Open Journal of Obstetrics and Gynecology
Vol.06 No.05(2016), Article ID:66105,8 pages
10.4236/ojog.2016.65042
The Influence of Progesterone Gel Therapy in the Treatment of Fibrocystic Breast Disease
Milena Brkic1, Svetlana Vujovic2,3, Maja Franic Ivanisevic4, Miomira Ivovic2,3, Milina Tancic Gajic2,3, Ljiljana Marina2,3, Marija Barac2,3, Branko Barac5, Alekasandar Djogo6, Gabrijela Malesevic7, Damir Franic8,9*
1Medical Faculty, University of Banja Luka, Banja Luka, Bosnia and Herzegovina
2Clinic of Endocrinology, Clinical Center of Serbia, Belgrade, Serbia
3School of Medicine, University of Belgrade, Belgrade, Serbia
4Clinic of Gynecology and Obstetrics, Clinical Center Serbia, Belgrade, Serbia
5Institute of Reumatology, Belgrade, Serbia
6Clinical Center of Montenegro, Podgorica, Montenegro
7Department for Diabetes with Endocrinology, University of BanjaLuka, Banja Luka, Republika Srpska
8Outpatient Clinic of Obstetrics and Gynecology, Rogaska Slatina, Slovenia
9School of Medicine, University of Maribor, Maribor, Slovenia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 1 March 2016; accepted 25 April 2016; published 28 April 2016
ABSTRACT
The effect of progesterone therapy on E2/P ratio changes during the luteal phase, and its consequences are on mastalgia and cyst, within a fibrocystic breast disease (FBD). Fifty women with FBD were included. Information for mastalgia and mastodynia were checked with a questionnaire. All women had (E2) and (P) concentration checked before and during the therapy on the 21st and 24th day of a cycle, ultrasound measured size and number of cysts before and during the therapy. T-test, X2-test, McNemar test, Wilcoxon test and Friedman test were used for statistics. There was a decrease E2/P relation during the therapy vs. before the treatment p < 0.01, as well as the decrease of E2 level on the 24th day during the therapy vs. same day of E2 level, before the therapy (p = 0.164). There was an increase of p level on the 24th day vs. on 24th day before the therapy (p < 0.001). During the therapy, it was found decrease in pain and tension (p < 0.001), and the number and size of the cysts (p < 0.001). Mastalgia and mastodynia significantly decreased during the local p gel therapy, and there was a significantly lower number and size of the cysts in patients with FBD.
Keywords:
Progesterone, Mastalgia, Mastodynia, Fibrocystic Breast Disease

1. Introduction
Fibrocystic breast disease (FBD) is a condition with high incidence in women during the reproductive age [1] . The incidence of benign breast disease within a population is 13.5% to 42% [2] . The hormonal changes, predominantly in estradiol and progesterone, as well as growth factors like the epidermal growth factor (EGF) and tissue growth factor α (TGFα) are the key factors in understanding the development and etiology of FBD. It is believed that higher concentrations of estradiol and lower concentration of progesterone in the midluteal phase, their disbalance and changed ratio can be the key factor in etiology of FBD [3] . Literature data are not consistant about these changes and further studies are needed. Having in mind the incidence of fibrocystic breast disease, detection of sex hormone in more details is very important. Nevertheless, relative hyperestrogenia and hypoprogesteronaemia, as well as changed estradiol/progesterone (E2/P) ratio, encouraged some researchers to base the therapeutic approach on the use of progesterone preparations. Recently, there was more data about exogenous natural progesterone and synthetic selective progesterone that they do not stimulate cell proliferation [4] , decrease number and expression of the estrogen receptors, or in the other words have anti estrogen effect [5] [6] . Progesterone used in a form of gel acts locally on the epithelium and breast vessels. Due to solubility characteristics, progesterone gel has effects on the site of applications and doesn’t have a systemic effect. It results in increase of 17-β-hydroxysteroid dehydrogenase which is the most important factor in estradiol metabolism. Adding progesterone there is short-term increase in differentiation and proliferation of epithelial tissue within the first 24 hours followed by a strong inhibition [7] - [9] . Progesterone antiproliferative effect on epithelial breast cells depends on kind of preparation and way of using, dose and duration of its administration [10] . There are no consistant data in the literature about the effects of oral contraceptive (OC) on benign breast disease. The low estrogen dose within combined OC, lower than 50 mcg reduces the risk of atypical fibroadenoma [11] . A majority of studies examining estradiol and progesterone detected only on the 21st day of the cycle, coming to conclusion that cycle is ovulatory and there were no hormonal changes. During the luteal phase, the most prominent changes are from day 21st-24th and minimum of data have to be completed with detection estradiol and progesterone in these days. The objective of this study was to examine changes of E2/P ratio on days 21st and 24th, and compared it with the ratio during therapy with progesterone gel.
2. Methods
This retrospective study was done in Clinic of endocrinology, diabetes and diseases of metabolism, Clinical center of Serbia, Belgrade, Serbia. Fifty women with mastalgia were recruited from out patient subjects and tested before and during the sixth months of therapy with progesterone gel.They were younger than 40 years of age (32.5 ± 5.04). Median BMI was 22.4 ± 4.0 kg/m. The number of birth were 1.04 ± 0.5, most of them (45/90%) had regular menstrual cycle with premenstrual syndrome (PMS). The ultrasound examination confirmed presence of simple breast cysts. In order to find out the etiology of FBD, hormonal analysis were done during the 21st and 24th day of the menstrual cycle before and during the treatment. They were treated with local progesterone therapy in a form of progesterone gel application (natural micronized progesterone-1 g) from the 16th to 26th day of cycle during the luteal phase with the gel dose applicator which contains 0.025 g of progesterone, on both breast, except nipples, two times a day for a 6 month period.
Pregnant women, women with a recent skin changes or documented allergic reaction to some of the hormonal estrogen-progesterone preparations, women with absolute or relative contraindications for estrogen and progesterone therapy (breast cancer, other existing cancer, thrombosis and thrombophilia, porphyria and liver and kidney insufficiency, endometriosis, uterine myoma, endometrial hyperplasia, prolactinoma, hyperthyroxinemia, diabetes mellitus, hyperlipoproteinemia, hypertension, liver adenoma, previously present and cured melanoma, meningioma, epilepsy) were excluded from the study. Blood analysis for follicle stimulating hormone (FSH), luteinizing hormone ( LH), estradiol (E2), prolactin (PRL) dehydroepiandrosteron sulphate (DHEAS), free testosterone (T), androstendion, free thyroxine (fT4), thyreostimulating hormone (TSH) were taken at 8 am on day 2 of the cycle to exclude other diseases. Blood samples for E2, progesterone (P), PRL were taken at 8 am on the 21st and 24th day of the cycle. Hormonal analysis were done with RIA (ECLIA) method on Roshe immunoassay analyzer (modular analitics E170). Ultrasound examinations of the breast were done by Esaote My Lab 50 and linear multi frequency high resolution probe was used, LA 523 13-4 MHz, linear 46 mm. Statistical analysis was done with the Statistica StatSoft program. Results were presented as median value ± standard deviation. Statistical significance was set at P < 0.05. T test was used for determining the correlation and statistical significance of sex hormones before and during the treatment, size and number of cysts before and during the treatment. McNemar test and X2 was used for correlation between historical and clinical findings (pain, tension) before and during the treatment. Wilcoxon signed-rank test allowed us to determine the individual difference between the progesterone concentration levels.
3. Results
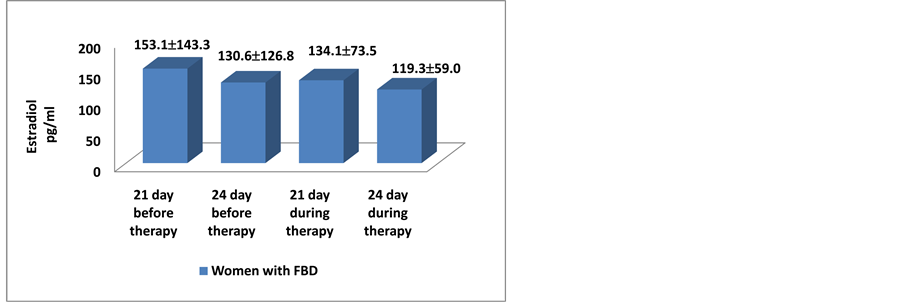
Prior to therapy estradiol levels were higher during the luteal phase, compared to period during the therapy.
Significant decrease of estradiol value on the 24th day during the therapy was found, compared to the same day before progesterone gel therapy (130.6 ± 126.8 pg/ml vs. 119.3 ± 59 pg/ml) (p = 0.164) (Figure 1).
The most important changes were detected in progesterone values. Significant increase for progesterone was found.
During the therapy on the 24th day compared to the same day before the therapy (6.9 ± 5.3: 15 ± 9.5 ng/ml), (p < 0.001) (Figure 2).
Having in mind the fact that estradiol and progesterone were hormones which controlled each others, number of receptors and activity, not just a simple values, but their ratio represented the most important factor leading to fibrocystic breast disease, myoma arising, luteal phase disturbances etc. In all tested women with FBD, E2/P increased from 21st to 24th day before therapy, while progesterone gel therapy induced decreasing of ratio and sharply reducing mastopathy and cysts volume (Figure 3).
Figure 1. Concentration of estradiol 21st and 24th day before and during the therapy.
Figure 2. Concentration of progesterone 21st and 24th day before and during the therapy.
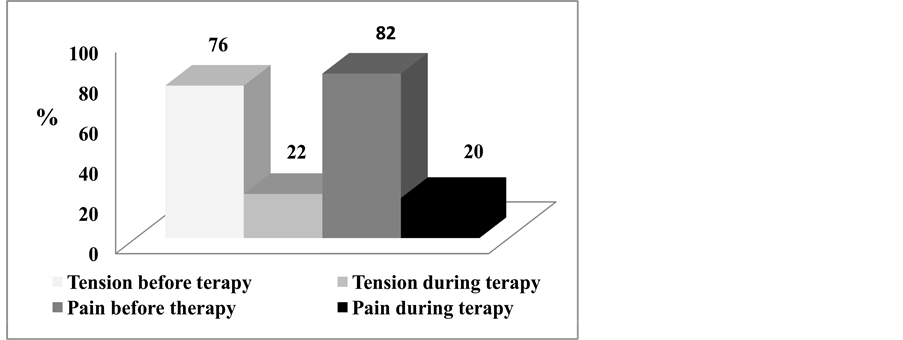
During the therapy statistically significant decreased in mastalgia and tension were found during sixth month of therapy (p < 0.001) (Figure 4).
During the therapy average cyst size had significantly decreased from 8.3 mm to 5 mm during only six months, but more important was the reduction of median cyst number from 7 to 2.8 during only 3 months of therapy (p < 0.001) (Figure 5).
Figure 3. E2/P ratio on 21st and 24th cycle days before and during the therapy.
Figure 4. Pain and tension (%) during luteal phase before and during the therapy.
Figure 5. Number and size of cysts before and during the therapy.
4. Discussion
The most important factor in etiology of FBD were dynamic changes and correlations of E2/P influenced by many other hormones (prolactin, insulin etc.) and growth factors. Only more detailed studies detecting more samples of estradiol and progesterone during the luteal phase, could confirm this. Some studies previously confirmed that fibrocystic breast disease occurred due to disbalance between high estradiol concentration and low progesterone concentrations during the luteal phase [2] . Other studies found no correlation between FBD and cyclic mastalgia with progesterone insufficiency during the luteal phase of menstrual cycle [12] . However these studies had E2/P determined only during the 21st day of the cycle and no dynamic changes were followed up during luteal phase. Due to insufficient data conclusion was not a correct one.
It was known that estradiol affect the breast lesion proliferation. It influenced on cell proliferation and differentiation during the cell cycle. It was likely that in benign proliferative breast disease steroid hormones antagonized cell differentiation and apoptosis. Dysregulation of this process lead to fibrocystic changes manifesting as thickening [13] .
Sixty years after the progesterone discovery its complete way of action is still controversial in breasts. Unfortunately, many studies based their data on detecting only serum analysis of estradiol and progesterone. In the breast tissue local concentration of estradiol is 20 time higher than in the circulation! So, the relation between E2 and P was being even higher!
Recent research showed that during the physiological increase of endogenous progesterone during the luteal phase, there was a decrease of breast epithelial cell proliferation [14] . Histological studies found an increase of breast cell apoptosis during the first 24 hours of luteal phase when a level of progesterone was increasing too. After that, there was significant decrease of number of apoptotic breast cells [15] [16] . Chang and associates tested the effects of estrogen and progesterone in woman before the breast surgery in double blind, placebo controlled study where patients were treated with placebo, estrogen and transdermal progesterone 10 - 13 days before the surgery. Estrogen had an impact on increase rate of breast cell proliferation by 230%, while progesterone had an impact on decrease rate of cell proliferation by 400%. When given together with estradiol, progesterone inhibited estrogen induced breast cell proliferation [17] , what was coherent with the results of our study. Similarly, in randomized, double blind study, Foidart and associates also showed that progesterone decreased breast cell proliferation previously triggered by estradiol [18] .
In most of the studies, changed relation between E2 and P during the luteal phase was not investigated, only absolute values were taken. This study confirmed the great importance of E2/P during the luteal phase, as the etiological factor for FBD. Changing the E2/P ratio was the key factor for treating women with FBD, cyst reduction and cessation of mastalgia.
Complex breast cysts with a less than 2% risk for malignant disease, are marked as BIRADS 3 category, and could be followed [19] [20] , whereas more complex cysts with intracystic Doppler effect and more complex echo characteristics with a risk for malignancy more than 2%, required biopsy [21] [22] . Having of all this in mind, ultrasound method was considered relevant for simple cyst diagnosis before and during the treatment in our study. More significant decrease of cysts number and the size can be explained with decrease of the E2/P ratio, during the luteal phase.
For a long time, the transdermal approach is known to be a successful alternative to conventional therapy, including oral therapy. Progesterone was a first hormone tested as a local therapy of benign breast diseases [23] . Progesterone limited proliferative action of estrogen on the breast tissue [24] .
Also, progesterone had natriuretic effect due to suppression of renal tubular reabsorption and increase of cell filtration, which prevented retention of liquid within a breast gland component and subsequently prevented occurrence of pain syndrome (mastalgia and mastodynia). In addition, transdermal application of progesterone had an effect on vascular network by decrease the capillary permeability and subsequent breast tissue edema. All those, consequently decreased the occurrence of mastalgia symptoms what was confirmed in this study.
The majority of authors found correlations between mastalgia and reproductive period, which was in accordance with our study [25] .
During that period woman was most exposed to the hormonal fluctuations. Clinical mastalgia, like pain and breast tension usually occured within a third or fourth decade of life and symptoms continue. Usually, they begun during the luteal phase of a menstrual cycle, with increasing of intensity during the midluteal phase and disappear at the beginning of the menstruation [26] [27] . In only 14% of women with this problem, symptoms spontaneously resolved. Moreover, in 42% of the women symptoms started during the climacterium, period preceding the menopause, characterized by the anovulatory cycles, hypoprogesteronemia and normal or higher estradiol levels, confirming the importance of E2/P ratio. Davies suggested non-pharmacological and pharmacological measures for symptom alleviation [28] [29] .
Multicentric research was conducted in women who received medroxyprogesterone acetate (Depo-Provera) for contraception purposes, versus control group without the treatment. Significantly lower occurrence of breast pain (9%) figured out in women who used Depo-Provera versus control group (21%) [30] .
In double blind study, Winkler et al. treated 31 women with mastopathy with medrogesterone or dydrogesterone (10 mg/day) from 14th to 25th day of the cycle for 6 cycles. They concluded that cyclic use of low dose of progestin shown to be effective and reliable therapy for mastodynia and mastopathy. In more than 50% of patients it was found improvement of objective parameters . The improvement was especially noted in women with low levels of progesterone in the second half of the cycle. After 6 cycle therapy, 75% of patients treated with dydrogesterone and 86% of patients treated with medrogesterone did not have breast pain. Our study correlated with this data, since 60% of patients who used transdermal progesterone gel did not feel the breast pain and tension [31] .
Limitations of our study are the number of patients as well as lack of randomization in a prospective manner. Therefore, the same women were used as a control group (before the therapy) and as a study group (after the therapy).
Recently this type of fibrocystic breast disease and mastalgia treatment was very common in France. Some random control “cross-over” studies did not find such a significant improvement with this type of the therapy versus placebo group [32] . However, in double blind study in Italy it was noted that the use of micronized progesterone vaginal cream decreased breast pain by 64.9% in patients with mastalgia versus 22% in control group [33] .
Use of OC pills was not connected with benign proliferative breast disease unless there was a very low level of estrogen and progesterone in contraceptive pills in the range 20 - 30 mcg [10] [34] .
It was also important relative E2/P ratio, which has not been previously discussed in the studies. Study of Carbonaro concluded the same as earlier clinical trials, that in women who used hormonal contraceptive preparations there was no development of new benign breast changes nor worsening of preexisting, so the neutral effect was shown. However, there was a mastodynia reduction in patients who used OC for 36 months [35] . In our study, use of progesterone local therapy for 6 months showed no solid benign or malignant thickening, which was in accordance with the above mentioned research. Researchers agreed that natural estrogens and progesterone should be used due to less negative effect on breast.
5. Conclusion
During the local progesterone gel therapy mastalgia mastodynia, average size and cyst number, had significantly decreased and disappeared. The value of this study was paying attention on dynamic changes of E2/P ratio during the luteal phase, being the most important etiological factor for FBD. Including local progesterone gel, many unnecessary surgical cysts removal could be prevented. Understanding of FBD etiology showed completely new way of preserving women’s health.
Acknowledgements
The authors owe special thanks to Professor Svetlana Vujovic who generously helped with her experience and knowledge in creating and writing of this paper!
Conflicts of Interest
The authors declare that no conflicts of interest exist.
Funding
No funding has been received for the conduct of this study and/or preparation of this manuscript.
Ethical Approval
Research was conducted according to ethical principles, and was approved by The Ethics Committee Faculty of medicine, University of Belgrade in 2012, reference number 29/XI?8.
Cite this paper
Milena Brkic,Svetlana Vujovic,Maja Franic Ivanisevic,Miomira Ivovic,Milina Tancic Gajic,Ljiljana Marina,Marija Barac,Branko Barac,Alekasandar Djogo,Gabrijela Malesevic,Damir Franic,1 1,1 1, (2016) The Influence of Progesterone Gel Therapy in the Treatment of Fibrocystic Breast Disease. Open Journal of Obstetrics and Gynecology,06,334-341. doi: 10.4236/ojog.2016.65042
References
- 1. Marchant, D.J. (2002) Benign Breast Disease. Obstetrics & Gynecology Clinics of North America, 29, 1-20.
http://dx.doi.org/10.1016/S0889-8545(03)00048-2 - 2. Valea, F.A. and Katz, V.L. (2007) Breast Diseases: Diagnosis and Treatment of Benign and Malignant Disease. In: Katz, V.L., Lentz, G.M., Lobo, R.A., Gershenson, D.M., Eds., Comprehensive Gynecology, 5th Edition, Pa. Mosby Elsevier, Philadelphia.
http://dx.doi.org/10.1016/B978-0-323-02951-3.50018-2 - 3. Wipych, K., Kuzlik, R. and Wipyh, P. (2002) Hormonal Abnormalities in Women with Breast Cyst. Ginekologia Polska, 73, 1117-1125.
- 4. Walsh, P.V., Bulbrook, R.D., Stell, P.M., Wang, D.Y., McDicken, I.W. and George, W.D. (1984) Serum Progesterone Concentration during the Luteal Phase in Women with Benign Breast Disease. European Journal of Cancer and Clinical Oncology, 20, 1339-1343.
http://dx.doi.org/10.1016/0277-5379(84)90051-8 - 5. Katz, V.L. and Dotters, D. (2012) Breast Diseases: Diagnosis and Treatment of Benign and Malignant Disease. In: Katz, V.L., Lentz, G.M., Lobo, R.A., Gershenson, D.M., Eds., Comprehensive Gynecology, 6th Edition, Elsevier Mosby, Philadelphia.
- 6. Plu-Bureau, G., Le, M.G., Sitruk-Ware, B. and Thalabard, J.С. (2006) Cyclical Mastalgia and Breast Cancer Risk: Results of a French Cohort Study. Cancer Epidemiology, Biomarkers & Prevention, 15, 1229-1231.
http://dx.doi.org/10.1158/1055-9965.EPI-05-0745 - 7. Graham, J.D. and Clarke, C.L. (1997) Physiological Action of Progesterone in Target Tissues. Endocrine Reviews, 18, 502-519.
http://dx.doi.org/10.1210/er.18.4.502 - 8. Clarke, C.L. and Sutherland, R.L. (1990) Progestin Regulation of Cellular Proliferation. Endocrine Reviews, 11, 266-301.
http://dx.doi.org/10.1210/edrv-11-2-266 - 9. Yu, S., Lee, M., Shin, S. and Park, J.-S. (2001) Apoptosis Induced by Progesterone in Human Ovarian Cancer Cell Line SNU. Journal of Cellular Biochemistry, 82, 445-451.
http://dx.doi.org/10.1002/jcb.1171 - 10. Sitruk-Ware, R. and Plu-Bureau, G. (2000) Epidemiology and Treatment of Benign Breast Disease: Effect of Progestins on Breast Disease. In: Sitruk-Ware, R. and Mishelle Jr., D.R., Eds., Progestins and Antiprogestins in Clinical Practice, Marcel Dekker, New York, 327-340.
- 11. Vessey, M. and Yeates, D. (2007) Oral Contraceptives and Benign Breast Disease: An Update of Findings in a Large Cohort Study. Contraception, 76, 418-424.
http://dx.doi.org/10.1016/j.contraception.2007.08.011 - 12. Kumar, S., Mansel, R.E., Wilson, D.W., Read, G.F., Truran, P.L., Hughes, L.E. and Griffiths, K. (1986) Daily Salivary Progesterone Levels in Cyclical Mastalgia Patients and Their Controls. British Journal of Surgery, 73, 260-263.
http://dx.doi.org/10.1002/bjs.1800730406 - 13. Thomas, E.R., Abdissa, N., Chlebowski, R.T., Lasser, N.L., McTiernan, A., Schenken, R.S., et al. (2008) Estrogen plus Progestin and Risk of Benign Proliferative Breast Disease. Cancer Epidemiology, Biomarkers & Prevention, 17, 2337-2343.
http://dx.doi.org/10.1158/1055-9965.EPI-08-0380 - 14. Campagnoli, C., Clavel-Chapelon, F., Kaaks, R., Peris, C. and Berrino, F. (2005) Progestins and Progesterone in Hormone Replacement Therapy and the Risk of Breast Cancer. The Journal of Steroid Biochemistry and Molecular Biology, 96, 95-108.
http://dx.doi.org/10.1016/j.jsbmb.2005.02.014 - 15. Soderqvist, G., Isaksson, E., Von Schoulz, B., et al. (1997) Proliferation of Breast Epithelial Cells in Healthy Women during the Menstrual Cycle. American Journal of Obstetrics & Gynecology, 176, 123-128.
http://dx.doi.org/10.1016/S0002-9378(97)80024-5 - 16. Ferguson, D.J. and Anderson, T.J. (1981) Morphological Evaluation of Cell Turnover in Relation to the Menstrual Cycle in the “Resting” Human Breast. British Journal of Cancer, 44, 177-181.
http://dx.doi.org/10.1038/bjc.1981.168 - 17. Chang, K.J., Lee, T.T., Linares-Cruz, G., Fournier, S. and de Lignieres, B. (1995) Influences of Percutaneous Administration of Estradiol and Progesterone on Human Breast Epithelial Cell Cycle in Vivo. Fertility and Sterility, 63, 785-791.
- 18. Foidart, J.M., Colin, C., Denoo, X., Desreux, J., Béliard, A., Fournier, S. and de Lignières, B. (1998) Estradiol and Progesterone Regulate the Proliferation of Human Breast Epithelial Cells. Fertility and Sterility, 69, 963-969.
http://dx.doi.org/10.1016/S0015-0282(98)00042-9 - 19. Berg, W.A., Campassi, C.I. and Loffe, O.B. (2003) Cystic Lesions of the Breast: Sonographic-Pathologic Correlation. Radiology, 227, 183-191.
http://dx.doi.org/10.1148/radiol.2272020660 - 20. Venta, L.A., Kim, J.P., Pelloski, C.E. and Morrow, M. (1999) Management of Complex Breast Cysts. AJR American Journal of Roentgenology, 173, 1331-1336.
http://dx.doi.org/10.2214/ajr.173.5.10541113 - 21. Graf, O., Helbich, T.H., Fuchsjaeger, M.H., Hopf, G., Morgun, M., Graf, C., Mallek, R. and Sickles, E.A. (2004) Follow-Up of Palpable Circumscribed Noncalcified Solid Breast Masses at Mammography and US: Can Biopsy Be Averted? Radiology, 233, 850-856.
http://dx.doi.org/10.1148/radiol.2333031845 - 22. Doshi, D.J., March, D.E., Crisi, G.M. and Coughlin, B.F. (2007) Complex Cystic Breast Masses: Diagnostic Approach and Imaging-Pathologic Correlation. Radiographics, 27, S53-S64.
http://dx.doi.org/10.1148/rg.27si075508 - 23. Sitruk-Ware, R., Seradour, B. and Lafaye, C. (1980) Treatment of Benign Breast Disease by Progesterone Applied Topically. In: Mauvais-Jarvis, P., Vickers, C.F. and Wepierre, J., Eds., Percutaneous Absorption of Steroids, Academic Press, London, 219-229.
- 24. Mauvais-Jarvis, P., Kuttenn, F. and Gompel, A. (1986) Antiestrogen Action of Progesterone in Breast Tissue. Breast Cancer Research and Treatment, 8, 179-188.
http://dx.doi.org/10.1007/BF01807330 - 25. Ader, D.N., South-Paul, J., Adera, T. and Deuster, P.A. (2001) Cyclical Mastalgia: Prevalence and Associated Health and Behavioral Factors. Journal of Psychosomatic Obstetrics & Gynecology, 22, 71-76.
http://dx.doi.org/10.3109/01674820109049956 - 26. Smith, R.L., Pruthi, S. and Fitzpatrick, L.A. (2004) Evaluation and Management of Breast Pain. Mayo Clinic Proceedings, 79, 353-372.
http://dx.doi.org/10.4065/79.3.353 - 27. Tihomirov, A.L. and Lubnin, D.M. (2003) Mastopathia and Mastodynia. Trudni Pacient, 4, 29-30. (In Russian)
- 28. Khalid, R.M. (2011) A Review of Mastalgia in Patients with Fibrocystic Breast Changes and the Non-Surgical Treatment Options. Journal of Taibah University Medical Sciences, 6, 1-18.
http://dx.doi.org/10.1016/S1658-3612(11)70151-2 - 29. Davies, E.L., Gateley, C.A., Miers, M. and Mansel, R.E. (1998) The Long-Term Course of Mastalgia. Journal of the Royal Society of Medicine, 91, 462-464.
- 30. Euhus, D.M. and Uyehara, C. (1997) Influence Parenteral Progesterones on the Prevalence and Severity of Mastalagia in Premenopausal Women: A Multi-Institutional Cross-Sectional Study. Journal of the American College of Surgeons, 184, 596-604.
- 31. Winkler, U.H., Schindler, A.E., Brinkmann, U.S., Ebert, C. and Oberhoff, C. (2001) Cyclic Progestin Therapy for the Management of Mastopathy and Mastodynia. Gynecological Endocrinology, 15, 37-43.
http://dx.doi.org/10.1080/gye.15.s6.37.43 - 32. McFadyen, I.J., Raab, G.M., Macintyre, C.C. and Forrest, A.P. (1989) Progesterone Cream for Cyclic Breast Pain. BMJ, 298, 931.
http://www.ncbi.nlm.nih.gov/pubmed/2497864
http://dx.doi.org/10.1136/bmj.298.6678.931 - 33. Nappi, C., Affinito, P., Di Carlo, C., Esposito, G. and Montemagno, U. (1992) Double Blind Controlled Trial of Progesterone Vaginal Cream Treatment for Cyclical Mastalgia in Women with Benign Breast Disease. Journal of Endocrinological Investigation, 15, 801-806.
http://dx.doi.org/10.1007/BF03348808 - 34. Rohan, T.E. and Miller, A.B. (1999) A Cohort Study of Oral Contraceptive Use and Risk of Benign Breast Disease. International Journal of Cancer, 82, 191-196.
http://dx.doi.org/10.1002/(SICI)1097-0215(19990719)82:2<191::AID-IJC7>3.0.CO;2-F - 35. Carbonaro, A., Ciotta, L., Stracquadanio, M., Formuso, C., Giunta, M.R. and Agati, A.D. (2012) Oral Contraception and Benign Breast Diseases. American Journal of Nursing Science, 1, 1-4.
http://dx.doi.org/10.11648/j.ajns.20120101.11
NOTES

*Corresponding author.