Open Journal of Psychiatry
Vol. 2 No. 3 (2012) , Article ID: 21339 , 6 pages DOI:10.4236/ojpsych.2012.23032
Dendritic and spine alterations in areas 9 and 17 in schizophrenia and Huntington chorea and the role of neuroleptic exposure
![]()
1Department of Biology/MLT, Bronx Community College, New York, USA
2Department of Biological Sciences, Lehman College, New York, USA
Email: *latchman.somenarain@bcc.cuny.edu, liesl.jones@lehman.cuny.edu
Received 25 May 2012; revised 29 June 2012; accepted 7 July 2012
Keywords: Schizophrenia; Huntington; Neuroleptic; Spines; Dendrites; Pyramidal cells; Prefrontal Cortex; Area 9 and Area 17
ABSTRACT
Recent morphological studies in schizophrenia suggest atrophic changes in the neuropil of the prefrontal cortex. Most recently, we showed a schizophreniaassociated decrease in MAP2 in schizophrenia, which we believed is not due to neuroleptic exposure. MAP2 is a very important protein in the assembly of microtubule in neurons; therefore, it plays a major role in neuronal processes like dendrites, spines and synapses. Additionally, recent studies from our lab showed decreases in dendrites in area 32 and area 9. In this study we examined the dendrites and spines in area 9 and 17 to determine if neuroleptic drugs play a role. Huntington’s patients take neuroleptics similar to schizophrenics; therefore, by comparing the two groups to controls we can determine if neuroleptics play a role in the deficits reported in schizophrenia. Our results showed a significant decrease in both basal dendrites and spines for both layers III and V in area 9 in schizophrenia compared to controls. The Huntington’s brains, on the other hand, showed no significant difference compared to controls. In area 17, there was also no significant difference when comparing the three groups. The data suggest that neuroleptic drugs may not be responsible for the changes observed in schizophrenia.
1. INTRODUCTION
The neuroanatomical basis of schizophrenia is still a mystery to researchers. However, much research over past few decades were designed to examine various cortical areas looking at blood flow in the brain; differential neuronal counts and density; neuronal size and volume; dendritic architecture; spine counts; transmitter systems; and several structural and synaptic proteins [1-16]. As a result, much is known about the cortex of the brain. There are two types of neurons in the cortex, pyramidal and non-pyramidal neurons. The pyramidal neurons constitute approximately 70% of the cortical neurons and the non-pyramidal neurons account for about 25% [17]. The pyramidal neurons are named for their pyramidal shape cell body; they have an apical dendrite extending towards the pial layer as well as basal dendrites on the base of the cell body. Spines are protrusions of the neuronal membrane consisting of a head connected to the neuron by a thin spine neck (Figure 1). They can be found on the dendrites, the soma and on the axon hillock [18].

Figure 1. Micrograph showing spines on the basal dendrites of a pyramidal cell. Scale bar = 0.01 mm.
Spines are the site of synaptic transmission and about 90% of the synapses on spines are excitatory [18]. There are three types of spines: mushroom, stubby and filopodium [19]. In the DLPFC in schizophrenia, structural imaging studies suggest a decrease in volume in this part of the brain [20,21]; whereas, most neuron density measurements showed an increase in neuronal density without a change in the total number of neurons [21]. Thus, researchers theorized that there are changes within the neuropil. Loss of spines, dendrites and axons would strongly suggest altered connectivity in patients with schizophrenia. Previously we reported a decrease in MAP2 in area 9 and 32 in schizophrenics, which we believe is not due to neuroleptic exposure [11,15,16]. We believe that this decrease in MAP2 in schizophrenia will result in consequences reflected in the cytoskeletal processes of the pyramidal cells, like the dendrites and spines. Our lab had previously reported decreases in dendrites in area 32 of the prefrontal cortex [14] and the issue of neuroleptic exposure remains unanswered.
This study employs Golgi silver Impregnation to analyze the dendrites and spines of the pyramidal cells in area 9 of the PFC. We employed a modify Golgi-Cox method by Armstrong and Parker and counted basal dendrites and spines on pyramidal cells in area 9 layers III and V in cohorts of Huntington Chorea, schizophrenia and control. Both Huntington’s patients and Schizophrenics take similar neuroleptic drugs; therefore, by comparing the two groups’ one can begin to see if neuroleptics play a role in the changes observed in schizophrenia.
2. METHODS
2.1. Subjects and Tissue
Postmortem brain tissues from 5 subjects diagnosed with Huntington’s chorea, 5 subjects diagnosed with schizophrenia and 5 controls were collected from The Harvard Brain Tissue Resource Center (HBTRC). Tissues from all three groups were matched for age, sex and postmortem interval (Table 1). All tissues were collected at the time of autopsy in accordance with Partners Healthcare System Institutional Review Board protocol. Diagnoses conformed to DSM-IV criteria and were established retrospectively by reviewing medical records and interviewing knowledgeable individuals. Tissue was not included in the study if there was evidence of neuropathology (e.g. Alzheimer’s disease) or substance dependence as determined by the HBTRC. The brain bank coded the tissues and the codes were maintained by one of the authors who did not conduct quantitative assessment (L.J.) until quantitative procedures had been completed.
Blocks of 1 cm thick sections were dissected in the
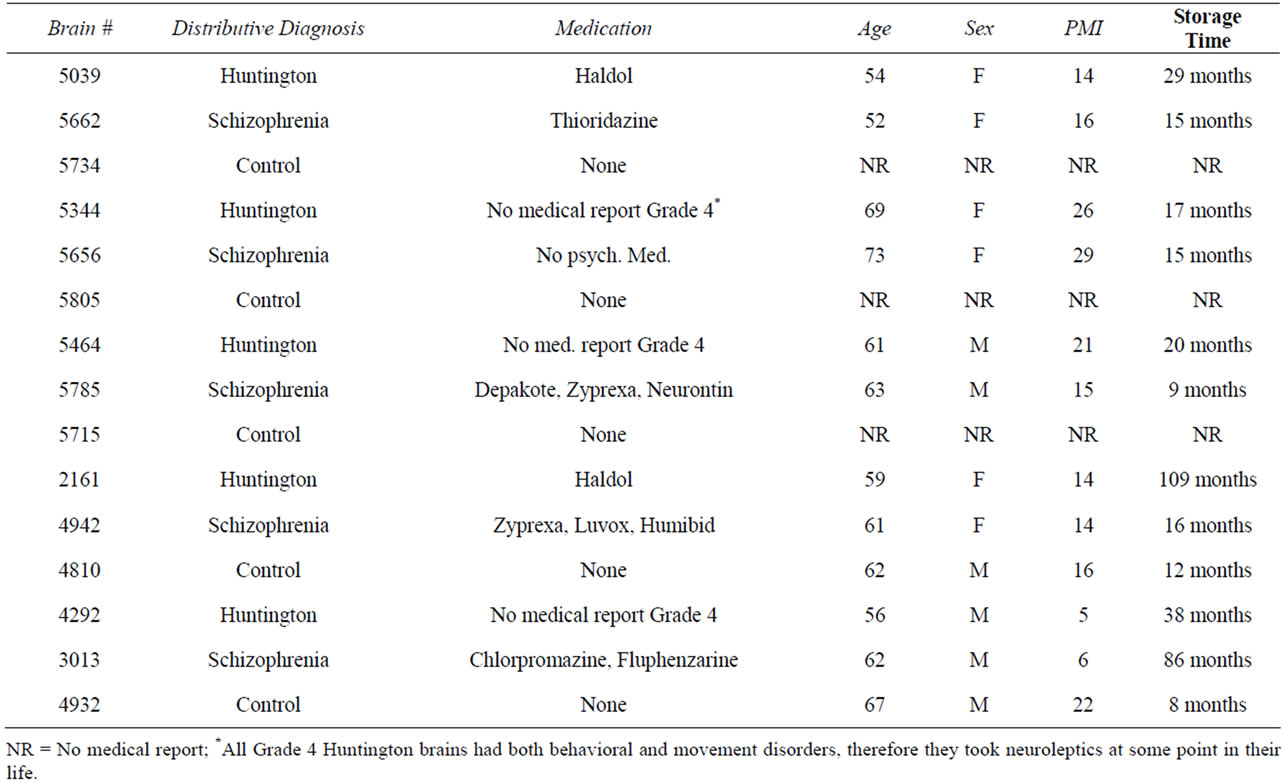
Table 1. Demographic data for the matched brains used in the Golgi projects.
coronal plane by a qualified neuropathologist at the HBTRC and formalin-fixed in a consistent manner for shipment. The accuracy of the dissections was confirmed by examining thionin-stained sections prepared from each block. Specimens from area 9 were taken from its dorsolateral extent, which is found on the middle third of the superior frontal gyrus. Compared with the medial region, the lateral region of area 9 is more differentiated. In addition to having a distinguishable layer IV, it has a Layer II with a distinct upper margin and the supragranular cell density is conspicuously higher than the infragranular cell density. Area 17 is situated on the banks of the calcarine sulcus and is unambiguously identified by the presence of the line of Genari which can be seen with the naked eye.
Layers III and V in both areas 9 and 17 were selected to be studied. Layer III pyramidal neurons are the corticocortical projections; they play a critical role in information processing such as working memory. Layer V pyramidal neurons are the main projection cells from the cortex to other subcortical and cortical areas; therefore changes in information in one cortical area could affect many brain regions.
2.2. Golgi Silver Impregnation
The brains were processed in groups (control, schizophrenia and Huntington). The tissue was cut into 2 mm slabs then wrapped in gauze. All of the glassware used in this experiment was acid washed. The tissue was first placed in a solution containing 100 ml dH2O, 3 g potassium dichromate, 12.5 g sucrose, 5 ml 37% formalin, and 7.5 ml of 3% H2O2 and incubated in an oven at 55˚C for 6 hours. The tissue was removed from the oven and placed in fresh solution and left at room temperature overnight. The following day the tissue was placed in a second solution containing 100 ml dH2O, 3 g potassium dichromate and 12.5 g sucrose and incubated in an oven at 55˚C for 6 hours. Following the incubation, the tissue was placed in fresh solution and left again at room temperature overnight. The tissue was then rinsed in 0.75% silver nitrate and placed in fresh 0.75% silver nitrate solution. Because silver nitrate is light sensitive, the jars were wrapped in aluminum foil and left at room temperature for 5 days. Finally the tissue was serially sectioned on a vibratome into 150 μm thick sections and collected in 70% alcohol. The sections were dehydrated in graded alcohol starting at 70%, defatted in xylene, mounted onto slides and coverslipped with permount solution.
2.3. Quantification of Dendrites and Spines
The material was analyzed using a Bioquant Image Analysis System interfaced with an Olympus AX70 microscope connected to a Ludl Motorized stage and a Sony 3-chip camera. Three measurements were performed on Golgi material, 1) number of primary basilary dendrites, 2) number of secondary basilary dendrites and 3) spine density along the primary and secondary basilary branches. Fifty cells per brain were chosen randomly to be analyzed for dendritic measurements and ten cells per brain for the spine measurements. The cells must meet the following criteria to be analyzed: the cell body must be easily seen with no secondary branches being obscured by background or by another cell and the apical dendrite must be visible from the soma to the pial surface. All measurements were made using stereologic methods. The Bioquant system comes with a stereologic package that was used to make measurements. The total area, that contains cells, was traced. A 100/100 grid was placed within the contour. The computer randomly marked grids to be measured, a counting box with a set z range was placed inside the marked grids and the cells within the grid which meet criteria were measured. Two different measurements were made for each cell at one time. The numbers of primary and secondary basilar branches were counted and an average for each brain was determined. The counting of spines along the primary and secondary basilary dendrites was done at 100× under oil. Ten cells per brain were chosen randomly. Dendrites whose cell meets criteria were measured for their length in microns at 60× under oil and have their spines counted manually at 100×. By moving through the focal plane we counted all of the spines visible along the dendrite using a hand counter. In addition, the numbers of spines counted were entered manually into the Bioquant to keep track of the number of spines counted. The data was expressed as average number of spines per millimeter of dendrite in each lamina in the different brain regions.
2.4. Statistical Analysis
Standard descriptive statistics were used in all analysis. Calculations and data analysis were carried out using StatView (V5.0) for Windows (Abacus Concepts Software/SAS). Dendritic and spine data were taken from layers III and V of areas 9 and 17 and were expressed as means for each of the Huntington, schizophrenia and control groups. A one-way analysis of variance (ANOVA) was used to test for differences between groups at 95% confidence limit. All of the tissues were prepared and analyzed in matched groups; therefore, we believe that the ANOVA is a more sensitive test for intergroup comparison. Additionally, a power analysis of the spine data showed that a minimum sample size of five was enough to yield levels of significance with a 95% confidence limit. Similar studies by [5] for spine density measurements used a sample size of four schizophrenics and a MAP2 study by [22] used only six brains.
3. RESULTS
3.1. Dendrites and Spines in Area 9
The number of dendrites and spines were obtained from 5 Huntington, 5 schizophrenic and 5 controls in both layer III and V. The data is expressed as average number of dendrites per brain. Compared to controls the schizophrenic brains showed a 33% decrease (P-value 0.007, SD ± 0.5) in the primary dendrites in layer III and a 32% decrease (P-value 0.01, SD ± 0.67) in layer V (Table 2). Compared to controls the Huntington brains showed no significant changes in the primary dendrites in both layers III and V (Table 2). For the secondary dendrites, both layers III and V in schizophrenia showed a trend towards a decrease compared to controls. There were no significant differences seen in Huntington’s brains compared to controls in the secondary dendrites for both layers III and V.
The spine data is expressed as number of spines per millimeter of dendrite.
Compared to controls the schizophrenic brains exhibited a 37% decrease (P-value 0.0001, SD ± 32) in primary spines in layer III and a 38% decrease (P-value, SD ± 31) in layer V (Table 3). The Huntington brains showed no significant changes in primary spines in both layers III and V compared to controls (Table 3). For the secondary spines, the schizophrenic brains the exhibited a 37% decrease (P-value, SD ± 39) in layer III and a 37% decrease (P-value, SD ± 58) in layer V compared to controls (Table 4). The Huntington brains showed no

Table 2. Golgi data showing means and P-value for primary dendrites in layers III and V in area 9 (n = 5).

Table 3. Spine counts showing means and P-value for primary spine counts per millimeter of dendrite in layers III and V in area 9 (n = 5).
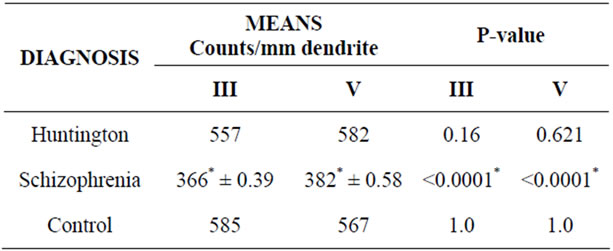
Table 4. Spine data showing means and P-value for secondary spines counts/mm dendrite in layers III and V in area 9 (n = 5).
significant changes for secondary spines in both layers III and V compared to controls (Table 4).
3.2. Dendrites and Spines in Control (Area 17)
The number of primary and secondary basilary dendrites was obtained from five Huntington, five schizophrenic and five controls in both layer III and V in area 17. The data is expressed as number of dendrites per brain. In both layers III and V there were no significant difference for primary and secondary dendrites in Huntington and schizophrenia compared to controls. Similarly, there was no significant difference in primary and secondary spines in both layers for Huntington and schizophrenia compared to controls.
4. DISCUSSION
The primary dendritic data in area 9 in both layers III and V showed a significant decrease in schizophrenia compared to controls; whereas, there was a trend towards a decrease in the secondary basilary dendrites. We believe that these data support what was reported in area 32, where a decrease in primary and secondary basilary dendrites was reported [11]. Furthermore, this decrease in dendrites directly correlates with the decrease in MAP2 [11]. Because MAP2 is involved in the cytoskeletal processes of the cell, a decrease will most likely impact the dendritic processes of the pyramidal cells. The Huntington’s brains on the other hand, did not show any significant difference in their dendritic processes compared to the control brains. Similarly, the spine data suggest that there is a schizophrenia-associated decrease in spines in area 9, which support what was reported by [5, 8]. There was also no significant difference in spine counts in the Huntington brains compared to controls. Together, because we are not seeing any difference between Huntington and controls suggest that neuroleptic drugs might not be responsible for the dendritic and spine alterations seen in schizophrenia.
Neuroleptics drugs, because of their mode of action on metabotropic neurotransmitter receptors, can phosphorylate proteins like MAP2 [23]. Thus, they have the ability to alter neuronal processes such as dendrites and spines. Both Huntington and Schizophrenic patients take comparable dosages of about 407 chlorpromazine equivalents per day and for at least two years of continuous use, which is consider long term usage [24]. We believe that this data support the hypothesis that neuroleptic drugs are not responsible for the dendritic and spine alterations seen in schizophrenia and that they are due to the disease.
The loss of dendritic spines on pyramidal cells in schizophrenia has several implications. The pyramidal cells in the PFC are glutamatergic and they project between various cortical regions; whereas, layer V pyramidal cells project to various cortical areas as well as subcortical areas. A loss of spines reflects a loss of excitatory input to these neurons; therefore, it is expected that cognitive information processing might be disturbed as seen in schizophrenia. This is consistent with previous reports of decrease synaptophysin, a 38-kd integral membrane protein of small synaptic vesicles, which is important in calcium-dependent synaptic transmission [4]. Moreover, the spines of basal dendrites receive both dopamine and glutamate afferents [25]; thus, this give credence to possible disturbances in these transmitter systems in schizophrenia. Additionally, the basal dendrites are the site for afferents from the MD nucleus of the thalamus, an area of the brain that has consistently shown neuroanatomical deficits in schizophrenia [26-33]. The data further support the hypothesis that neuroleptic drugs might not be responsible for the decrease in spines seen in schizophrenia since there were no significant differences in the Huntington spine counts and the control spine counts. Moreover, in area 17, there were no changes seen in dendritic or spine measurements in Huntington and schizophrenia compared to control in both layers III or V. Together, these data provide additional insightful information for both Huntington’s disease and schizophrenia.
REFERENCES
- Pakkenberg, B. (1993) Total nerve cell number in neocortex in chronic schizophrenics and controls estimated using optical dissectors. Biological Psychiatry, 34, 768-772. doi:10.1016/0006-3223(93)90065-L
- Goldman-Rakic, P.S. and Selemon, L.D. (1997) Functional and anatomical aspects of prefrontal pathology in schizophrenia. Schizophrenia Bulletin, 23, 437-458. doi:10.1093/schbul/23.3.437
- Beasley, C.L. and Reynolds, G.P. (1997) Parvalbuminimmunoreactive neurons are reduced in the prefrontal cortex of schizophrenic. Schizophrenia Research, 24, 349-355. doi:10.1016/S0920-9964(96)00122-3
- Glantz L.A. and Lewis, D.A. (1997) Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Archives of General Psychiatry, 54, 943-952. doi:10.1001/archpsyc.1997.01830220065010
- Garey, L.J., Ong, W.Y., Patel, T.S., Kanani, M., Davis, A. and Mortimer, A.M. (1998) Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of Neurology Neurosurgery Psychiatry, 65, 446-453. doi:10.1136/jnnp.65.4.446
- Selemon, L.D. and Goldman-Rakic, P.S. (1999) The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biological Psychiatry, 45, 17-25. doi:10.1016/S0006-3223(98)00281-9
- Buxhoeveden, D., Ror, E. and Switala, A. (2000) Reduced interneuronal space in schizophrenia. Biological Psychiatry, 47, 681. doi:10.1016/S0006-3223(99)00275-9
- Glantz, A.L. and Lewis, D.A. (2000) Decrease dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of General Psychiatry, 57, 65-73. doi:10.1001/archpsyc.57.1.65
- Andreasen, N.C. (2000) Schizophrenia: The fundamental questions. Brain Research Review, 31, 106-112. doi:10.1016/S0165-0173(99)00027-2
- Thune, J.J., Uylings, H.B.M. and Pakkenberg, B. (2001) No deficit in total number of neurons in the prefrontal cortex in schizophrenics. Journal of Psychiatric Research, 35, 15-21. doi:10.1016/S0022-3956(00)00043-1
- Jones, L., Johnson, N. and Byne, W. (2002) Alterations in MAP2 immunocytochemistry in area 9 and 32 of schizophrenic prefrontal cortex. Psychiatry Research Neuroimaging, 9, 3621-3625.
- Broadbelt, K. and Jones, L.B. (2008) Evidence of altered calmodulin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. Journal of Psychiatric Research, 42, 612-621. doi:10.1016/j.jpsychires.2007.07.006
- Broadbelt, K., Rampersaud, A. and Jones, L.B. (2006) Evidence of altered neurogranin immunoreactivity in areas 9 and 32 of schizophrenic prefrontal cortex. Schizophrenia Research, 87, 6-14. doi:10.1016/j.schres.2006.04.028
- Broadbelt, K., Byne, W.B. and Jones, L.B. (2002) Evidence for a decrease in primary and secondary basilar dendrites on pyramidal cells in area 32 of schizophrenic prefrontal cortex. Schizophrenia Research, 58, 75-81. doi:10.1016/S0920-9964(02)00201-3
- Somenarain, L. and Jones, L. (2010) A comparative study of MAP2 immunostaining in areas 9 and 17 in schizophrenia and Huntington chorea. Journal of Psychiatric Research, 44, 694-699. doi:10.1016/j.jpsychires.2009.12.006
- Somenarain, L. (2011) Neuropathology of the prefrontal cortex neuropil in schizophrenia. Psychiatric Disorders Trends and Developments, Intech, 1-18.
- Powell, T.P.S. (1981) Certain aspects of the intrinsic organization of the cerebral cortex. Raven Press, New York, 1-19.
- Mates, S.L. and Lund, J.S. (1983) Spine formation and maturation of type I synapses on spiny stelate neurons in primary visual cortex. Journal of Comparative Neurology, 221, 91-97. doi:10.1002/cne.902210107
- Peters, A. and Kaiserman-Abramof, I.R. (1970) The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. American Journal of Anatomy, 127, 321-355. doi:10.1002/aja.1001270402
- Daviss, S.R. and Lewis, D.A. (1995) Local circuit neurons of the prefrontal cortex in schizophrenia: Selective increase in the density of calbindin-immunoreactive neurons. Psychiatric Research, 59, 81-96. doi:10.1016/0165-1781(95)02720-3
- Selemon, L.D., Rajkowska, G. and Goldman-Rakic, P.S. (1995) Abnormally high neuronal density in the schizophrenic cortex: A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry, 52, 805-818. doi:10.1001/archpsyc.1995.03950220015005
- Arnold, S.E., Hyman, B.T., Van Hoesen, G.W. and Damasio, A.R. (1991) Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Archives of General Psychiatry, 48, 625-632. doi:10.1001/archpsyc.1991.01810310043008
- Lidow, M.S., Songa, Z., Castnerb, S.A., Allenc, P.B., Greengarde, P. and Goldman-Rakic, P.S. (2001) Antipsychotic treatment induces alterations in dendrite and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biological Psychiatry, 49, 1-12. doi:10.1016/S0006-3223(00)01058-1
- Gur, R.E., Maany, V., Mozley, P.D., Swanson, C., Bilker, W. and Gur, R.C. (1998) Subcortical MRI volumes in neuroleptic-naïve and treated patients with schizophrenia. American Journal of Psychiatry, 155, 1711-1717.
- Smiley, J.F., Williams, S.M. and Szigeti, K. (1992) Light electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. Comparative Neurology, 321, 325-335. doi:10.1002/cne.903210302
- Andreasen, N.C., Arndt, S., Swayze, V., Cizadlo, T., Flaum, M. and O’Leary, D. (1994) Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science, 266, 294-298. doi:10.1126/science.7939669
- Buchsbaum, M.S., Someya, T., Teng, C.Y., Abel, L., Chin, S. and Najafi, A. (1996) PET and MRI of the thalamus in never-medicated patients with schizophrenia. American Journal of Psychiatry, 153, 191-199.
- Byne, W., Jones, L.B., Kemether, E., Haroutunian, V. and Davis, K.L. (1997) Towards localization of thalamic pathology in schizophrenia. APA Abstract, NR544.
- Byne, W., Buchsbaum, M.S., Kemether, E., Hazlett, E.A., Shinwari, A., Mitropoulou, V. and Siever, L.J. (2001) Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Archives of General Psychiatry, 58, 133-140. doi:10.1001/archpsyc.58.2.133
- Byne, W., Buchsbaum, M.S., Mattiace, L. A., Hazlett, A., Kemether, E., Elhakem, S.L., Purohit, D.P., Haroutunian, V. and Jones, L. (2002) Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. American Journal of Psychiatry, 159, 59-65. doi:10.1176/appi.ajp.159.1.59
- Jones, G.E. (1997) Cortical development and thalamic pathology in schizophrenia. Schizophrenia, 23, 483-501. doi:10.1093/schbul/23.3.483
- Popken, J.G., Bunney, E.W., Potkin, G.S. and Jones, G.E. (2000) Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proceedings of the National Academy of Sciences USA, 97, 9276-9280. doi:10.1073/pnas.150243397
- Young, K., Manaye, K.F., Liang, C., Hicks, P.B. and German, D.C. (2000) Reduced number of MD and anterior thalamic neurons in schizophrenia. Biological Psychiatry, 47, 944-953. doi:10.1016/S0006-3223(00)00826-X
NOTES
*Corresponding author.

