Journal of Materials Science and Chemical Engineering
Vol.03 No.11(2015), Article ID:61535,6 pages
10.4236/msce.2015.311010
Physicochemical Properties of Thin Rhenium Chalcogenides Coatings
E. A. Salakhova, D. B. Tagiyev, P. E. Kalantarova, K. F. Ibrahimova
Lab. of Electrodeposition of Rhenium Alloys and Electrocatalysis, Institute of Catalysis and Inorganic Chemistry, NAS of Azerbaijan, Baku, Azerbaijan

Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).


Received 17 September 2015; accepted 24 November 2015; published 27 November 2015

ABSTRACT
Some physicochemical properties of thin layers of rhenium chalcogenides alloys such as micro- hardness and inner tension have been investigated. An influence of electrolyte composition and content of rhenium in the alloy on inner tension and microhardness of rhenium chalcogenides alloy was studied. It is established that the electrolytic alloys of rhenium chalcogenides obtained from different electrolytes possess inner tension of stretching. The most favourable condition for reaching the uniform coating of rhenium chalcogenides from sulphate and chloride-sulphate electrolyte using dispersive capacity (DC) of electrolyte has been found out. The best DC in chloride-sulphate and sulphate electrolyte takes place at current density of 0.8 A/dm2 at 75˚C.
Keywords:
Electrochemistry, Thin Films, Rhenium Chalcogenides, Rhenium Alloys

1. Introduction
In modern engineering semiconductor materials take a particular place. An actual issue is creation and investi- gation of the new materials including the semiconducting ones possessing the valuable properties and possible practical application.
In connection with that alloys of rhenium chalcogenides are semi-conductor materials broadly used in modern technologies, their application in different fields is conditioned by their physico-chemical properties [1] [2] . Therefore, for deciding the question on using semi-conductor materials, it is necessary first to pay attention to their physico-chemical properties. The study of electro-physical properties of semi-conductor materials pursues two aims. Firstly these properties make it possible to cognize deeper physico-chemical nature of a substance: kind of chemical bond, break of sto-ichiometric composition, influence of their admixtures and so on. Secondly, the fields of technical application of semi-conductors are mainly determined by their electrophysical and optical properties. Therefore, these properties of semi-conductor materials are considered in that minimum volume which is necessary for the right orientation of their practical use.
We have also investigated the dispersive capacity of electrolytes. Dispersive capacity (DC) of electrolyte is one of the most important technological indices, characterizing an evenness of current distribution and thickness of galvano deposits on a surface of coated articles. To the factors which determine aquality of the electrolytic deposit, they should refer to an evenness of their distribution along the surface as well. At estimation of evenness of distributing metal along an electrode surface the various terminologi is used, including current distribution, dispersive capscity, cutting capacity and so on.
In practice current is distributed unevenly on a surface of electrode, especially under coating the articles of complicated confiquration. Hence, the factual current density and thickness of a coating at different sections of a cathode are different, at some they exceed an average value, at others they are less. This may be negatively reflected in anticorrosive, protective, mechanical and other properties of a coating as at some sections its thickness may be less than minimum admissible.
Electrolytic capacity to change the primary distribution of a current, conditioned only by ratio of the geome- trical parameters, is termed as a dispersive capacity of electrolyte (DC).
The dispersive capucity (DC) of electrolyte is one of the most important technological indices, characteriring an evenness of current distrubution and thickness of galvanodeposits on a surface of coated articles. Therefore, definition of DC of electrolyte in different conditions is of great practical importance. In the literature there are no almost data on the question of DC of chloride-sulphate (of Re-Te alloy) electrolytes for drawing the rhenium chalcogenides alloys.
So, determination of DC value in different conditions of electrolysis is of great practical importance [3] [4] . Earlier [5] -[8] we obtained thin coatings of rhenium chalcogenides from different electrolytes.
The present work is dedicated to studying some physico-chemical properties of thin electrolytic coatings of rhenium chalcogenides, microhardness, inner tension of the alloys. We have studied an influence of electrolyte composition and content of rhenium in an alloy on inner tension and microhardness of the alloy. The study of microhardness and inner tension displayed that the physicomechanical properties of electrolytic deposited alloys differed, to a great extent, from those of the alloys obtained by other ways. As well, the physicomechanical pro- perties of the electrolytic alloys may change considerably depending on conditions and the rate of electrolysis. It should be noted that microhardness of the electrolytic alloys is somewhat higher than that of the metallurgical alloys. Arise in microhardness of the electrolytic deposite is connected with inclusion of hydrogen and diverse outside admixtures, for instance, the surfactants which cause distortion of a crystalline lattice. Hardness depends on a grain thickness, the smaller crystalline deposit is, the higher its hardness is.
By electrochemical way it is possible to get the coatings with diverse and preliminarily given properties.
Among other physicochemical properties, inner tensions of electrolytic material, greately affecting the quality of electrolytic coatings, present a particular interest. They understand inner tension as a strained condition of a deposit as a result of diffent structural distortions, what is expressed in compression or tensility of a deposit in comparison with a normal state.
In case of a stretched state, when metal tensions of stretching present, the deposit strives to be compressed or to reduce its volume, while in case of a compressed state, i.e. at tensions of compression, the deposit will aspire to extend and thus will increase in volume.
The emergence of inner tensions is usually associated with some causes. However, the reasons causing inner tensions may be very different. They are mainly as follows:
1) change of lattice parameters;
2) change of distances between crystals of a deposit in the process of deposition;
3) growing of deposit crystals as a consequence of a merge of small crystals;
4) formation of chemical compounds of metal with admixtures, accompanicd by a change of a deposit volume.
And so, finding out the reasons of emerging the inner tensions in each concrete case may further be the choice of conditions for the electrodeposition of metals with set values of inner tensions.
To obtain thin coatings of Re-Te alloys the electrolysis is to be carried out at current density of 2 - 10 mA/cm2 at 75˚C from the electrolyte with composition (mol/l):
 .
.
To obtain thin coatings of Re-Se alloy there was used electrolyte with composition (mol/l): 0.05NH4ReO4 + 0.5SeO2 + 1NaOH at current density of 4 - 20 mA/cm2, 75˚C of temperature. And for obtaining thin semi- conductor coatings of Re-S alloy there was used electrolyte with composition (mol/l): 1.0 × 10−3 NH4ReO4 + 1.5 × 10−3 (NH2)2CS + 1 × 23 × 10−3 H2SO4, current density of 35 - 40 mA/sm2, temperature of 60˚C.
2. Methods of Experiment
Inner tension of thin coatings of rhenium chalcogenides was investigated by the method of a flexible cathode deforming [9] . For accurate determination of a value of deflection, counting out was made by using the horizontal microscope with a micrometric scale of MIR-2 mark. Measurement of alloys microhardness was carried out on the device PMT-3 by way of pressing into of diamond square pyramid under load of 0.005 - 0.5 kgs [10] . The diamond tip in a form of right tetrahedral pyramid was pressed into the tested section of the sample under load attached within the definite time. To get the most precise results, the average of 10 definitions was taken as an index of microhardness. In the present work for defining DC of sulphate and chloride-sulphate electrolyte, the experiments conducted in Herring,s and Bloom’s right-angled cell [4] .
3. Results and Discussions
The electrolytic alloys on content of rhenium in alloy is presented. As is seen in Figure 1, depending on electrolyte composition and content of rhenium in alloy the change of inner tension of alloy is different. The curve of inner tension of the alloy has maxima. The maximum on curve of inner tension corresponds to the alloy, which meets the compound ReT2, ReS2 and ReSe2 according to X-ray structural analysis. We may assume that a high value of inner tension in deposits of such composition is caused by the presence of chemical compound, and as we have established, the electrolytic alloys ReX2 have inner tension of stretching. Inner tensions in the electrolytic deposits characterizing their compressed or stretched state, emerge almost in all cases. As a tesult there takes plase the cracking of deposits, leading to increasing the number of pores, what decreases the corro- sion-protective qualities of a coating. An emergence of inner tensions weakens very often cohesion of a deposit wiht a base.
Calculation of a value of inner tensions of the electrochemical deposits was made by inclination of a cathode. The inner tensions in a deposit cause tensions of the opposite sign in a lining, which lead to its bend and change of sizes. It is necessary to note that at beginning of the tension of stretching in the deposits in the layers directly adjacent to the deposit, there arise the forces of the opposite direction striving for the reduction of a volume,
Figure 1. Dependence of composition of alloy Re-X (X-S, Se, Te) on inner tension. 1: Re-Te (alkaline electrolyte) pH = 10; 2: Re- Te (chloride-sulphate electrolyte) pH = 1.0; 3: Re-Se (sulphate electrolyte pH = 1.2); 4: Re-Se (alkaline electrolyte pH = 10); 5: Re-S (thiourea electrolyte pH = 1.5).
while on the opposite side of a lining the forces emerge to wards direction analogous to the forces in the deposit. Hence, in the lining a layer is, which is neutral one and its state is determined by ratio of thickness of lining and coating, as well correlation of forces values. If an alloy is deposited on flexible lining, then as a result of tensions emerged in it. The deposit will be deformed taking off tension by this. Depending on a degree of lining elastisity there will be observed distortion of a value of true inner tensions, to a various degree.
The reasons, causing inner tensions in the electrolytic alloys may be explained by the formation of chemical compound of rhenium chalcogenides ReX2. In the present work for the purpose of establishing interaction between rhenium and chalcogen, the microhardness of the alloys depending on composion were measured. As is known the dependence between the physicochemical properties on composition of alloy is determined by the character of their interaction. The character of dependence of microhardness on composition of alloy changes according to a kind of interaction of the components of the electrolytic alloy. The dependence of the alloys, forming mechanical mixtures of the components, is of linear character. i.e. its value presents arithmetrical means from a value of microhardnes of the components forming an alloy-mixture. At the formation of solid solutions a line of microhardness measurement as far as a change of alloy composition presents bended curve. At the formation of a chemical compound in the system, the dependence between microhardness and composition of alloy bears spasmodic character, i.e. microhardness prossesses the properties inherent in a definite compound.
Figure 2 shows the dependence of microhardness of cathodic deposits of the alloys of rhenium chalcogenides on content of rhenium in alloy. As is seen in Figure 2. depending on a composition of electrolyte and content of rhenium in the alloys changes of microhardness may be different.
With increasing the content of rhenium from 20% to 30% microhardness of alloy the change from 50 to 90 kg/sm2. A curve of change of microhardness of rhenium chalcogenides alloys goes through maximum on microhardness curves corresponds to an alloy which meets ReS2, ReSe2 and ReTe2 according to the x-ray structural analysis. It is possible to suppose that high value of microherdness in the alloys of such composition is connected with the presence of a chemical compound on this section.
The obtained data confirm once more that during the clectrodeposition of rhenium with chalcogens on a cathode, there are received thin chemical componnds of semi-conductor coatings of rhenium chalcogenides. The data cited show that microhardnes,as well as inner tension of the electrolytic deposits R-X may be mostly diverse depending on content of rhenium in the alloy.
For the purpose of revealing the most favourable conditions to obtain the even coatings of rhenium chalcogenides, we have undertaken an attempt to estimate if only for the first approach of current density and temperature, DC of sulphate and chloride-sulphate electrolytes. For determining the dispersive capacity of the electrolytes the method of Herring and Bloom, described in the collection applied electrochemistry [4] .
Figure 2. Dependence of microhareh alloy R-X (X-S, Se, Te) on composition 1: Re-Te (alkaline electrolyte) pH = 10; 2: Re-Te (chlo- ride-sulphate electrolyte) pH = 1.0; 3: Re-Se (sulphate electrolyte) pH = 1.2; 4: Re-Se (alkaline electrolyte) pH = 10, 5: Re-S (thiourca electrolyte pH = 1.5).
The experiments were carried out in the rectangular cell with two parallel engaged fixed cathodes and the meshed anode between them. The distance between anode and cathodes was equal to 4:1, i.e. anode was moved off from cathode 4 times farther, than from other. At this ratio of distances in case of the primary current distribution on a near cathode, metal’s deposit will be 4 times more than on a remote one. Dispersive capacity by current was determined according to formula:

where k is ratio of distances between electrodes (primary distribution of current), In, Id―secondary (factual) distribution of current.
There is dependence between distribation of metal and factual distribution of current:
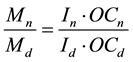
where OCn, OCd correspondingly output by current on a near and distant cathodes.
An cases, when output by current doesn’t depend on current density, the dispersive capacity of electrolyte is defined by formula:

where Mn, Md -additional weights at a near and distant cathodic sections.
The experiments were carried out in the rectangular cell of Herring and Bloom, prepared frow plexiglass and thermostated within t = 0.20. Nickel plate of 4.0 - 4.5 sm2 served as a cathode and platinum plate as anode.
Discrepancy of DC values in the parallel experiments didnot exceed 2% - 3%. The distance between anode and cathode was 4:1, i.e. anode moved off one cathode 4 times farther than from the other one. The volume of electrolyte made up 50 ml. For determination of DC there was used electrolyte with composition (mol/l)
1) For the alloy Re-Se sulphate; (mol/l) 0.01 - 0.10 NH4ReO4, 0.01 - 0.10 SeO2, 2H2SO4,
ik = 5 - 20 mA/cm2 duration of electrolysis 30 min.
2) For the alloy Re-Te sulphate: (mol/l) 0.01 - 0.8 NH4ReO4, 0.01 - 0.08 TeO2,
1 - 5 H2S04, 1 - 5 HCL, 0.02 - 0.05 (NH4)2SO4,
ik = 1 - 10 mA/sm2 duration of electrolysis 30 min.
Duration of deposition is 30 minutes. At changing a value of one of parameters,the values of therest ones were kept constant. The obtained results are presented in Figure 3. Dispersive capacity of bath turned positive in all cases. In the range of current densities of 5 - 10 A/dm2 in the investigated electrolyten output of alloy by current made up about 100%. So, with increasing current density DC of an electrolyte increases,and with further increase in current density, an output by current, in connection with isolation of hydrogen on a surface of electrode gradually decreases as a result of what DC decreases as well. Besides, as seen in Figure 3 with a rise in temperature of electrolyte, DC somewhat increases.
It is probably, connected with that a rise in temperature, electroconductivity of electrolyte increases, what improves distribution of cathodic current. And this, in its turn, provides an even distribution of a deposit on a surface of cathode.
On the basis of the experimental data it is established that dispersive capacity of bath turned to be positive in all cases. In the range of current densities of 0.2 - 1.2 A/dm2 in the investigated electrolytic, an output by current of alloy made up about 100%. The got results are presented in Figure 3.
Thus, the best DC in chloride-sulphate and sulphate-electrolyte takes place at current density of 0.8 A/dm2 at 75˚C.
4. Conlusions
1) An influence of electrolyte and the rhenium content in alloy on inner tension and microhardness of thin
Figure 3. Dependence of the dispersive capacity of sul- phate (1 - 3) and chloride-sulphate (1¢-3¢) electrolyte on current density at different temperatures.
rhenium chalcogenides has been investigated. It is established that electrolytic alloys of rhenium chal- cogenides, obtained from acidic electrolytes, have inner tension of stretching.
2) Thus, the best DC in chloride-sulphate and sulphate electrolyte takes place at current density of 0.8 A/dm2 and temperature of 75˚C.
Acknowledgements
This work was supported by the Science Development Foundation under the President of the Republic of Azerbaijan (Grant No EIF-2013-9(15)-46/19/4).
Cite this paper
E. A.Salakhova,D. B.Tagiyev,P. E.Kalantarova,K. F.Ibrahimova, (2015) Physicochemical Properties of Thin Rhenium Chalcogenides Coatings. Journal of Materials Science and Chemical Engineering,03,82-87. doi: 10.4236/msce.2015.311010
References
- 1. (1975) Col. Investigation and Application of the Rhenium Alloys. Transactions of the III All-Union Conference on the Problem of Rhenium. Nauka, Moscow, 203.
- 2. (1970) Col. Rhenium in the New Technologies. Transactions of the III All-Union Conference on the Problem of Rhenium. Nauka, Moscow, 203.
- 3. (1974) Applied Electrochemistry. 3rd Edition, Chemistry, Leningrad 137.
- 4. Col (1980) Practical Work on Applied Electrochemistry. Training Aid for Institutes of Higher Education. 2nd Edition, Chemistry, Leningrad, 5.
- 5. Naor, A. and Eliaz, N. (2010) Properties and Applications of Rhenium and Its Alloys. Ammtiac Quarterly, 5, 11-15
- 6. Salakhova, E.A. (2003) Electrodeposition of Thin Re-Se Films. Inorganic Materials, 39, 99-103.
http://dx.doi.org/10.1023/A:1022178109180 - 7. Salakhova, E.A. and Majidzada, V.A. (2011) Electrochemical Preparation of Thin Rhenium-Tellurium Coatings Chloride-Borate Electrolyte. Russian Journal of Electrochemistry, 47, 877-882.
http://dx.doi.org/10.1134/S1023193511080118 - 8. Salakhova, E.A. and Aliyev, A.M. (2012) Obtaining the Thin Semiconductive Covering Re-Se from Sulphate Electrolyte. Advances in Materials and Physics Chemistry, 2, 253-255.
http://dx.doi.org/10.4236/ampc.2012.24B064 - 9. Vagramian, A.T. and Solovyova, Z.A. (1960) Methods of Investigation of the Electrodeposition of Metals. AS USSR, 53.
- 10. Kudryavtseva, H.T. and Vyacheslavov, P.M. (1973) Practical Work on Applied Electrochemistry. Chemistry, Leningrad, 252.




