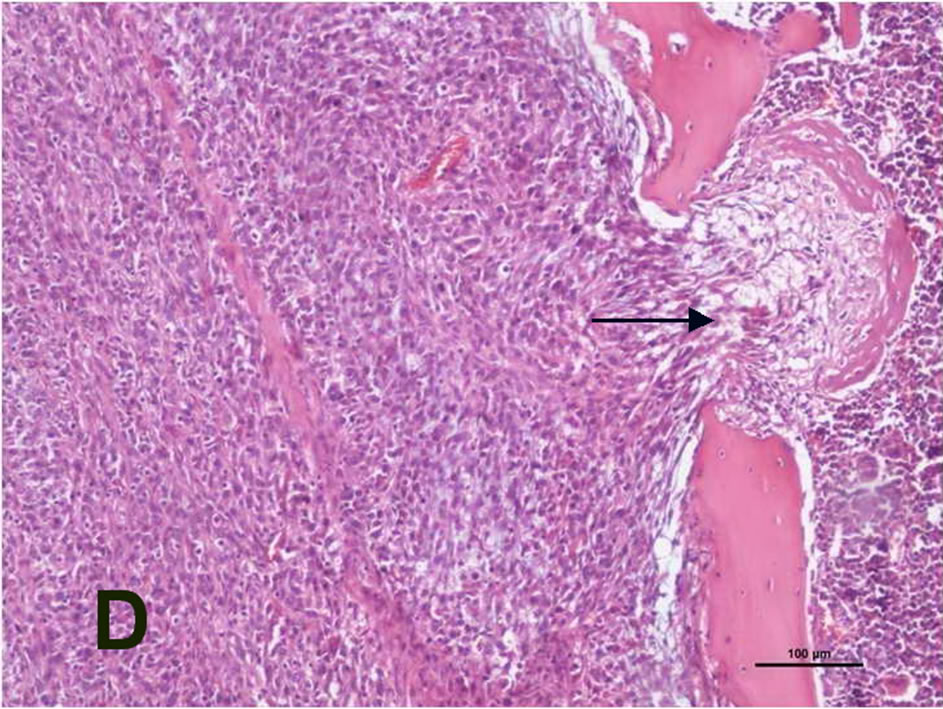
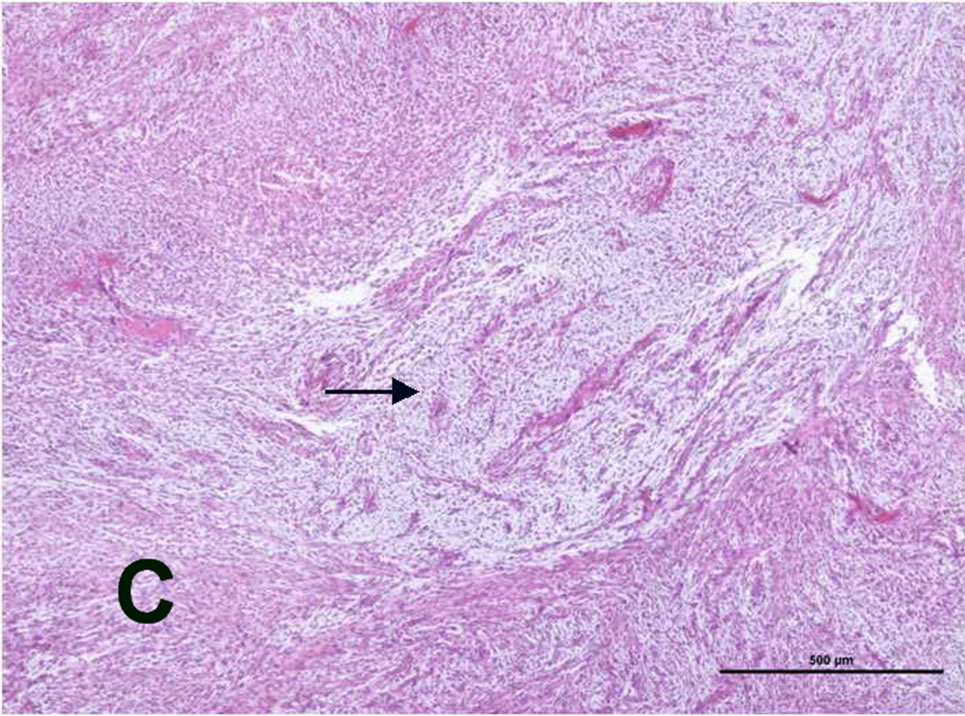

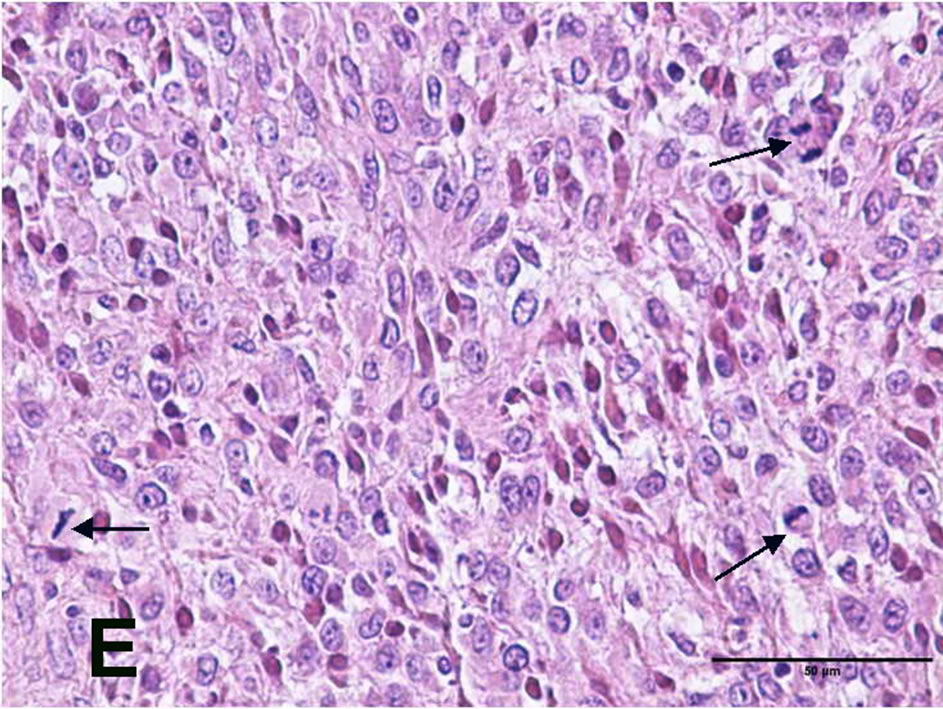
Figure 2. (A) Tumor tissue protruding from the skin; (B) tumor tissue separated from the surrounding tissue with a sharp demarcation and a dense increase in cellularity (H&E, original magnification ×4x); (C) cellularity changes less frequent monitored (→) and myxoid areas containing the tumor (H&E, original magnification ×4x); (D) tumor tissue infiltrating the bone (→) the (H&E, original magnification ×10x); (E) glial tumor cells, ovoid and round nuclei, pink fibrillary cytoplasm, moderate pleomorphism, and high mitotic activity (→) (H&E, original magnification ×40x); (F) necrosis area (*) and the tumor cells surrounded palizadik (→) (H&E, original magnification ×40x).
good for prognosis [15-17].
There have been limited advances in the diagnosis and management of spinal cord tumors because of the rarity of the disease. The best treatment for IMSCTs has so far to be determined. Hence, a suitable animal model of IMSCTs needs to be developed. In contrast to the many existing intracranial animal tumor models [17-19], the literature contains few animal models of IMSCTs [20-22]. Caplan et al. [22] found that animals injected with 9L or F98 consistently develop hind-limb paresis in a reliable and reproducible manner. The progression of neurological deficits was similar to that seen in patients with IMSCTs. They suggested that this model mimics the behavior of IMSCTs in humans and may be used to examine the efficacy of new treatment options for both lowand high-grade intramedullary tumors. Ren et al. [23] described a murine model of IMSCG using 9L gliosarcoma cells, which are syngeneic to Fischer 344 rats. They reported on the functional progression, neuroimaging, and histopathological characterization of this tumor model. The rats implanted with the 9L cells regularly developed paraplegia in a reliable and reproducible manner. In the present study, we found that the animals injected with the C6 tumor had a median onset of hindlimb paresis. The histopathological examination of the rats implanted with the C6 glioblastoma cells revealed intramedullary spinal cord tumors. The tumors displayed highly cellular, well-defined lesions with compression of the surrounding structures. Within the tumors, polymorphic malformed nuclei with clearly mitotic figures and multinucleated cells were observed. Endothelial proliferation was evident with necrosis also.
7. Conclusion
In conclusion, our results indicate that a rat C6 IMSCT model was successfully established. This current working rat model may resemble the behavior of IMSCTs in humans. However, we believe that further studies of the existing IMSCT model are needed.
REFERENCES
- D. C. Miller, “Surgical Pathology of Intramedullary Spinal Cord Neoplasms,” Journal of Neurooncology, Vol. 47, No. 3, 2000, pp. 189-194. http://dx.doi.org/10.1023/A:1006496204396
- A. Raco, V. Esposito, J. Lenzi, M. Piccirilli, R. Delfini and G. Cantore, “Long-Term Follow-Up of Intramedullary Spinal Cord Tumors: A Series of 202 Cases,” Neurosurgery, Vol. 56, No. 5, 2005, pp. 972-981.
- T. H. Schwartz and P. C. McCormick, “Intramedullary Ependymomas: Clinical Presentation, Surgical Treatment Strategies and Prognosis,” Journal of Neurooncology, Vol. 47, No. 3, 2000, pp. 211-218. http://dx.doi.org/10.1023/A:1006414405305
- A. A. Andrews, L. Enriques, J. Renaudin and U. Tomiyasu, “Spinal Intramedullary Glioblastoma with Intracranial Seeding,” Archives of Neurology, Vol. 35, No. 4, 1978, pp. 244-245. http://dx.doi.org/10.1001/archneur.1978.00500280062013
- A. Raco, M. Piccirilli, A. Landi, J. Lenzi, R. Delfini and G. Cantore, “High-Grade Intramedullary Astrocytomas: 30 Years’ Experience at the Neurosurgery Department of the University of Rome ‘Sapienza’,” Journal of Neurosurgical Spine, Vol. 12, No. 2, 2010, pp. 144-153. http://dx.doi.org/10.3171/2009.6.SPINE08910
- S. Yang, X. Yang and G. Hong, “Surgical Treatment of One Hundred Seventy-Four Intramedullary Spinal Cord Tumors,” Spine (Phila Pa 1976), Vol. 34, No. 24, 2009, pp. 2705-2710. http://dx.doi.org/10.1097/BRS.0b013e3181b43484
- R. F. Barth, “Rat Brain Tumor Models in Experimental Neuro-Oncology: The 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 Gliomas,” Journal of Neurooncology, Vol. 36, No. 1, 1998, pp. 91-102. http://dx.doi.org/10.1023/A:1005805203044
- A. T. Parsa, J. H. Chi, F. L. Acosta Jr., et al., “Intramedullary Spinal Cord Tumors: Molecular Insights and Surgical Innovation,” Clinical Neurosurgery, Vol. 52, No. 49-51, 2005, pp. 76-84.
- K. A. Schellinger, J. M. Propp, J. L. Villano and B. J. McCarthy, “Descriptive Epidemiology of Primary Spinal Cord Tumors,” Journal of Neurooncology, Vol. 87, No. 2, 2008, pp. 173-179. http://dx.doi.org/10.1007/s11060-007-9507-z
- G. Kopelson, R. M. Linggood, G. M. Kleinman, J. Doucette and C. C. Wang, “Management of Intramedullary Spinal Cord Tumors,” Radiology, Vol. 135, No. 2, 1980, pp. 473-479.
- G. Kopelson and R. M. Linggood, “Intramedullary Spinal Cord Astrocytoma versus Glioblastoma: The Prognostic Importance of Histologic Grade,” Cancer, Vol. 50, No. 4, 1982, pp. 732-735. http://dx.doi.org/10.1002/1097-0142(19820815)50:4<732::AID-CNCR2820500418>3.0.CO;2-0
- A. Di Marco, C. Griso, R. Pradella, F. Campostrini and G. F. Garusi, “Postoperative Management of Primary Spinal Cord Ependymomas,” Acta Oncology, Vol. 27, No. 4, 1988, pp. 371-375. http://dx.doi.org/10.3109/02841868809093557
- M. C. Chamberlain and T. L. Tredway, “Adult Primary Intradural Spinal Cord Tumors: A Review,” Current Neurology and Neuroscience Reports, Vol. 11, No. 3, 2011, pp. 320-328. http://dx.doi.org/10.1007/s11910-011-0190-2
- J. W. Henson, “Spinal Cord Gliomas,” Current Opinion in Neurology, Vol. 14, No. 6, 2001, pp. 679-682. http://dx.doi.org/10.1097/00019052-200112000-00001
- J. L. Slooff, J. W. Kernohan and C. S. MacCarty, “Primary Intramedullary Tumors of the Spinal Cord and Filum Terminale,” WB Saunders Company, Philadelphia, 1964.
- G. B. Rodrigues, J. N. Waldron, S. Wong and N. J. Laperriere, “A Retrospective Analysis of 52 Cases of Spinal Cord Glioma Managed with Radiation Therapy,” International Journal of Radiation Oncology, Biology and Physics, Vol. 48, No. 3, 2001, pp. 837-842. http://dx.doi.org/10.1016/S0360-3016(00)00690-8
- B. S. Carson, J. H. Anderson, S. A. Grossman, J. Hilton, C. L. White 3rd, O. M. Colvin, et al., “Improved Rabbit Brain Tumor Model Amenable to Diagnostic Radiographic Procedures,” Neurosurgery, Vol. 11, No. 5, 1982, pp. 603-608. http://dx.doi.org/10.1227/00006123-198211000-00003
- W. Griffitt, R. P. Glick and T. Lichtor, “Development of a New Mouse Brain Tumor Model Using Implantable Micro Cannulas,” Journal of Neurooncology, Vol. 41, No. 2, 1999, pp. 117-120. http://dx.doi.org/10.1023/A:1006115221761
- G. I. Jallo, M. Penno and L. Sukay, “Experimental Models of Brainstem Tumors: Development of a Neonatal Rat Model,” Child’s Nervous System, Vol. 21, No. 5, 2005, pp. 399-403. http://dx.doi.org/10.1007/s00381-004-1100-6
- M. Salcman, E. Botero, K. C. Rao, R. D. Broadwell and E. Scott, “Intramedullary Canine Spinal Cord Tumor Model,” Journal of Neurooncology, Vol. 61, No. 4, 1984, pp. 761-766. http://dx.doi.org/10.3171/jns.1984.61.4.0761
- G. Mavinkurve, G. Pradilla, F. G. Legnani, B. M. Tyler, C. A. Bagley and H. Brem, “A Novel Intramedullary Spinal Cord Tumor Model: Functional, Radiological, and Histopathological Characterization,” Journal of Neurosurgical Spine, Vol. 3, Journal of Neurooncology, Vol. 3, No. 2, 2005, pp. 142-148. http://dx.doi.org/10.3171/spi.2005.3.2.0142
- J. Caplan, G. Pradilla, A. Hdeib, B. M. Tyler, F. G. Legnani, C. A. Bagley, et al., “A Novel Model of Intramedullary Spinal Cord Tumors in Rats: Functional Progression and Histopathological Characterization,” Neurosurgery, Vol. 59, Journal of Neurooncology, Vol. 59, No. 1, 2006, pp. 193-200. http://dx.doi.org/10.1227/01.NEU.0000219276.44563.DA
- T. J. Ren, Z. C. Wang, Y. Z. Zhang, D. Li, H. Y. Wang and Z. Z. Li, “Establishment of Intramedullary Spinal Cord Glioma Model in Rats,” Chinical Medicine Journal (English), Vol. 123, No. 18, 2010, pp. 2580-2585.
NOTES
*Corresponding author.

