Open Journal of Pathology
Vol. 2 No. 3 (2012) , Article ID: 21142 , 4 pages DOI:10.4236/ojpathology.2012.23019
Tetramethrin Identified in Fat Adjacent to Mammary Carcinoma in a Male Dog—Case Report
![]()
1School of Veterinary Medicine and Animal Science, São Paulo State University (UNESP), Botucatu, Brazil; 2Maranhão State University (UEMA), São Luis, Brazil.
Email: *rochanoeme@fmvz.unesp.br
Received April 7th, 2012; revised May 7th, 2012; accepted May 16th, 2012
Keywords: Tetramethrin; Mammary Tumor; Male Dog; Environmental Contaminants
ABSTRACT
Canine mammary tumors (CMT) are very common in female dogs, representing approximately 50% of all tumors in this species, but are highly uncommon in male dogs and are primarily related to hyperestrogenism. There is considerable scientific interest in the possible role of environmental contaminants in the etiology of mammary tumors, specifically in relation to synthetic chemical substances released into the environment to which living beings are either directly or indirectly exposed. In this study, the presence of pyrethroid insecticide was observed in adipose tissue adjacent to canine mammary tumors. High Precision Liquid Chromatography—HPLC was adapted to detect and identify environmental contaminants in adipose tissue adjacent to malignant mammary tumor in one male dog, Akita, 12 years old. After surgery, the mass was carefully examined for malignant neoplastic lesions. Five grams of adipose tissue adjacent to the tumor was collected to detect environmental contaminants. The pyrethroid was identified as tetramethrin, at 0.20 μg/g. This is the first report in which the environmental contaminant level was detectable in adipose tissue of this male dog with a malignant mammary tumor, by HPLC. Results suggest the possible involvement of pyrethroid in the canine mammary tumor carcinogenesis since this animal did not present any other tumor that could cause hyperestrogenism.
1. Introduction
Canine mammary tumors (CMT) are very common in female dogs, representing approximately 50% of all canine tumors [1-3] and are highly uncommon in male dogs [4,5]. Misdorp reports that breast neoplasias can be found in male dogs if hyperestrogenism is present [6]. Male breast cancer (MBC) accounts for less than 1% of all cancers in men and less than 1% of all diagnosed breast cancers [7]. However, the incidence of MBC, like female breast cancer, seems to be increasing [8]. As to the females, middle-aged and older dogs are more affected by mammary neoplasias since they are more predisposed to obesity and hormonal disturbances; however, there is no breed-based predisposition [1]. About half of all CMT cases are malignant, with prognoses varying from guarded to poor [3].
The cause of mammary gland neoplasias remains unknown [4]. Mammary tumors can be influenced by genetic or external factors including environmental contaminants, which can augment or diminish individual responses [5,9]. Among the routinely utilized environmental contaminants, pyrethroids stand out given their increased use in recent years to control pests in agriculture, livestock and domestic animals [10]. The dissemination of the use of this pollutant is associated with its efficiency in controlling pests and the shortness of its half-life [11,12]. Its absorption in humans and in other animals is principally via the skin and by the digestive and respiratory tracts. Once absorbed, it is distributed into several tissues, but becomes concentrated especially in fat tissue. The International Agency for Research on Cancer (IARC) includes deltamethrin and cypermethrin in risk group number three, which is inconclusive as a carcinogen in humans, while the World Health Organization (WHO) classifies deltamethrin as an insecticide of moderate risk [13,14].
Garey et al. (1998) [15] emphasized that the pyrethroids can induce a breakdown in the homeostasis of hormones such as estrogen and indirectly influence cellular proliferation in breast tissue, thus initiating a neoplastic process.
Currently, the literature provides little information on what types of mammary tumors occur most frequently in male dogs. SILVA et al. (2004) [16] stress the importance of CMT to researchers, given its particular similarity to human breast cancer, making the dog, therefore, an excellent model for studying this disease, given the similarity of the epidemiological, clinical, biological and, apparently, genetic characteristics between the human and canine forms of the affliction. For all of these reasons, and because the number of men with breast neoplasia increases each year, the study of CMT in male dogs has also become extremely important.
The present work aims to report one case of environmental contamination in the adipose tissue adjacent to a mammary tumor located in the right cranial thoracic, caudal thoracic and cranial abdominal breasts in a male 12-year-old Akita dog weighing 22 kg.
2. Case Report
The 22 kg male dog of the Akita breed, aged 12 years, was attended at the Veterinary Hospital of the School of Veterinary Medicine and Animal Husbandry—UNESP— Botucatu, Sao Paulo, presenting enlargement of the cranial thoracic, caudal thoracic and cranial abdominal mammary of the right mammary chain with the presence of ulcers and myiasis. After one week of antibiotic and anti-inflammatory treatment the animal was referred for cytopathological collection and examination, which showed epithelial cells with nuclei containing loose chromatin, evident nucleoli, anisocytosis and anisokaryosis, accenttuated pleomorphism with cell clusters forming acini and loose cells in some fields, constituting a diagnosis of mammary carcinoma. Afterwards, unilateral mastectomy of the entire right mammary chain was performed. Fragments of the tumor were collected for histopathology (Figure 1) whereas peri-tumoral fat (adipose tissue adjacent to the tumor) was removed for subsequent analysis of the presence of pyrethroid contamination by the technique of High Precision Liquid Chromatography (HPLC) following the method of Bissacot and Vassilieff (1997) [17] at the CEATOX laboratory—UNESP—Botucatu. The tumor was diagnosed histopathologically as simple carcinoma (Figure 2). Immunohistochemistry was performed on the estrogen receptors: Antigen retrieval was carried out by microwave treatment in a 10 mM citrate buffer, pH 6.0. Tissue sections were incubated with primary monoclonal antibodies against ER (NovocastraUK), clone LH2, in 1:40 dilution, incubated for 120 minutes and developed with polymer Novolink (Novocastra, UK) after which the tumors presented highly positive staining (Figure 3). Analysis by HPLC for the presence of pyrethroid in the fat tissue adjacent to the mammary tumor revealed the presence of tetramethrin at the concentration of 0.20 μg/g.
3. Discussion
The histological and cytological diagnosis of a mammary
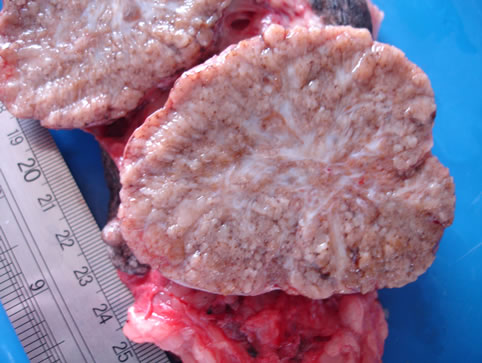
Figure 1. Macroscopy after collection of the adipose tissue adjacent to the tumor.
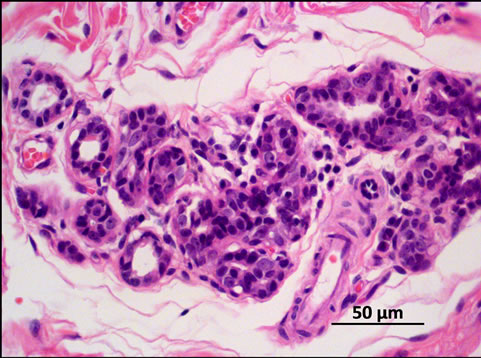
Figure 2. Tumor histology: Simple carcinoma, grade III, H & E stain. 400×.
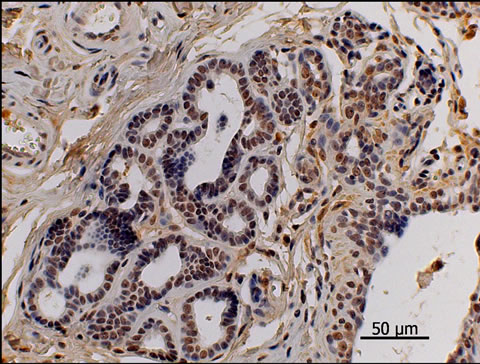
Figure 3. Estrogen receptors’ immunohistochemical picture. Observe the nuclear pattern stainning. Simple carcinoma, grade III, strongly positive for ER. 400×.
tumor does not differ between male and female dogs. The literature lacks a report on the histological types of male dog mammary tumors. Male dogs can present the development of mammary neoplasias on account of hyperestrogenism resulting from testicular neoplasias, such as sertolioma and Leydig cell tumor [18,19]. This specimen was a non-cryptorchidic non-neutered male dog that presented neither hormonal alterations nor clinical signs of feminization which, according to Misdorp, (2002) [6] tend to be frequent in animals with testicular tumorsresulting in hyperestrogenism. Thus, the hypothesis presented by Andrade et. al. (2010) [20]—in which contamination by pyrethroid insecticides that accumulate in peri-tumoral fat tissue may have an estrogenic effect on epithelial cells of mammary tissue and thereby induce their proliferation—is strongly supported since the immunohistochemical analysis of estrogen receptors produced positive staining in the tumoral tissue. As a function of these clinical and pathological findings, we sought to elucidate the genesis of this tumor.
In a short time, HPLC-aided confirmation of the presence of high levels of the pyrethroid tetramethrin in peri-tumoral fat provides us sufficient support to infer that the xenoestrogenic effect had made a determinative contribution to the promotion of mammary tumor in this male dog. Therefore, larger studies must be undertaken to assess the carcinogenesis of mammary tumors in male dogs, in relation to the absence of testicular tumors, in order to provide correct identification and better evaluation of substances that mimic estrogenic hormones.
4. Authors’ Contributions
FCF made substantial contributions to the conception, design, interpretation of histopathological and immunohistochemical data, and critically revised it for important intellectual content. PROB was involved in drafting the manuscript and critically revising it for important intellectual content. FHEA made substantial contributions to the conception, design and collection of samples, carried out the HPLC quantification and interpreted the data. NSR conceived the study, and participated in its design and coordination, and also helped to draft the manuscript. All authors read and approved the final version of the Manuscript.
5. Acknowledgements
We are grateful to FAPESP (2008/57.309-5) for the financial support.
REFERENCES
- C. R. Daleck, et al., “Aspectos Clínico e Cirúrgicos do Tumor de Mama Canino,” Ciência Rural, Santa Maria, Vol. 28, No. 1, 1998, pp. 95-100. doi:10.1590/S0103-84781998000100016
- F. Queiroga and C. Lopes, “Tumores Mamários Caninos—Novas Perspectivas,” Proceedings of the Veterinary Sciences Congress, Oeiras, 10-12 October 2002, pp. 183-190.
- J. Harvey, “Glândulas Mamárias,” In: M. J. Bojrab, Ed., Técnicas Atuais em Cirurgia de Pequenos Animais, 3rd Edition, Chapter 35, Roca, São Paulo, 2005, pp. 425-430.
- C. S. Hedlund, et al., “Cirurgia dos Sistemas Reprodutivo e Genital,” In: T. W. Fossum, Ed., Cirurgia de Pequenos Animais, 2nd Edition, Chapter 28, Roca, São Paulo, 2005, pp. 610-672.
- V. R. Nerurkar, A. R. Chitale, B. V. Jalnapurkar, S. N. Naik and V. S. Lalitha, “Comparative Pathology of Canine Mammary Tumors,” Journal of Comparative Pathology, Vol. 101, No. 4, 1989, pp. 389-397. doi:10.1016/0021-9975(89)90022-4
- D. J. Meuten, “Tumors in Domestic Animals,” 4th Edition, University of California, Berkeley, 2002, pp. 575-606. doi:10.1002/9780470376928
- G. Perkins and L. Middleton, “Breast Cancer in Men,” British Medical Journal, Vol. 327, No. 7420, 2003, pp. 239-240. doi:10.1136/bmj.327.7409.239
- S. H. Giordano, D. S. Cohen, A. U. Buzdar, et al., “Breast Carcinoma in Men: A Population-Based Study,” Cancer, Vol. 101, 2004, pp. 51-57. doi:10.1002/cncr.20312
- R. Schneider, C. R. Dorn and D. O. N. Taylor, “Factors Influencing Canine Mammary Cancer Development and Postsurgical Survival,” Journal of the National Cancer Institute, Vol. 43, 1969, pp. 1249-1261.
- Instituto Nacional do Câncer (INCA), “Estimativa para 2005 da Incidência e Mortalidade por Câncer no Brasil,” Ministério da Saúde, Rio de Janeiro, 2006.
- S. M. Goulart, M. E. L. R. Queiroz, A. A. Neves, J. H. Queiroz and I. C. Alves, “Anais da 27˚ Reunião Anual da SBQ e 26˚ Congresso Latino Americano de Química,” Salvador, 2004.
- J. W. Wright, “The WHO Program for the Evaluation and Testing of New Insecticides,” Bulletin of the World Health Organization, Vol. 44, 1997, pp. 11-12.
- International Agency for Research Cancer, IARC, “Overall Evaluations of Carcinogenicity to Humans,” IARC, Lyon, 2005. http://monographs.iarc.fr/ENG/Monographs/vol53/mono53-10.pdf
- Environmental Protection Agency, “Pyrethrins: Report of the Cancer Assessment Review Committee,” 3rd Evaluation, EPA, 2004. http://www.cepis.ops-oms.org/bvsacd/cd49/pesticide.pdf
- J. Garey and M. Wolff, “Estrogenic and Antiprogestagenic Activities of Pyrethroid Insecticides,” Biochemical and Biophysical Research Communications, Vol. 251, No. 3, 1998, pp. 855-859. doi:10.1006/bbrc.1998.9569
- A. E. Silva, et al., “Carcinogênese Hormonal e Neoplasias Hormônio-Dependentes,” Ciência Rural, Santa Maria, Vol. 34, No. 2, 2004, pp. 625-633. doi:10.1590/S0103-84782004000200048
- D. Z. Bissacot and I. Vassilieff, “HPLC Determination of Flumethrin, Deltamethrin, Cypermethrin, and Cyhalothrin Residues in Milk and Blood of Lactating Dairy Cows,” Journal of Analytical Toxicology, Vol. 21, No. 5, 1997, pp. 397-402.
- L. Ackerman, “Reproductive Disorders,” The Genetic Connection: A Guide to Health Problems in Purebred Dogs, AAHA Press, Colorado, 1999.
- G. Daugaard, V. Karas and P. Sommer, “Inguinal Metastases from Testicular Cancer,” BJU International, Vol. 97, 2006, pp. 724-726. doi:10.1111/j.1464-410X.2006.06017.x
- F. H. E. Andrade, F. C. Figueiroa, P. R. O. Bersano, D. Z. Bissacot and N. S .Rocha, “Malignant Mammary Tumor in Female Dogs: Environmental Contaminants,” Diagnostic Pathology, Vol. 5, No. 45, 2010.
NOTES
*Corresponding author.
Competing Interests: The authors declare that they have no competing interests.

