Advances in Infectious Diseases
Vol.2 No.4(2012), Article ID:25487,5 pages DOI:10.4236/aid.2012.24023
The Occurrence of Cryptosporidium and Giardia Infections among Patients Reporting Diarrheal Disease in Chobe District, Botswana
![]()
1Fisheries and Wildlife Conservation, Virginia Tech, Blacksburg, USA; 2Center for Conservation of African Resources, Animals, Communities, and Land use (CARACAL), Kasane, Botswana; 3Technology Laborites in Blacksburg, Blacksburg, USA; 4Virginia Maryland Regional College of Veterinary Medicine, Virginia Tech, Blacksburg, USA.
Email: kathyalx@vt.edu
Received September 27th, 2012; revised October 28th, 2012; accepted November 29th, 2012
Keywords: Giardia; Cryptosporidium; Botswana; Africa; Waterborne Disease; Diarrhea; Zoonotic Disease; HIV/AIDS
ABSTRACT
Diarrheal disease is a chronic public health problem in Chobe District, Botswana. Acute diarrheal outbreaks occur annually with a bimodal seasonal pattern coinciding with major hydrological phenomena. Information is lacking regarding the etiology of reoccurring outbreaks. Cryptosporidium and Giardia are recognized as important waterborne causes of diarrheal disease with Cryptosporidium transmission potentially involving zoonotic reservoirs. In Chobe District, municipal water is obtained from the Chobe River after it exits the Chobe National Park where high concentrations of wildlife occur. Using the Giardia/Cryptosporidium QUIK CHEK rapid antigen cartridge test, we evaluate the occurrence of Giardia and Cryptosporidium among patients (n = 153) presenting to medical facilities in Chobe District with diarrhea (August 2011-July 2012). Cryptosporidium was only identified in children less than two years of age (10%, n = 42, 95% CI 3% - 23%), with positive cases occurring during diarrheal outbreak periods. Infections were not identified in adults despite the high level of human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS) in the region (n = 117). Although not significant, cases were predominately identified in the wet season outbreak (p = 0.94, 25%, n = 12, 95% CI 5% - 57%, dry season outbreaks 3%, n = 30, CI 95% 0% - 17%). Giardia infections occurred across age groups and seasons (7%, n = 153, 95% CI 4% - 10%). This is the first published report of human infections with Cryptosporidium and Giardia in this area of Africa. This study suggests that Cryptosporidium may be a potentially important cause of diarrheal disease in children less than 2 years of age in this region. Further research is required to identify pathogen transmission and persistence dynamics and public health implications, particularly the role of HIV/AIDS and vulnerability to waterborne disease.
1. Introduction
Diarrheal disease continues to be identified as a primary global threat to public health, particularly for children less than 5 years of age. In Botswana, diarrhea is a leading cause of morbidity and mortality, the majority of deaths occurring in this same age group [1]. Botswana also has one of the highest prevelance levels of Human Immunodeficiency Virus infection (HIV) in the world (36%, [2]). This epidemic has impacted population vulnerability to infectious disease generally and diarrheal disease in particular [3]. In northern Botswana, seasonal, acute diarrhea in children under five years of age has occurred across years, coinciding with major hydrological phenomena occurring in the area (rainfall/flood recession, Alexander unpublished data). Despite the reoccurring nature of these outbreaks, little is known regarding causality of this disease syndrome in the region.
A number of important protozoan waterborne pathogens are associated with diarrheal disease outbreaks. Cryptosporidium, a protozoal pathogen of global importance, is increasingly implicated as a leading cause of diarrheal disease in developing nations, particularly Africa [4-7] .
This emerging group of protozoan parasites is extremely infectious, resistant to chlorine and filtration [8- 10] . While human-to-human transmission occurs, Cryptosporidium can spillover from wildlife and domestic animal reservoirs to exposed human populations through contaminated surface water [10-12] or contact with infected animal or human hosts [13].
Cryptosporidium infection is identified as an important threat to children, particularly those living with HIV and Acquired Immuno Deficiency Syndrome (AIDS) [11]. While infection in the immuno compentent person is normally selflimiting, co-infection with HIV/AIDS often results in chronic diarrhea, health complications, and mortality [14].
Giardia is also identified as an important protozoal cause of diarrheal disease associated with conditions of poverty, reduced hygiene, and contaminated water sources [15]. This pathogen is also noted as dispro-portionally affecting HIV/AIDS patients [16]. The zoonotic potential of Giardia is, however, uncertain [17,18].
In Botswana, Cryptosporidium has been reported in children during diarrheal outbreaks in Francistown [19], and among ostriches exported to Canada [20]. Giardia has also been described in children [21] during diarrhea outbreaks in the capital. Little is known regarding the occurrence of these pathogens in Northern Botswana and the role of these infections in recurrent outbreaks of human diarrheal disease.
In this study, we evaluate the occurrence of Cryptosporidium amd Giardia among patients presenting to medical facilities with diarrheal disease in Chobe District from August 2011-July 2012. Three diarrheal outbreaks occurred during this period. We discuss our results and provide recommendations for future study.
2. Methods
2.1. Study Site
Botswana is a politically stable, semi-arid, landlocked country located in sub-Saharan Africa. The study area (Figure 1) is located in Chobe District, the northern most district in the country. The relatively small district population (~18,500 people) is spread across two peri-urban
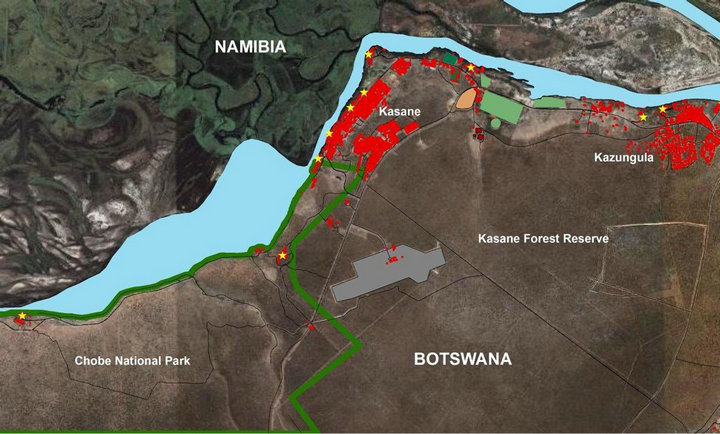
Figure 1. Chobe District, Botswana is located in Northern Botswana. Communities live around the Chobe National Park (green outline). The Chobe River (blue) flows through the Park to industrial, commercial, residential developments (red) and commercial agricultural (green) areas. Sewage treatment facilities are located downstream from the water abstraction point (brown). Tourism facilities are found within the developed and protected areas (yellow).
communities (Kasane and Kazungula) and seven smaller villages. The country has a subtropical climate with annual wet (November-March) and dry (April-October) seasons. The Chobe River is the only permanent surface water in the area. The river crosses through the Chobe National Park (a high density wildlife area) before reaching the towns of Kazungula and Kasane where water is abstracted for municipal use for the district. Piped water is available either through direct reticulation to residences or through a limited number of public taps. Government health services are freely available to the public with a nominal charge.
During the study period, three diarrheal outbreaks occurred with peaks in August 2011 (dry season), February 2012 (wet season), and July 2012 (dry season, Alexander unpublished data).
2.2. Sample Collection and Diagnostic Evaluations
Patients (all ages) presenting to Chobe District health facilities with diarrheal disease (August 2011-July 2012) were asked by medical staff to submit a single fecal sample as part of a prospective study of diarrhea in the District. Stool samples were requested from hospitalized patients as well as those presenting for outpatient treatment. A subset of these individuals returned a fresh stool to nursing or laboratory staff. Other than reporting diarrheal disease, no other health data could be secured on patients submitting stool samples for this study (e.g., HIV/AIDS status). Diarrheal disease was defined as the occurrence of at least 3 loose stools in a 24-hour period within the four days preceding the health visit. This study was conducted under permit from the Ministry of Health in Botswana and approval from the Virginia Tech Institutional Review Board (IRB# 11 - 573). All human associated data were anonymized.
Collected stool samples were frozen at –18˚C until testing. Samples were screened for the presence of Giardia and Cryptosporidium protozoa species using the Giardia/Cryptosporidium QUIK CHEK (TechLab, Inc., Blacksburg, VA) rapid antigen cartridge test in accordance with the manufacturer’s directions. Test specificity and sensitivity levels have previously been identified for both human associated Giardia (94% and 100%, respectively) and Cryptosporidium species (100% for both indices) [22].
2.3. Statistical Tests
Exact binomial 95% confidence intervals were calculated using the Epitools package in R [23]. Fisher’s Exact Test was used to evaluate differences between seasons [24].
3. Results
Stool samples were collected from 164 patients (children: <2 years = 42; >2 and <5 years = 2; >5 and <14 years = 3; and Adults: n = 117). Of those patients where data were available (n = 130), only 12% were hospitalized. The remaining patients were seen on an outpatient basis. With the exception of one adult, hospitalized patients were all reported to be 2 years or younger.
All of the Cryptosporidium positive samples came from children less than two years of age (mean = 13.5 months, standard deviation = 5.28 months). Positive cases were identified in both the dry and wet season outbreak periods of 2011 and 2012, respectively (10%, n = 42, 95% CI 3% - 23%).
While there was no significant difference between seasons (p = 0.094) among infected children <2 years, cases were predominately identified in the wet season outbreak (25%, n = 12, 95% CI 5 - 57, dry season outbreaks 3%, n = 30, CI 95% 0% - 17%). Infections were not identified in adults during this study period (n = 117, wet season n = 56).
Giardia infections were identified broadly across age groups (mean = 10.68 years, standard deviation = 11.72 years) and season (7%, 95% CI 4% - 10%). One child was co-infected with both Cryptosporidium and Giardia at the peak of the wet season outbreak in 2012.
4. Discussion
Water borne pathogens contribute significantly to diarrheal disease particularly in developing countries where declining water quality can be a persistent problem [25, 26]. Both Giardia and Cryptosporidium are considered important emerging protozoan pathogens contributing significantly to diarrheal disease outbreaks in both developed and developing nations across the globe [27].
In this study, Cryptosporidium and Giardia are identified among patients reporting diarrheal disease in Chobe District, Botswana. This study was conducted on a limited number of samples (n = 153) across age groups. Most of the stool samples originated from patients seen on an outpatient base (88%). Obtaining stool samples from outpatients is often difficult in more rural areas. In these settings, people are required to return largely on foot to the requesting health facility in order to submit these specimens. Medical and laboratory personnel are often overwhelmed with patients and laboratory duties, reducing interest in obtaining specimens for prospective research studies. The large sample sizes often associated with studies conducted in major hospitals are then often not possible in more remote, low-density locations. Small sample sizes then become an important limitation to health studies conducted in these more remote rural locations, limiting statistical power. Never the less, it is often these more remote locations where health impacts are the highest and knowledge of causation is the lowest, necessitating continuation of such studies, despite sample size limitations.
Whereas Giardia was found broadly among age groups and seasons, Cryptosporidium was found only in children less than two years of age. Previous studies have found similar associations with age in Cryptosporidium infections [14,28].
In Chobe, as in many locations across Africa, HIV/ AIDS has influenced population vulnerability to waterborne infections such as Cryptosporidium and Giardia directly and indirectly through reduced immunocompetence related to breastfeeding [29,30]. Despite high levels of HIV in this population, we did not identify any Cryptosporidium infections in adults (total n = 117, wet season n = 56). This could be a product of a reduced sample size. In addition, Botswana does offer universal access to antiretroviral therapy for HIV positive patients [31] and this may contribute to decreased infections in the face of the exceptionally high proportion of infected individuals in this region [32]. Further research is needed to understand these possible interactions.
The potential indirect affects of the HIV/AIDS epidemic have important implications to water borne disease and invasion potential. HIV-positive mothers are encouraged to use formula rather than breastfeed to reduce mother-to-child transmission of the virus. Natural maternal immunity, important in fighting water associated infections, is therefore absent in a great percentage of children in Africa, particularly in Botswana [33]. In a survey in 2006 in Chobe, 48% of children reported not being breast-fed [34]. In Botswana in 2006, 537 children under five years of age died during a diarrheal outbreak linked to rainfall flooding of pit latrines. In this outbreak, most affected children had not been breastfed (88%) and Cryptosporidium was one of the most common infecting agents identified [19].
More research is needed to understand the role of HIV in the temporal and spatial dynamics of these protozoan infections. It is likely, that HIV/AIDS has a significant influence on the transmission dynamics of these pathogens, and both the direct and indirect mechanisms must be considered.
Humans, cattle, and wildlife can all contribute to environmental transmission of Cryptosporidium, although species-specific transmission dynamics and host specificity influence the movement of these protozoan pathogens between hosts [35,36]. Seasonal changes in wildlife and domestic animal densities in water restricted environments and calving may influence the spatial and temporal dynamics of Cryptosporidium oocytes in the environment [37-39] and potential human exposure. Land use has also been identified as an important predictor of water contamination with Cryptosporidium oocytes [40]. Direct contamination of water resources with human fecal waste from direct defection on the ground, sewage spills, and pit latrines frequently occurs in this system and can also contribute or may dominate human-to-human transmission of these parasites. Molecular tools will ultimately be necessary to discriminate between protozoan species and subgenotypes as they occur in domestic and wildlife species, infected humans, and potentially connecting water resources [35,36]. This information is important to public health strategy development and a critical area of future research.
5. Conclusion
While this initial survey is limited in scope, it identifies two important findings. Firstly, Cryptosporidium and Giardia are identified as contributing to recurrent diarrheal disease in Chobe District Botswana. Secondly, Cryptosporidium appears to be a potentially important source of diarrheal disease for children less than 2 years of age but initial data did not identify any adult infections, despite high levels of HIV/AIDS in the region. Further research is required to fully understand pathogen transmission and persistence dynamics, the impact of HIV/ AIDS and interventions on transmission potential, and the public health implications.
6. Acknowledgements
We thank the Ministry of Health for permission to conduct this study. We also thank the nurses, laboratory technicians, doctors, and the District Health Team in Chobe for their invaluable assistance. We thank M. Pacheko and R. Sutcliffe for sample testing. We thank M. E. Vandewalle for comments on this manuscript. This project was funded under the National Science Foundation Coupled Human Environmental Systems Award # 114953 and WildiZe Foundation. J. Herbein is an employee of Tech Lab. This company owns the diagnostic tests donated for use in this study.
REFERENCES
- A. Pruss-Ustun and C. Corvalan, “How Much Disease Burden Can Be Prevented by Environmental Interventions,” Epidemiology, Vol. 18, No. 1, 2007, pp. 167-178. doi:10.1097/01.ede.0000239647.26389.80
- P. Piot, et al., “The Global Impact of HIV/AIDS,” Nature, Vol. 410, No. 6831, 2001, pp. 968-973. doi:10.1038/35073639
- R. Glass and B. Stoll, “The Protective Effect of Human Milk against Diarrhea: A Review of Studies from Bangladesh,” Acta Pædiatrica, Vol. 78, No. S351, 1989, pp. 131- 136. doi:10.1111/j.1651-2227.1989.tb11225.x
- J. Bogaerts, et al., “Cryptosporidium spp., a Frequent Cause of Diarrhea in Central Africa,” Journal of Clinical Microbiology, Vol. 20, No. 5, 1984, pp. 874-876.
- A. Samie, et al., “Cryptosporidium Species: Preliminary Descriptions of the Prevalence and Genotype Distribution among School Children and Hospital Patients in the Venda Region, Limpopo Province, South Africa,” Experimental Parasitology, Vol. 114, No. 4, 2006, pp. 314- 322. doi:10.1016/j.exppara.2006.04.007
- R. L. Guerrant, et al., “Diarrhea in Developed and Developing Countries: Magnitude, Special Settings, and Etiologies,” Review of Infectious Diseases, Vol. 12, No. S1, 1990, pp. S41-S50. doi:10.1093/clinids/12.Supplement_1.S41
- P. L. Meinhardt, D. P. Casemore and K. B. Miller, “Epidemiologic Aspects of Human Cryptosporidiosis and the Role of Waterborne Transmission,” Epidemiologic Reviews, Vol. 18, No. 2, 1996, pp. 118-136. doi:10.1093/oxfordjournals.epirev.a017920
- W. R. Mac-Kenzie, et al., “A Massive Outbreak in Milwaukee of Cryptosporidium Infection Transmitted through the Public Water Supply,” New England Journal of Medicine, Vol. 331, No. 3, 1994, pp. 161-167. doi:10.1056/NEJM199407213310304
- E. B. Hayes, et al., “Large Community Outbreak of Cryptosporidiosis Due to Contamination of a Filtered Public Water Supply,” New England Journal of Medicine, Vol. 320, No. 21, 1989, pp. 1372-1376. doi:10.1056/NEJM198905253202103
- L. Savioli, H. Smith and A. Thompson, “Giardia and Cryptosporidium Join the Neglected Diseases Initiative,” Trends in Parasitology, Vol. 22, No. 5, 2006, pp. 203- 208. doi:10.1016/j.pt.2006.02.015
- R. L. Guerrant, “Cryptosporidiosis: An Emerging, Highly Infectious Threat,” Emerging Infectious Diseases, Vol. 3, No. 1, 1997. p. 51. doi:10.3201/eid0301.970106
- R. B. Davies and C. P. Hibler, “Animal Reservoirs and Cross-Species Transmission of Giardia. Waterborne Transmission of Giardiasis,” Environmental Protection Agency, Cincinnati, 1979.
- J. E. Peeters, et al., “Effect of Disinfection of Drinking Water with Ozone or Chlorine Dioxide on Survival of Cryptosporidium Parvum Oocysts,” Applied and Environmental Microbiology, Vol. 55, No. 6, 1989, pp. 1519- 1522.
- T. Mahin, R. Peletz and R. Shaw, “Cryptosporidium Contamination of Water in Africa: Impact on Mortality Rates for Children with HIV/AIDS. 2009. Water, Engineering and Development Centre (WEDC),” Loughborough University of Technology, Loughborough, 2009.
- A. Mohammed Mahdy, et al., “Risk Factors for Endemic Giardiasis: Highlighting the Possible Association of Contaminated Water and Food,” Transactions of the Royal Society of Tropical Medicine and Hygiene, Vol. 102, No. 5, 2008, pp. 465-470. doi:10.1016/j.trstmh.2008.02.004
- G. Feitosa, et al., “High Prevalence of Giardiasis and Strongyloidiasis among HIV-Infected Patients in Bahia, Brazil,” Brazilian Journal of Infectious Diseases, Vol. 5, No. 6, 2001, pp. 339-344. doi:10.1590/S1413-86702001000600008
- P. R. Hunter and R. Thompson, “The Zoonotic Transmission of Giardia and Cryptosporidium,” International Journal for Parasitology, Vol. 35, No. 11-12, 2005, pp. 1181-1190. doi:10.1016/j.ijpara.2005.07.009
- P. Monis and R. Thompson, “Cryptosporidium and Giardia Zoonoses: Fact or Fiction,” Infection, Genetics and Evolution, Vol. 3, No. 4, 2003, pp. 233-244. doi:10.1016/j.meegid.2003.08.003
- T. L. Creek, et al., “Hospitalization and Mortality among Primarily Nonbreastfed Children during a Large Outbreak of Diarrhea and Malnutrition in Botswana, 2006,” Journal of Acquired Immune Deficiency Syndromes, Vol. 53, No. 1, 2010, p. 14. doi:10.1097/QAI.0b013e3181bdf676
- A. A. Gajadhar, “Cryptosporidium Species in Imported Ostriches and Consideration of Possible Implications for Birds in Canada,” The Canadian Veterinary Journal, Vol. 34, No. 2, 1993, pp. 115-116.
- A. Cooper and R. Johnson, “The Bacteria and Parasites Found in Botswana Children with Acute Gastro-Enteritis,” South African Medical Journal, Vol. 47, No. 37, 1973, pp. 1705-1707.
- J. Minak, et al., “Evaluation of Rapid Antigen Pointof-Care Tests for Detection of Giardia and Cryptosporidium Species in Human Fecal Specimens,” Journal of Clinical Microbiology, Vol. 50, No. 1, 2012, pp. 154-156. doi:10.1128/JCM.01194-11
- T. J. Aragon, and W. T. Enanoria, Applied Epidemiology Using R, 2007. http://www.medepi.net/epir/index.html
- A. Agresti, “An Introduction to Categorical Data Analysis,” John Wiles and Sons Inc., New York, 1996.
- M. A. Montgomery and M. Elimelech, “Water and Sanitation in Developing Countries: Including Health in the Equation,” Environmental Science & Technology, Vol. 41, No. 1, 2007, pp. 17-24. doi:10.1021/es072435t
- S. Sharma, P. Sachdeva and J. S. Virdi, “Emerging WaterBorne Pathogens,” Applied Microbiology and Biotechnology, Vol. 61, No. 5, 2003, pp. 424-428.
- M. Steiner, M. Thielmanand M. Guerrant, “Protozoal Agents: What Are the Dangers for the Public Water Supply,” Annual Review of Medicine, Vol. 48, No. 1, 1997, pp. 329- 340.
- R. D. Newman, et al., “Longitudinal Study of Cryptosporidium Infection in Children in Northeastern Brazil,” Journal of Infectious Diseases, Vol. 180, No. 1, 1999, pp. 167-175. doi:10.1086/314820
- C. Petersen, “Cryptosporidiosis in Patients Infected with the Human Immunodeficiency Virus,” Clinical Infectious Diseases, Vol. 15, No. 6, 1992, pp. 903-909. doi:10.1093/clind/15.6.903
- B. E. Laughon, et al., “Prevalence of Enteric Pathogens in Homosexual Men with and without Acquired Immunodeficiency Syndrome,” Gastroenterology, Vol. 94, No. 4, 1988, pp. 1865-1871.
- W. R. Wolfe, et al., “The Impact of Universal Access to Antiretroviral Therapy on HIV Stigma in Botswana,” Journal Information, Vol. 98, No. 10, 2008, pp. 1865-1871.
- O. Ntshebe and K. Bainame, “Components of Regional Difference in HIV Prevalence Rates in Botswana,” Southern African Journal of Demography, Vol. 13, No. 1, 2012, pp. 59-82.
- R. L. Shapiro, et al., “Low Adherence to Recommended Infant Feeding Strategies among HIV-Infected Women: Results from the Pilot Phase of a Randomized Trial to Prevent Mother-to-Child Transmission in Botswana,” AIDS Education and Prevention, Vol. 15, No. 3, 2003, pp. 221- 230. doi:10.1521/aeap.15.4.221.23830
- Central Statistics Office, Botswana Family Healtgh Survey IV Report, Government of Botswana, Botswana, 2007.
- H. V. Smith, et al., “Tools for Investigating the Environmental Transmission of Cryptosporidium and Giardia Infections in Humans,” Trends in Parasitology, Vol. 22, No. 4, 2006, pp. 160-167. doi:10.1016/j.pt.2006.02.009
- L. Xiao, et al., “Host Adaptation and Host-Parasite CoEvolution in Cryptosporidium: Implications for Taxonomy and Public Health,” International Journal for Parasitology, Vol. 32, No. 14, 2002, pp. 1773-1785. doi:10.1016/S0020-7519(02)00197-2
- T. Heitman, et al., “Prevalence of Giardia and Cryptosporidium and Characterization of Cryptosporidium spp. Isolated from Wildlife, Human, and Agricultural Sources in the North Saskatchewan River Basin in Alberta, Canada,” Canadian Journal of Microbiology, Vol. 48, No. 6, 2002, pp. 530-541. doi:10.1139/w02-047
- I. S. Hamnes, et al., “Prevalence of Cryptosporidium and Giardia in Free-Ranging Wild Cervids in Norway,” Veterinary Parasitology, Vol. 141, No. 1, 2006, pp. 30-41.
- E. Atwill, et al., “Age, Geographic, and Temporal Distribution of Fecal Shedding of Cryptosporidium Parvum Oocysts in Cow-Calf Herds,” American Journal of Veterinary Research, Vol. 60, No. 4, 1999, pp. 420-425.
- D. A. T. Phillip, et al., “Relative Importance of the Various Environmental Sources of Cryptosporidium Oocysts in Three Watersheds,” Journal of Water and Health, Vol. 6, No. 1, 2008, pp. 23-34. doi:10.2166/wh.2007.016

