Advances in Biological Chemistry
Vol.4 No.1(2014), Article ID:43304,12 pages DOI:10.4236/abc.2014.41010
Responses of bioenergy sorghum cell wall metabolism to agronomic practices
![]()
1Department of Soil and Crop Sciences, Texas A&M University, College Station, USA
2Biochemistry Laboratory, Cooperative Agricultural Research Center, Prairie View A&M University, Prairie View, USA
Email: *goosuji@pvamu.edu
Copyright © 2014 Jason P. Wight et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Jason P. Wight et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received 9 December 2013; revised 13 January 2014; accepted 21 January 2014
KEYWORDS:Peroxidase Enzymology; Free Solution Isoelectric Focusing; Lignin and Cellulose; Biochemical Mechanism of Crop Rotation
ABSTRACT
Maximum lignocellulose yield of biomass sorghum [Sorghum bicolor L. (Moench.)] is hampered by complex biological phenomena related to rotation, nitrogen (N) fertilization, soil tillage, and excessive biomass removal. The biochemical basis of the effects of agronomic practices on sorghum production was studied by the enzymology of the active peroxidase (EC 1.11.1.7) isoenzymes that synthesize lignin. All studied practices altered the peroxidase pI values. Control sorghum without rotation and without N fertilization had the most inhibited peroxidase with very low maximum velocity (Vmax) value (3.10 mmol·min−1), and very low lignin (857 kg·ha−1) yield, which could decrease soil organic carbon possibly leading to adverse changes in soil chemistry. Corn-sorghum rotations with and without N fertilization increased the Vmax values of peroxidase and lignin and cellulose yields. Rotated sorghum subjected to 50% residue return (the percentage of crop residue was returned to the plot immediately after grinding at harvest) and 280 kg·ha−1 N fertilization possessed very active peroxidase (Vmax value 66.4 mmole·min−1) and the highest lignin (1387 kg·ha−1) yield. The 25% residue return rate without N fertilization induced high lignin (1125 kg·ha−1) and cellulose (11,961 kg·ha−1) but the 25% residue return rate with 280 kg·ha−1 N fertilization induced lower lignin (1046 kg·ha−1) yield. Continuously cropped sorghum treated with 336 kg·N·ha−1 produced active peroxidase that shared competitive inhibition relationship with the peroxidase of the 84 kg·N·ha−1 treatment. Ridge tillage combined with 280 kg·ha−1 N fertilization under continuous sorghum resulted in inhibited peroxidase possessing low Vmax value (13.0 µmole·min−1). Changing to conventional tillage combined with 280 kg·ha−1 N fertilization relieved the inhibition and increased the Vmax value to 23.7 mmol·min−1. These biological anomalies of sorghum cell wall related to agronomic practices originated from doubly inhibited sorghum peroxidases. This understanding may guide the choice of sustainable agronomic practices for maximizing lignocellulose yields for the bioenergy industry while protecting the environment.
1. INTRODUCTION
Bioenergy (biomass) sorghum is a novel second-generation energy crop [1] that is particularly suited to bioenergy production because it produces large quantities of biomass and also large quantities of carbohydrates that can be readily converted to ethanol. Currently, sorghum is cultivated on 2.6 million acres annually in Texas, accounting for ~28% of all sorghum acreage in the USA [2]. Biomass sorghum has yielded three times more aerial dry material than similar corn-based systems, but also depleted soil macronutrients more than corn [3,4]. Therefore, sorghum production has the potential to affect soil chemistry. Other complex biological changes surrounding biomass sorghum production include the observations that in monoculture, biomass sorghum exhibited greater soil carbon and total nitrogen sequesteration than when in biannual rotation with corn [3], and that excessive removal of biomass substantially decreased subsequent biomass (cell wall) yield and soil quality [5]. Agronomic practices that impede biomass sorghum cell wall biology need to be better understood so that production can be efficiently increased as the bioenergy industry expands and demands more biomass sorghum as feedstock [6]. Very little biochemical information is available about the responses of biomass sorghum cell wall metabolism to rotation with corn, continuous monoculture sorghum, residue return (where the designated percentage of crop residue was returned to the plot immediately after weighing and grinding at harvest), nitrogen (N) fertilization rates, soil tillage intensity and combinations thereof. Although crop rotation generally increases crop yield and soil carbon sequestration [7-9], the biochemical mechanisms of the responses have not been reported. Over-application of fertilizer reduces farmers’ economic margin [10], and nitrogen fertilizer when improperly managed has the potential to increase environmental degradation [11]. A better understanding of sorghum’s biochemistry could help optimize production management practices and lignocellulose yield, while minimizing costs of bioenergy production, protecting the soil from erosion [12], improving soil carbon content and water retention [13], enhancing nutrient cycling and possibly reducing global greenhouse gas emissions. An all encompassing enzymatic denominator for evaluating the responses of lignocellulose yield to the natural environment could help explain the complex biological changes surrounding biomass sorghum production and quality.
The purification and determination of active peroxidase isoenzymes [14] opens the way for broad-based studies on responses of crop cell wall metabolism to the biological environment. Peroxidases (EC 1.11.1.7) are a multi-isoenzymatic family of cell wall-bound oxidoreductase enzymes that participate in lignin biosynthesis [15-17] and stiffening of the cell wall. Since the lignin of crop residues increases soil organic carbon content because it is slow to degrade [18], agronomic management practices that enhance peroxidase activity might improve both biomass yield and soil carbon sequestration; whereas those that inactivate the enzyme might minimize lignin contents of crop residues, thereby resulting in less carbon sequesteration or possibly even promoting soil organic carbon depletion with time. However, only about 30% of sorghum peroxidase isoenzymes are active [14]. This lowered activity assures moderate levels of lignin in sorghum as higher levels could be detrimental to conversion to bio-ethanol. Inability to distinguish active from inactive isoenzymes has hampered the utility of this enzyme as a general purpose biochemical tool for studying the responses of cell wall biology to the plant’s environment. The overall objective of this project was to determine the common biochemical responses induced by agronomic management practices (environment) on lignocellulose (cell wall) productivity and potential sustainability of biomass sorghum production. The enzyme kinetics responses of active peroxidase isoenzymes to residue return, crop rotation, nitrogen fertilization rate, and soil tillage practices in relation to lignocellulose yields of biomass sorghum were determined.
2. MATERIALS AND METHODS
Agronomic management changes: The experiment used a combination of three sub-experiments, each being a randomized block design. Study 1 consisted of three treatment factors: crop rotation, nitrogen fertilizer rate, and residue return (where the designated percentage of crop residue was returned to the plot immediately after weighing and grinding at harvest). Crop sequences were either continuous bioenergy sorghum or biannual rotation with corn. The agronomic systems were originally implemented in 2008. Sorghum was planted in the continuous sorghum treatments each spring, while corn was planted in the rotation treatment in spring of 2008 and 2010. Nitrogen was applied at 0, or a non-limiting rate of 280 kg·N·ha−1. Nitrogen application to corn was either 0 or 168 kg·N·ha−1. Residue return rates were 0%, 25%, or 50% of sorghum biomass yield at harvest or a treatment of 100% mineral nutrients in aerial biomass being returned to the soil. These 100% mineral nutrient return plots also received the non-limiting N rate. All corn stover was returned. Each possible combination of residue return, fertilization, and crop rotation was replicated four times. Study 2 consisted of four N fertilizer rates: 0, 84, 252, and 336 kg·ha−1. Study 3 consisted of plots receiving N fertilizer at 280 kg·ha−1 and either conventional tillage or ridge-till, which is a form of conservation tillage where only interrows were tilled as needed to maintain planting beds. Nitrogen as urea was subsurface banded 15-cm deep approximately 6 weeks after planting at the 4-to-5-leaf stage for sorghum and the 6-to-8-leaf stage for corn.
Plots were 9.14-m long, four rows wide and planted on 102-cm row centers. The bioenergy sorghum used was “4-Ever Green”, a modern photoperiod-sensitive, onecross hybrid with high biomass yield and low lodging potentials (Walter Moss Seed Co, Waco, TX, USA). Planting dates ranged from late March to late April, with a seeding rate of 65,000 seed ha−1. Bioenergy sorghum and corn were managed under conventional disk tillage in studies 1 and 2. After the harvest each year, plots that were not under ridge-till were disked three times to a depth of 15 to 20 cm, and bedded. Furrow irrigation was minimally performed as needed.
Biomass sorghum plots were harvested each year in September or October using a New Holland model 707U forage chopper (New Holland, PA, USA) and cut to leave a short stubble height (ca. 10 cm). For determination of yield, the harvest from the two inner rows of each plot were weighed using a weigh bucket and Avery Weigh-Tronix model RD40RF scale indicator (Illinois Tool Works Inc., Glenview, IL, USA) that were coupled with the harvester. Residue corresponding to 25% and 50% of harvested biomass was returned to appropriate plots after each harvest. Water content of aerial plant biomass from each plot was determined by randomly selecting 5 plants, chopping in a commercial chipper/ shredder, and taking an approximately 600-g subsample. Subsamples were weighed, oven dried at 60˚C for 7 days, and then re-weighed to determine moisture content. For peroxidase assay, a sample of sorghum leaf tissue was taken immediately prior to mechanical harvest in September 2011. Leaf tissue samples were taken by randomly selecting the youngest fully mature leaf from five sorghum plants within each plot. These entire five leaves were removed, placed in a sealed storage container, and immediately brought to −200˚C by submersion of the sample container in liquid nitrogen. Peroxidase purification and assays were completed in <6 weeks after sorghum leaf samples were stored at −80˚C.
Purification and assay of peroxidase: Peroxidase was extracted from sorghum leaves (50 g) by high speed homogenization with 100 mL of ice-cold 50 mM Na2HPO4 buffer pH 6.0 containing 25% polyvinylpyrrolidone (w/v) and 0.1% β-mercaptoethanol [19]. The homogenate was centrifuged (4000 g, 30 min, 4˚C) to pellet the cell debris. The supernatant was frozen at −80˚C, thawed at 5˚C, and centrifuged (9000 g, 30 min, 4˚C). The supernatant was made 50% saturated with solid (NH4)2SO4, and the protein precipitated was pelleted by centrifugation at 9000 g for 30 min at 4˚C. The pellet was dissolved in a minimum volume of extraction buffer, and dialyzed against 3 changes of 10 mM Tris-HCl buffer (pH 8.0) at 5˚C over 48 h, each change being 4 L. Protein precipitates at the end of dialysis were removed by centrifugation (9000 g, 30 min, 4˚C).
Partially purified peroxidase containing ~1 g protein in ~30 mL was made 4 M with deionized urea and 2% with Bio-Lyte ampholyte (pH 3 - 10, 40% w/v). This solution was applied to Rotofor cell (Bio-Rad Laboratories, Hercules CA, USA), and focused for 3.5 h at 15 W constant power and 4˚C. Rotofor fractions were harvested and their pH (isoelectric point pI) values measured. Ampholyte and urea were removed from the fractions by dialyzing at 4˚C against 3 changes of 10 mM Tris-HCl buffer (pH 8.0) over 48 h, with each change being 4 L. Dialyzed Rotofor fractions were stored at 4˚C, and peroxidase remained fully active for at least 3 weeks.
Dialyzed Rotofor fractions of peroxidase (0.2 mL) were prepared with bromophenol blue-glycerol protein loading buffer [20] and loaded into the wells of a slab of 7.5% native PAG, and electrophoresed (Bio-Rad protean ii cell, constant 100 V at 4˚C) until the bromophenol blue dye was at the lower edge of the gel. Peroxidase activity was detected by staining [21] the electrophoresed gel in a solution of 50 mM Na2HPO4 containing 6.0 mM o-dianisidine and 8.8 mM hydrogen peroxide at room temperature in the dark until the peroxidase bands became visible (30 - 45 min). The stained gel was rinsed with distilled water, photographed, and the peroxidase bands digitized using UN-SCAN-IT software (Silk Scientific, Utah, USA).
Peroxidase activity was determined at fixed 0.6 mM o-dianisidine concentration, and varied (0.3 - 1.8 mM) hydrogen peroxide concentrations, in 3 mL of 50 mM Na2HPO4 solution pH 6.0. The activity [21] was measured by photometry (Nanodrop spectrophotometer) at 27˚C and calculated at A460 (Є460 nm: 11.3 mM−1cm−1) for double reciprocal plots. Protein concentrations were determined using the Folin-Ciocalteau reagent, and lysozyme as the protein standard.
Determination of cellulose and lignin: A 20 g subsample of dry, composited and ball-milled sorghum shoots (600 g-harvested) per field plot was custom analyzed (Universal Testing, Illinois, USA) for lignin, acid detergent fiber (ADF) and neutral detergent fiber (NDF) using proprietary modification of standard gravimetric methods.
3. RESULTS
Cell wall-bound peroxidase isoenzymes: The active isoenzymes of sorghum peroxidase were separated from the inactive ones by Rotofor (free solution) isoelectric focusing (IEF) technology that has been very successful in fractionating the complex 28 isoenzymes of glutamate dehydrogenase [22]. Rotofor IEF separated the isoenzyme population into 9 - 15 charge isomers similar to other peroxidases [23]. Isoelectric focusing arranged the isoenzymes in three groups: negatively charged, neutral, and mildly alkaline isoenzymes (Figures 1-4). Most of the inactive isoenzymes were acidic (pI < 4.0), and the active isoenzymes (boxed) had higher pI values. Chromatographic and two-dimensional purification procedures had been inadequate for concentrating the active isoenzymes, and removing them from the inactive forms [23-25]. Native polyacrylamide gel electrophoresis (PAGE) of the Rotofor fractions revealed the two groups (anionic and cationic) of isoenzymes that make up the charge isomers (Figures 1-4). The highly acidic (pI < 4.5) isomers contained predominantly anionic isoenzymes; the weakly basic (pI > 7.5) contained predominantly cationic isoenzymes; while neutral consisted of a graded mix of anionic and cationic isoenzymes. The isoenzymes varied in relative concentration and distribution patterns/ profiles according to crop rotation and other agronomic
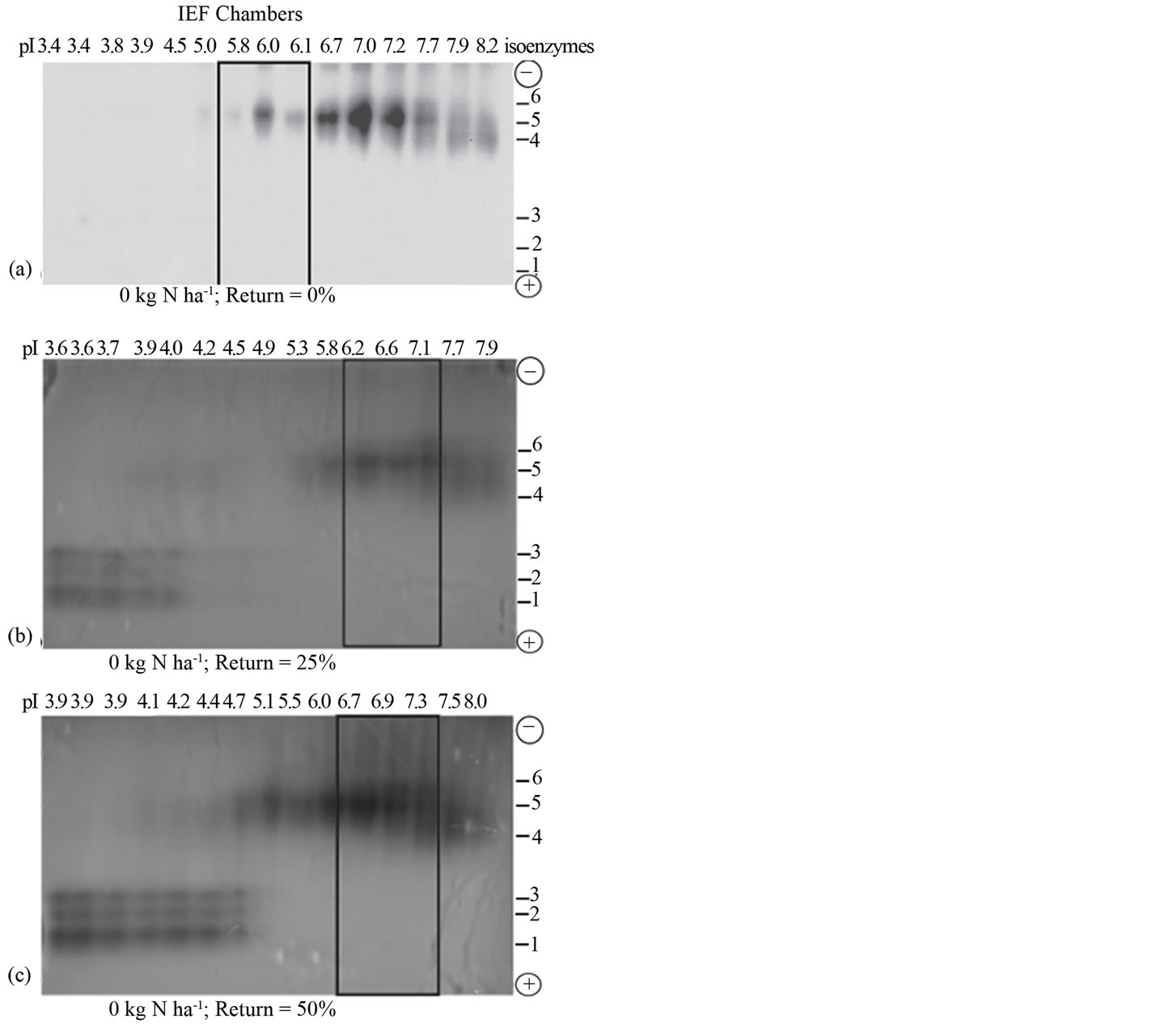
Figure 1. Residue return without N fertilization: Distribution pattern of active and inactive peroxidase isoenzymes from rotated biomass sorghum without N fertilization, but with (a) 0% sorghum residue return; (b) 25% sorghum residue return; (c) 50% sorghum residue return. Partially purified peroxidase extract from the leaves was subjected to free solution IEF followed by native PAGE. The electrophoresed gel was stained with o-dianisidine. The active charge isomers of peroxidase are boxed.
practices (Figures 1-4).
Responses of cell wall metabolism to agronomic management of sorghum: The active peroxidase isoenzyme distribution patterns responded in different ways to the various agronomic management practices (Figures 1-4). Digital quantitation of the isoenzyme bands showed that corn-sorghum rotation, residue return, nitrogen fertilization, tillage and combinations thereof altered some of the chemical properties of the isoenzyme (Figures 1-4).
Residue return without N fertilization: For rotated sorghum, no residue return and no nitrogen fertilization resulted in a family of predominantly cationic active peroxidase isoenzymes with isoelectric point (pI value) of 5.9 (Figure 1(a)). Returning 25% of the biomass without N fertilization increased the pI value of the active peroxidase to 6.6 compared with the control sorghum, and also doubled the yield of the active peroxidase (Figure 1(b)). Returning 50% of the biomass without N fertilization also increased the pI value of the active peroxidase to 6.9 compared with the control (Figure 1(a)), and quadrupled the yield of the active peroxidase (Figure 1(c)). These results redefine the agronomic practice of crop residue return by presenting it in the light of cell wall metabolic responses.
Nitrogen fertilization: For rotated sorghum, 280 kg·ha−1 nitrogen fertilization without residue return shifted the pI value of the active peroxidase to 6.7 (Figure 2(a)) compared with the control sorghum (Figure 1(a)), dramatically increased the anodal isoenzymes, and doubled the yield of the active peroxidase. Fertilization with 280 kg·ha−1 N in combination with 25% residue return (Figure 2(b)) increased the active peroxidase pI value to 7.1 compared with the control (Figure 1(a)), but did not increase its yield. The combination of 280 kg·ha−1 N fertilization and returning mineral nutrients to the soil increased the active peroxidase pI value to 7.7 and quadrupled their yield (Figure 2(c)) compared with the control sorghum. In all the responses of the sorghum cell wall metabolism to the agronomic management practices outlined above, the resultant active peroxidases consisted mainly of the neutral-mildly alkaline cationic isoenzymes. A combination of 280 kg·ha−1 N fertilization and 50% residue return lowered the active peroxidase pI value from 5.9 to 4.7, expanded the active peroxidase composition to include anionic and cationic isoenzymes, and doubled their yield (Figure 2(d)).
For continuously cropped sorghum without N fertilization, the pI value of the active peroxidase was 5.9 (Figure 3(a)). Change of agronomic practice to fertilization with 84 kg·N·ha−1 increased the pI value of the active peroxidase from 5.9 to 6.7 without changing the yield of the active peroxidase (Figure 3(b)). Fertilization with 252 kg·N·ha−1 shifted the pI value of the active peroxidase to 5.5, expanded the active peroxidase composition to include both anionic and cationic isoenzymes and doubled the active peroxidase yield (Figure 3(c)). The highest N rate (336 kg·ha−1) increased the pI value of the active peroxidase to 6.6 (Figure 3(d)) without changing its yield. These results suggest that the isoelectric points of the active peroxidase isoenzymes are the common biological denominators for describing the responses of biomass sorghum cell wall metabolism to different rates of N fertilization.
Corn-sorghum rotation: A significant difference noted between rotated and continuous sorghum was that the peroxidase isoenzyme profile of sorghum rotated with corn (Figure 1(a)) contained little to no anionic isomers,
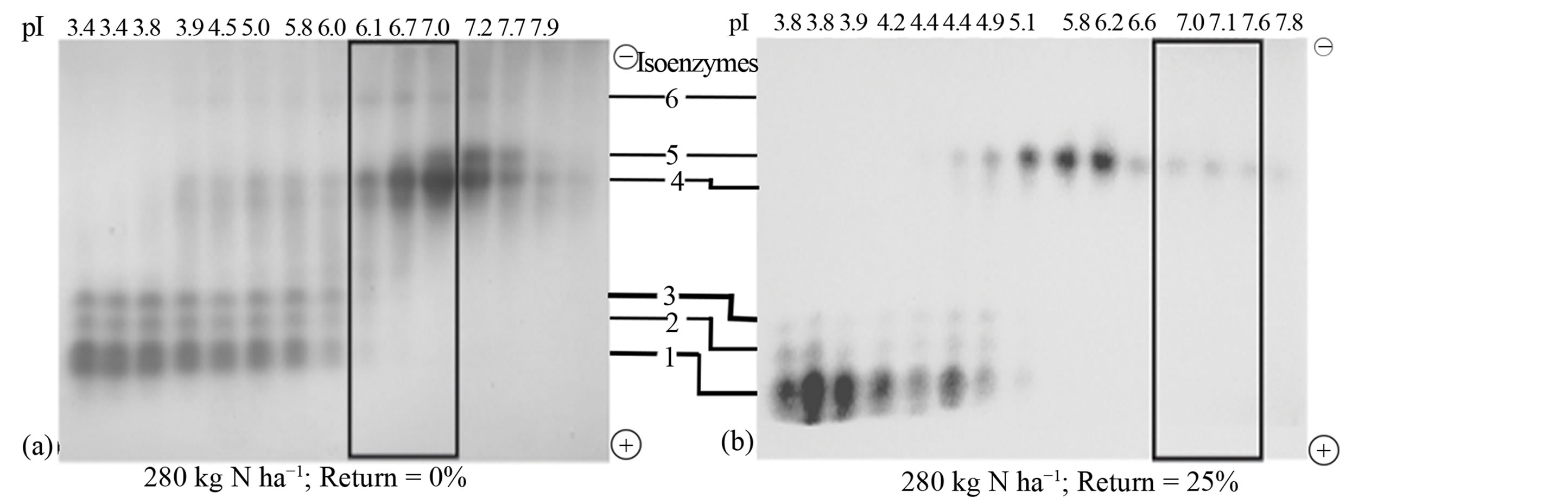
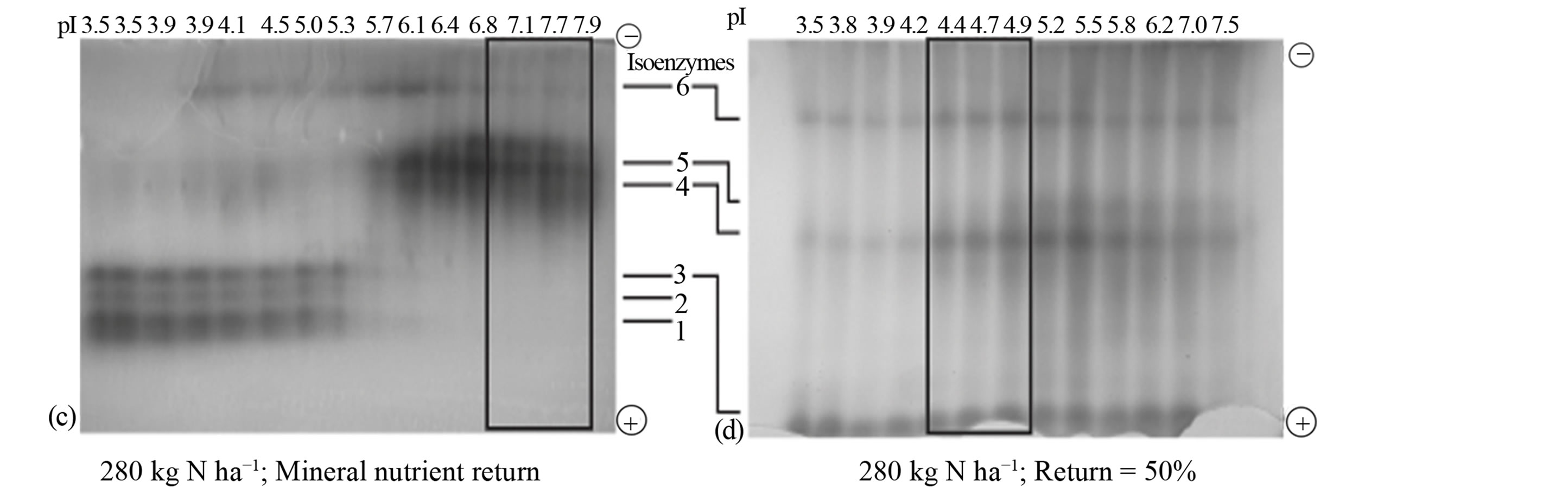
Figure 2. Residue return and N fertilization: Distribution patterns of active and inactive peroxidase isoenzymes from rotated biomass sorghum combined with N fertilization and (a) 0% sorghum residue return; (b) 25% sorghum residue return; (c) 100% mineral uptake from previous crop applied to the soil; (d) 50% sorghum residue return. The partially purified peroxidase extract from the leaves was subjected to free solution (Rotofor) IEF followed by native PAGE. The electrophoresed gel was stained with o-dianisidine. The active charge isomers of peroxidase are boxed.
while that of continuous sorghum (Figure 3(a)) contained a very large amount of strongly acidic anodal isomers. However, the pI values of the active peroxidases were similar thus suggesting that rotation of sorghum did not affect the pI values of the active peroxidase.
Soil tillage intensity: Responses of sorghum’s active peroxidase to different tillage intensities for continuously cropped bioenergy sorghum (Figure 4) were similar to those of N rates (Figure 3). For ridge-till (no plowing prior to row creation so that rows remain undisturbed from one year to the next) with 280 kg N fertilization ha−1, the pI value of the active peroxidase was 6.6 (Figure 4(a)). Conventional tillage (disk tillage) together with N of 280 kg·ha−1 decreased the pI value to 5.7, and also doubled the enzyme yield (Figure 4(b)).
Peroxidase kinetics: The qualitative biochemical changes in peroxidase activities were transformed to quantitative terms by subjecting them to MichaelisMenten kinetics (Figures 5 and 6). Assays at constant o-dianisidine and variable hydrogen peroxide concentrations [19] showed that only 20% - 30% of the isoenzymes displayed normal Michaelis-Menten kinetics while the majority displayed aberrant kinetics. In all the experiments, almost all the strongly acidic charge isomers were inactive, suggesting that they were some of the degradation products of the inactivated isoenzymes. In the mechanism of the peroxidase reaction [14], when the oxidized peroxidase and partially reduced peroxidase become oxidized by hydrogen peroxide, dead-end enzyme-substrate complexes are formed [26]. Inactive forms of enzymes are targeted for degradation, producing progressively lower molecular weight polypeptides [27,28]. Double reciprocal plots for the active peroxidase isomers produced distinct groups of sorghums, one member of the group being the sorghum with the inhibited [29] peroxidase (Figures 5 and 6).
Residue return for the rotated sorghum with and without N fertilization induced noncompetitive (same Km but decreasing Vmax values) inhibition of the active peroxidase isoenzymes (Figure 5). Returning 25% of the biomass without N fertilization (Figure 5(a)) induced a low maximum velocity (Vmax) value (15.7 mmol·min−1). Returning 50% of the biomass without N fertilization (Figure 5(c)) decreased the inhibition by increasing the peroxidase Vmax (29.5 mmol·min−1) value without changing the Km (0.93 µM) value. Control sorghum without residue return or N fertilization exhibited the most inhibited peroxidase (Figure 5(b)) with the lowest
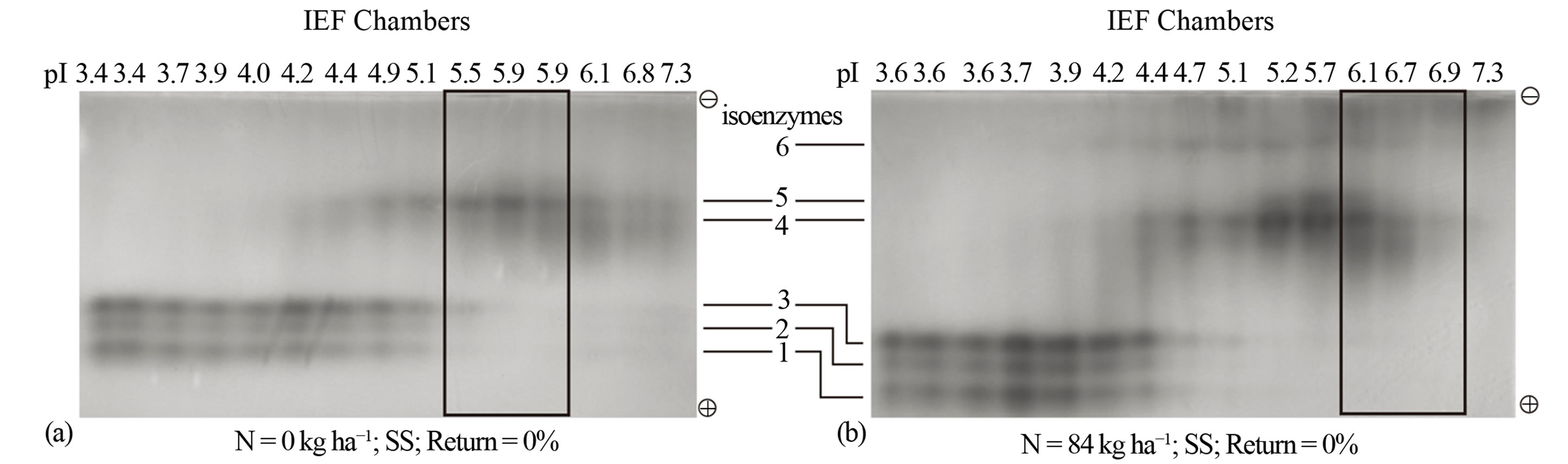
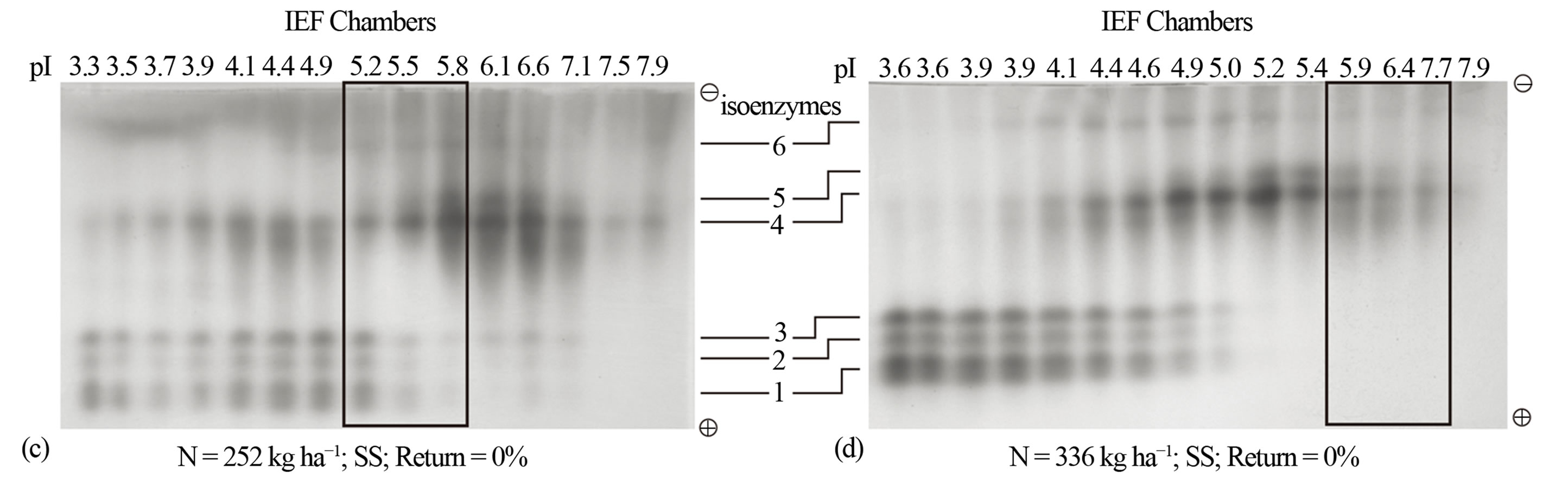
Figure 3. Nitrogen fertilization without residue returned: Distribution patterns of active and inactive peroxidase isoenzymes from continuous biomass sorghum subjected to different rates of nitrogen fertilization (a) 0 kg·N·ha−1; (b) 84 kg·N·ha−1; (c) 252 kg·N·ha−1; (d) 336 kg·N·ha−1. The partially purified peroxidase extract from the leaves was subjected to free solution (Rotofor) IEF followed by native PAGE. The electrophoresed gel was stained with o-dianisidine. The active charge isomers of peroxidase are boxed. SS is continuous sorghum.
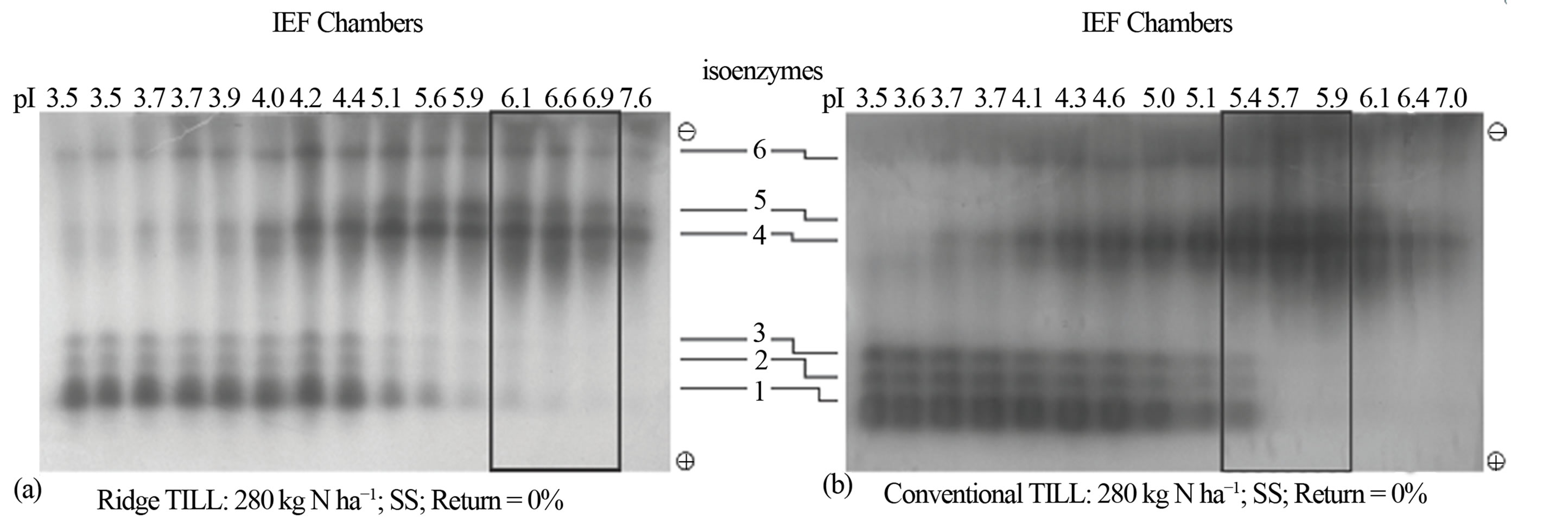
Figure 4. Soil tillage intensity: Distribution patterns of active and inactive peroxidase isoenzymes from biomass sorghum grown using (a) ridge tillage; (b) conventional disk tillage. The partially purified peroxidase extract from the leaves was subjected to free solution (Rotofor) IEF followed by native PAGE. The electrophoresed gel was stained with o-dianisidine. The active charge isomers of peroxidase are boxed. SS is continuous sorghum.
Vmax (3.1 mmol·min−1) and Km values (0.08 µM). Nitrogen fertilization (280 kg·ha−1) without returning residue (Figure 5(d)) increased the peroxidase Vmax value (33.2 mmol·min−1) compared with control sorghum (Figure 5(b)). Fertilization with 280 kg·ha−1 N in combination with 25% residue return (Figure 5(e)) increased the Vmax value further (37.3 mmol·min−1). Combination of 280 kg·ha−1 N fertilization and returning mineral nutrients to the soil (Figure 5(f)) also increased the peroxidase Vmax value (39.6 mmol·min−1) without changing the Km value. Combination of 280 kg·ha−1 N fertilization and 50% residue return (Figure 5(g)) increased the peroxidase Vmax value to the highest level (66.4 mmol·min−1). These kinetic responses of sorghum peroxidase isoenzymes were in agreement with their respective changes in pI values and yield (Figures 1 and 2). Biomass return exponentially increased and maximized the velocity of the peroxidase reaction (Figure 5) leading
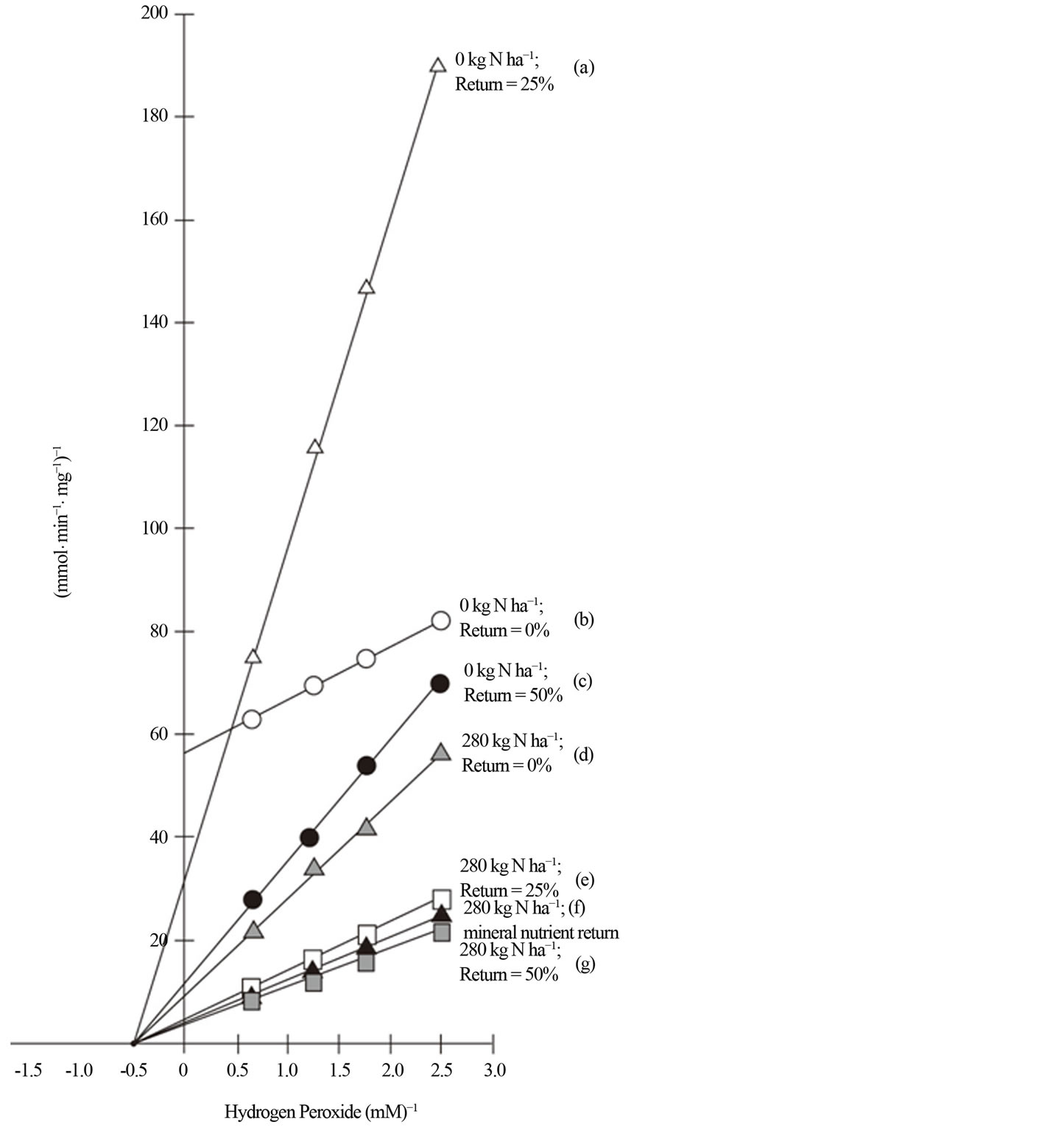
Figure 5. Kinetics of the peroxidase of rotated sorghum. Inhibition kinetics of active peroxidase from rotated biomass sorghum without and with different percentage residue return and rates of N fertilization (a) 0 kg·N·ha−1 and 25% sorghum residue return; (b) 0 kg·N·ha−1 without residue return; (c) 0 kg·N·ha−1 and 50% residue return; (d) 280 kg·N·ha-1 without residue return; (e) 280 kg·N·ha-1 and 25% residue return; (f) 280 kg·N·ha−1 and 100% mineral uptake from previous crop applied to the soil; (g) 280 kg·N·ha−1 and 50% residue return. O-dianisidine saturations of peroxidase activities at varied hydrogen peroxide concentrations (0.4, 0.57, 0.85, and 1.7 mM) were performed. Calculated Vmax and Km values are listed in Table 1.
directly to increased cell wall yield. The results may also explain the observations by [5] that excessive removal of biomass substantially decreased subsequent biomass (cell wall) yield. Therefore, the ability of biomass sorghum to increase its aerial dry material [3,4] could be at least partly attributable to peroxidase activity.
For continuously cropped sorghum, the peroxidase activity of control sorghum without N fertilization was inhibited (Figure 6(a)), possessing a low Vmax value (31.3 mmole·min−1). Changes associated with 84, and
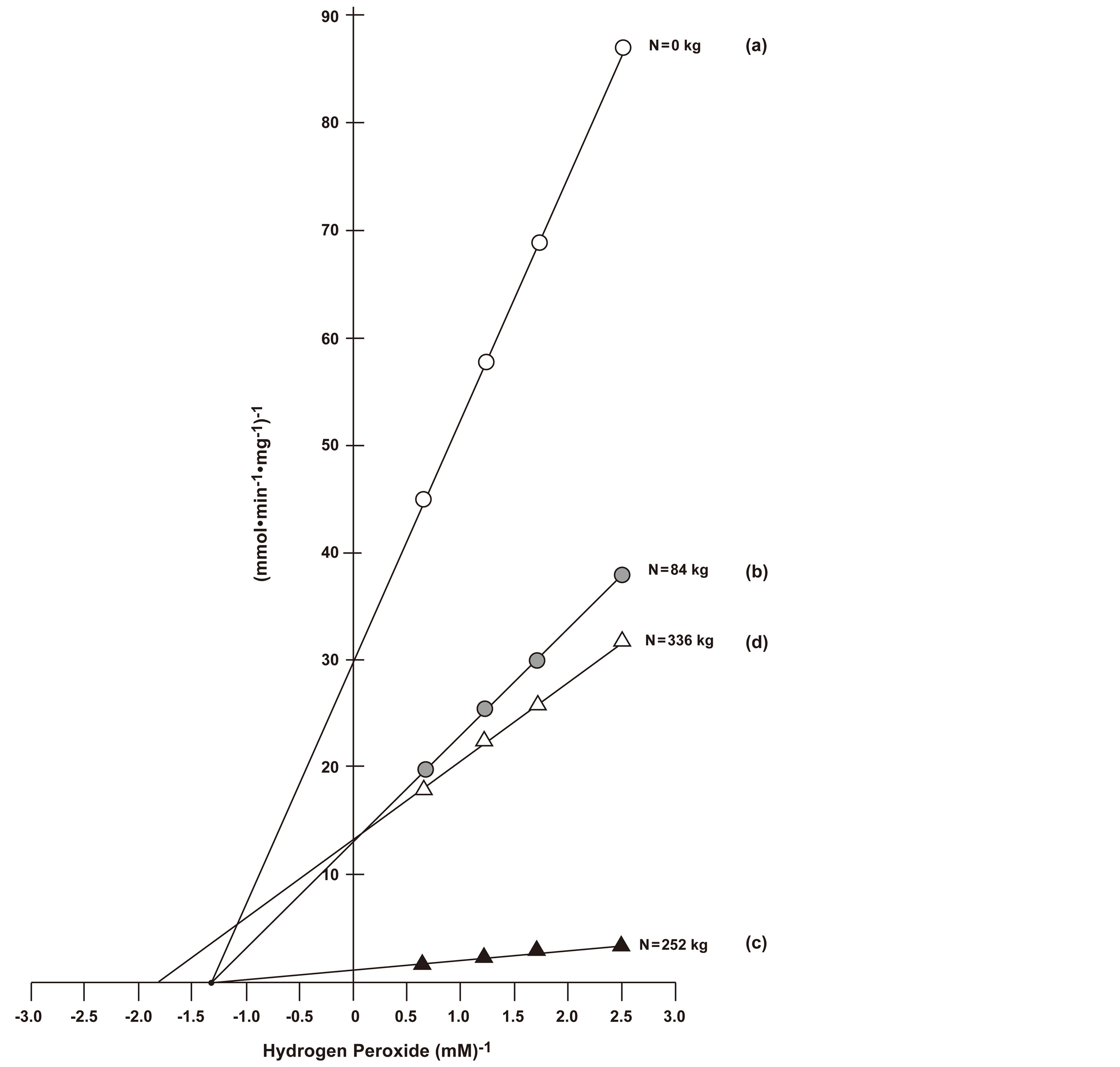
Figure 6. Kinetics of the peroxidase of continuously cropped sorghum. Inhibition kinetics of active peroxidase from continuous biomass sorghum under different rates of N fertilization (a) 0 kg·N·ha−1; (b) 84 kg·N·ha−1; (c) 252 kg·N·ha−1; (d) 336 kg·N·ha−1. O-dianisidine saturations of peroxidase activities at varied hydrogen peroxide concentrations (0.4, 0.57, 0.85, and 1.7 mM) were performed. Calculated Vmax and Km values are listed in Table 1.
252 kg·ha−1 N reduced the inhibition by exponentially increasing the Vmax value to 666.0 mmoles·min−1 for sorghum receiving the latter N rate (Figure 6(c)). But treatment with 336 kg·N·ha−1 produced active peroxidase (Figure 6(d)) that shared a competitive inhibition relationship with the peroxidase of the 84 kg·N·ha−1 treatment (Figure 6(b)), which in turn shared a noncompetition inhibitive relationship with the peroxidase of control sorghum (Figure 6(a)). These observations may help explain some of the biological responses of sorghum to agronomic management changes [3-5].
Observed differences related to crop rotation included much lower Vmax and Km values (3.1 mmol·min−1, 0.08 µM respectively) for the active peroxidase in rotated compared with continuous sorghum (31.3 mmol·min−1, 0.31 µM respectively). These enzymatic responses exerted profound effects on lignocellulose yields (Table 1).
Ridge tillage combined with 280 kg·N·ha−1 under continuous sorghum resulted in inhibited peroxidase (Figure not shown) possessing a low Vmax value (13.0 µmole·min−1). Changing to conventional tillage combined with 280 kg·N·ha−1 reduced the inhibition and increased the Vmax value to 23.7 mmol·min−1 without changing the Km value (Table 1). Increased soil tillage intensity increased the maximum velocity of the peroxidase reaction without altering the Km value (non-
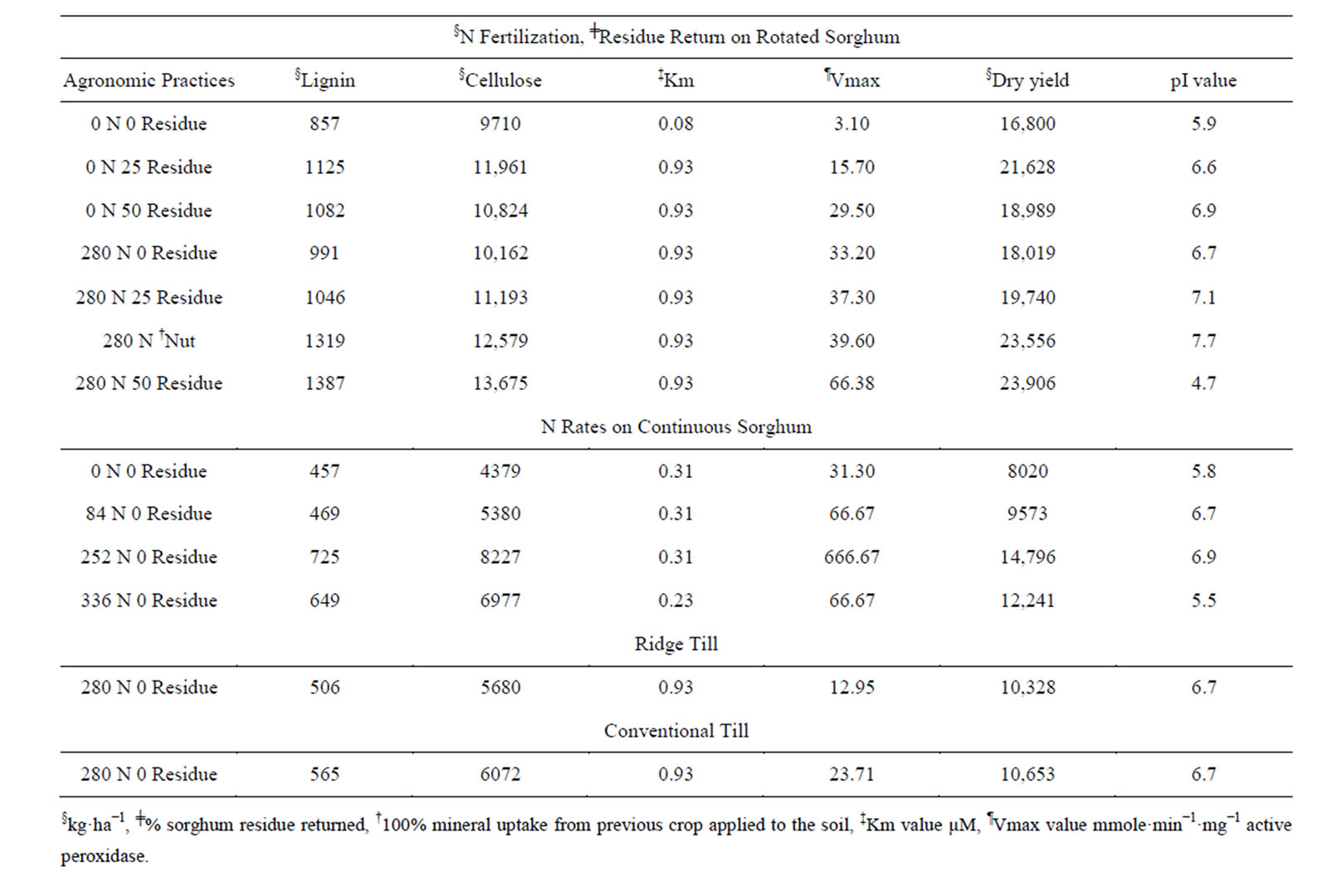
Table 1. Biomass sorghum cell wall metabolic (active peroxidase kinetics, lignin, cellulose and dry matter) responses to agronomic changes (crop rotation, residue return, N fertilization, soil tillage).
competitive inhibition).
Cell wall metabolism: Lignin and cellulose yields: Peroxidases are cell wall-bound enzymes that participate in lignin and suberin biosynthesis [15,17,19]. Changes in their activity altered the lignin and cellulose yields (Table 1) and the cellulose/lignin ratios. Without residue return and N fertilization, control rotated sorghum whose peroxidase was the least active (Figure 5(b)), produced low lignin (857 kg·ha−1) yield and consequently a high cellulose/lignin ratio (11.3). Rotated sorghum with and without N fertilization successively increased peroxidase activities (Figure 5); the lignin yield of sorghum receiving 50% residue return and 280 kg·N·ha−1 (Figure 5(g)) possessed the most active peroxidase and the highest lignin (1387 kg·ha−1) yield (Table 1) and consequently a low cellulose/lignin ratio (9.8). Although 280 kg·N·ha−1 and 50% residue return (Figure 5(g)) produced ~350 kg·ha−1 more biomass than the sorghum treated with 280 kg·N·ha−1 combined with 100% sorghum mineral return (Figure 5(f)), the treatment that included 50% residue return (Figure 5(g)) produced ~1096 kg·ha−1 more cellulose than the treatment that included 100% sorghum mineral residue return (equivalent to 100% return, without the C or N) thus highlighting the chemical importance of biomass return on subsequent sorghum biomass (cell wall) yield [5], and the biological disadvantage of excessive biomass removal. Based strictly on nutrient availability, 100% mineral return could be expected to induce more sorghum growth than the 50% biomass return treatment. The observed peroxidase kinetics (Figure 5) helped explain this biological anomaly.
Without N fertilization, returning 25% of the biomass produced resulted in ~1888 kg·ha−1 more sorghum dry matter, ~768 kg·ha−1 more cellulose, and ~79 kg·ha−1 more lignin than 25% residue return combined with 280 kg ha-1 N fertilization (Table 1) and also changed peroxidase kinetics (Figures 1(b), 2(b), 5(a) and (e)). This result could increase farmers’ economic return [10], while minimizing possible environmental damage associated with N-based fertilizer [11]. Rotated sorghum with 50% residue return combined with 280 kg·ha−1 N fertilization produced only ~2278 kg·ha−1 (10.5%) more dry matter, ~1714 kg·ha−1 (14.3%) more cellulose, and ~262 kg·ha−1 (23.3%) more lignin than 25% residue return without N fertilization (Table 1), possibly indicating that economic margins could be increased if producers do not apply excess N to rotated sorghum. Peroxidase kinetics (Figures 1(b), 2(d), 5(e) and (g)) were also altered by these treatments.
The effect of residue return (Figure 5) on sorghum biology was to maximize the yields of cell wall lignocellulosic biomass by relieving the inhibition of the active peroxidase isoenzymes. These results indicated the importance of peroxidase as the target enzyme on which agronomic practices revolve in efforts aimed at dramatic improvement of cellulosic biomass feedstock yields.
In the continuous sorghum N rate study, absence of N fertilization inhibited the peroxidase activity (Figure 6(a)), thus producing low lignin (457 kg·ha−1) and cellulose (4379 kg·ha−1) yields (Table 1), and a low cellulose/ lignin ratio (9.5). Treatment of the sorghum with 252 kg·ha−1 N relieved the inhibition by increasing the peroxidase maximum velocity (Figure 6(c)), the cellulose (8227 kg·ha−1) and lignin (725 kg·ha−1) yields, and the cellulose/lignin ratio to 11.4 similar to the 84 kg·ha−1- treated sorghum. Although the 252 kg·ha−1 fertilizer N treatment (Figure 6(c)) produced ~2555 kg·ha−1 more biomass than the sorghum treated with 336 kg N fertilizer (Figure 6(d)), the 252 kg·ha−1 fertilization rate induced the peroxidase to produce ~1250 kg·ha−1 more cellulose than the 336 kg fertilizer treatment, thus again highlighting the possible economic improvement for the grower. The greater yield of biomass induced by the 252 kg·ha−1 fertilization was 50% richer in cellulose. This anomaly was due to the competitive inhibition relationship between the peroxidases of the sorghums from the 84 and 336 kg·ha−1 N applications on the one hand and the noncompetitive inhibition relationship between the peroxidases of the sorghums from the control, 84 kg, and 252 kg·ha−1 N treatments on the other hand (Figure 6). The peroxidase from the 84 kg·ha−1 N rate was doubly inhibited, with inhibition spilling over to the peroxidase from 336 kg·ha−1 N-fertilized sorghum. The peroxidase of the control sorghum was the most inhibited (Figure 6(a)) thus explaining the anomalous low cellulose/lignin ratio for the sorghum. Agronomic anomalies (complex biological phenomena) are reflections of deep-seated biochemical root causes.
Rotated sorghum (Figure 5(b)) yielded 120% more cellulose, 87% more lignin, and 100% more dry matter than continuously cropped sorghum (Figure 6(a)). This was because the active peroxidase of the rotated sorghum had a much higher affinity for hydrogen peroxide (lower Km value, 0.08 µM) than the peroxidase (higher Km value, 0.31 µM) of the continuously cropped sorghum (Table 1). These considerations begin to provide a biochemical explanation for the repeated observation that crop rotation generally increases crop yield and soil carbon sequestration [7-9]. Results also showed that crop rotation induced much more dry matter, cellulose, and lignin yields than nitrogen rate and is in agreement with the negative effects of nitrogen fertilizer when combined with 25% crop residue return (Table 1). The causative factors lie in part in the mineral nutrients absorbed as will be discussed later.
Conventional tillage combined with 280 kg·ha−1 N induced higher Vmax values of the peroxidase and accordingly high lignin (565 kg·ha−1) and cellulose (6072 kg·ha−1) yields than ridge tillage (Table 1). Ridge-tillage (less soil disturbance), combined with 280 kg·N·ha−1 inhibited peroxidase compared with the fertilized conventional tillage treatment, and accordingly produced less lignin (506 kg·ha−1) and less cellulose (5680 kg·ha−1). Soil tillage increased the maximum velocity of the peroxidases.
Therefore, the observed peroxidase kinetics agreed with the known peroxidase biological role as cell wallbound enzymes that stiffen cell walls by participating in the biosynthesis of lignin, suberin and other cellulosic materials [16-19].
The greatest lignocellulosic yield was produced by the combination of 280 kg·ha−1 N with 50% sorghum residue return, which shifted the pI value of the active peroxidase to 4.6 because the Vmax increased exponentially to 66.0 mmole mg−1 enzyme while the Km value remained unchanged at 0.90 µM (Table 1).
4. DISCUSSION
Peroxidase kinetics and lignin yield: The Km values (Table 1) of hydrogen peroxide are much lower than earlier reports [25] probably because the inactive isoenzymes of the peroxidase had been removed through isoelectric focusing (Figures 1-4). This further supports the observed increases in the Vmax values. The lowering of the Km values for hydrogen peroxide increased the affinity of peroxidases and their capacity to synthesize lignin and suberin for the stiffening of mechanical supportive and transport tissues [15,17,19]. Crop residue return, nitrogen fertilization rate, corn-sorghum rotation or continuous sorghum cropping, and soil tillage are physically different agronomic practices but they acted in concert on the wall-bound peroxidase isoenzymes, whose maximum velocities changed in response to these practices. Inhibition kinetics (Figures 5 and 6) under variable hydrogen peroxide concentrations were consistent with known mechanisms of active peroxidase involving oxidized peroxidase and partially reduced peroxidase as enzyme-substrate intermediate complexes that frequently form dead-end products with hydrogen peroxide [30]. Therefore, there were agreements between peroxidase isoenzyme patterns, their activation, kinetics, and the yields of lignocellulose (biological systems) in the responses of sorghum to agricultural management practices (environment). IEF purification of active peroxidase could constitute an analytical biochemistry framework for expanding the utility of peroxidase enzymology for performance evaluation of lignocellulose production in the pulp/paper, and bioenergy industries [6,14,31], and generally for monitoring the effects of the environment on plant cell wall metabolism.
Adverse agronomic changes: The understanding that lignin is slowly degraded in the soil [18], thereby increasing the half-life of organic carbon and nitrogen in the soil, provides a frame-work for minimizing some of the potentially adverse biological effects of bioenergy crop production on the environment. The quantity of lignin produced per hectare was decreased up to 50% by N fertilization of the sorghum, by tillage of the soil, and by avoidance of crop rotation (Table 1). These decreases in lignin quantity could potentially lead to decreased soil organic carbon in plots with N fertilization. Lower soil organic carbon decreases nutrient cycling [5] and acelerates soil erosion [12] because soil water retention capacity is decreased [13]. Therefore, sorghum residue return to the soil minimizes some of the adverse biological effects of total aboveground plant removal by increasing the soil organic carbon and the lignin yield of the next harvest of sorghum.
Biochemical mechanisms of different agronomic practices: Results discussed in Figures 1-6 and Table 1 begin to provide some biochemical explanation for previous observations [3-5] that return of sorghum residues to the soil improved soil organic carbon contents, nutrient cycling, and the biomass yield of the next crop of sorghum. Lignin is a major component of crop residue, and it is slow to degrade in soil [7,18] thereby enhancing higher soil carbon contents in the long-term for efficient macroand micro-nutrient exchange. In this regard, return of high-lignin rotated sorghum residue (1387 kg·ha−1 from 280 kg fertilizer-50% return) to the soil should be more beneficial than the low-lignin continuous sorghum residue (457 kg·ha−1 from the 0 fertilizer-0% return).
The biochemical mechanisms of the different agronomic practices focused on the pI values of the peroxidase isoenzymes (Figures 1-4). The active peroxidase from 0 kg·ha−1 N fertilization had same pI value (5.9) as that from 0% return rotated sorghum (Table 1). Similarly, active peroxidases that had the pI 6.7 were from the sorghum produced under different agronomic practices including 280 kg·N·ha−1 without residue return, 25% residue return without N, 50% residue return without N, 84 kg·ha−1 N, and ridge tillage. Also, acidic active peroxidase isoenzymes were generally more efficient than the mildly acidic isoenzymes in the synthesis of lignin (Table 1). Therefore, by differentially selecting the isoenzymes to be inactivated (Figures 1-4), and altering the pI values of the active isoenzymes, the different agronomic practices controlled lignocellulose yield via a pervasive network of competitive, non-competitive, and uncompetitive hydrogen peroxide inhibition kinetics interacting with peroxidases (Figures 5 and 6). Peroxidase pI values were the common thread linking the active peroxidase isoenzymes in the plots of sorghum. The coordinating ability of the peroxidase pI values extended far and beyond, and helped explain the high lignocellulose yields in continuous sorghum with and without N fertilization [3-5] as they also did in the cases of rotated sorghum, nitrogen rates, soil tillage and combinations thereof (Table 1).
ACKNOWLEDGEMENTS
This research was funded by USDA AFRI (grant #2011-67009-30050).
REFERENCES
- Rooney, W.L., Blumenthal, J. and Bean, B. (2007) Designing sorghum as a dedicated bioenergy feedstock. Biofuels, Bioproducts and Biorefining, 1, 147-157. http://dx.doi.org/10.1002/bbb.15
- NASS (2008) Cr Pr 2-5 (6-08). USDA, National Agricultural Statistics Service, Washington DC. http://www.usda.gov/nass/PUBS/TODAYRPT/acrg0608.txt
- Wight, J.P., Hons, F.M., Storlien, J.O., Shahandeh, H., Dou, F. and Provin, T.L. (2012) Bioenergy sorghum management affects yield, nutrient uptake and soil quality. Annual Meeting ASA, CSSA, and SSSA, Cincinnati, 21-24 October 2012.
- Wight, J.P., Hons, F.M., Storlien, J.O., Provin, T.L., Shahandeh, H. and Wiedenfeld, R.P. (2012) Management effects on bioenergy sorghum growth, yield, and nutrient uptake. Biomass and Bioenergy, 46, 593-604. http://dx.doi.org/10.1016/j.biombioe.2012.06.036
- Powell, J.M. and Hons, F.M. (1991) Sorghum stover removal effects on soil organic-matter content, extractable nutrients and crop yield. Journal Sustainable Agriculture, 2, 25-39. http://dx.doi.org/10.1300/J064v02n01_04
- Wright, L. (2006) Worldwide commercial development of bioenergy with focus on energy crop-based projects. Biomass and Bioenergy, 30, 706-714. http://dx.doi.org/10.1016/j.biombioe.2005.08.008
- Wright, A.L., Dou, F. and Hons, F.M. (2007) Crop species and tillage effects on carbon sequesteration in subsurface soil. Soil Science, 172, 124-131. http://dx.doi.org/10.1097/SS.0b013e31802d11eb
- Dou, F., Wright, A.L. and Hons, F.M. (2008) Sensitivity of labile soil organic carbon to tillage in wheat-based cropping systems. Soil Science of America Journal, 72, 1445-1453. http://dx.doi.org/10.2136/sssaj2007.0230
- Franzluebbers, A.J., Hons, F.M. and Zuberer, D.A. (1995) Soil organic carbon, microbial biomass, and mineralizable carbon and nitrogen in sorghum. Soil Science Society America Journal, 159, 460-466. http://dx.doi.org/10.2136/sssaj1995.03615995005900020027x
- Hirel, B., Bertin, P., Quillere, I., et al. (2001) Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiology 125, 1258-1270. http://dx.doi.org/10.1104/pp.125.3.1258
- Good, A.G., Shrawat, A.K. and Muench, D.G. (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? TRENDS in Plant Science, 9, 597-605. http://dx.doi.org/10.1016/j.tplants.2004.10.008
- Kimble, J.M., Lal, R. and Mausbach, M. (2001) Erosion effects on soil organic carbon pool in soils of Iowa. In: Scott, D.E., Mohtar, R.H. and Steinhardt, G.C., Eds., Stustaining the Global Farm, Purdue University and USDA-ARS National Soil Erosion Research Laboratory, 472-475.
- Rawls, W.J., Pachepsky, Y.A., Ritchie, J.C., Sobecki, T.M. and Bloodworth, H. (2003) Effect of soil organic carbon on soil water retention. Geoderma, 116, 61-76. http://dx.doi.org/10.1016/S0016-7061(03)00094-6
- Wight, J.P., Hons, F.M., South, S.M. and Osuji, G.O. (2012) Purification of active peroxidase isoenzymes and their responses to nitrogen fertilization and rotation of biomass sorghum. American Journal of Plant Sciences, 3, 1422-1429. http://dx.doi.org/10.4236/ajps.2012.310172
- Andrews, J., Malone, M., Thompson, D.S., Ho, L.C. and Burton, K.S. (2000) Peroxidase isoenzyme patterns in the skin of maturing tomato fruit. Plant, Cell and Environment, 23, 415-422. http://dx.doi.org/10.1046/j.1365-3040.2000.00555.x
- Dunford, H.B. (1991) Horseradish peroxidase: Structure and kinetic properties. In: Everse, K.E. and Grisham, M.B., Eds., Peroxidase in Chemistry and Biology, CRC Press, Boca Raton, 1-24.
- Whitten, R.W., MacKay, J.J. and Sederoff, R.R. (1998) Recent advances in understanding lignin biosynthesis. Annual Review Plant Physiology Plant Molecular Biology, 49, 585-609. http://dx.doi.org/10.1146/annurev.arplant.49.1.585
- Hatakka, A. (2001) Biodegradation of lignin. In: Hofrichter, M. and Steinbuchel, A., Eds., Biopolymers, Vol. 1: Lignin, Humic Substances and Coal, Wiley-VCH, Weinheim, 129-180.
- Quiroga, M., Guerrero, C., Botella, M.A., et al. (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiology, 122, 1119-1127. http://dx.doi.org/10.1104/pp.122.4.1119
- Davis, L.G., Dibner, M.D. and Battey, J.F. (1986) Basic methods in molecular biology. Elsevier, New York. Amsterdam, London.
- Quesada, M.A., Tigier, H.A., Bukovac, M.J. and Valpuesta, V. (1990) Purification of an anionic isoperoxidase from peach seeds and its immunological comparison with other anionic isoperoxidases. Plant Physiology, 79, 623- 628. http://dx.doi.org/10.1111/j.1399-3054.1990.tb00035.x
- Osuji, G.O., Brown, T.K., South, S.M., Duncan, J.C. and Johnson, D. (2011) Doubling of crop yield through permutation of metabolic pathways. Advances Bioscience and Biotechnology, 2, 364-379. http://dx.doi.org/10.4236/abb.2011.25054
- Gramss, G. and Rudeschko, O. (1998) Activities of oxidoreductase enzymes in tissue extracts and sterile root exudates of three crop plants and some properties of peroxidase component. New Phytology, 138, 401-409. http://dx.doi.org/10.1046/j.1469-8137.1998.00128.x
- Omidiji, O., Okpuzor, J. and Otubu, O. (2002) Peroxidase activity of germinating Sorghum bicolor grains: Effect of some cations. Journal Science of Food Agriculture, 82, 1881-1885. http://dx.doi.org/10.1002/jsfa.1144
- Maciel, H.P.R., Gouvea, C.M.C.P., Toyama, M., Somlka, M., Marangoni, S. and Pastore, G.M. (2007) Extraction, purification and biochemical characterization of a peroxidase from Copaifera langdorffi leaves. Quimica Nova, 30, 1067-1071. http://dx.doi.org/10.1590/S0100-40422007000500003
- Sakharov, I.Y., Vesga, M.K., Galaev, B., Sakharova, I.V. and Pletjushkina, O.Y. (2001) Peroxidase from leaves of palm tree. Plant Science, 161, 853-860. http://dx.doi.org/10.1016/S0168-9452(01)00466-6
- Desimone, M., Henke, A. and Wagner, E. (1996) Oxidative stress induces partial degradation of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase in isolated chloroplasts of barley. Plant Physiology, 111, 789-796.
- Osuji, G.O., Braithwaite, C., Pointer, R. and Reyes, J. (1999) Pesticide inactivation of peanut glutamate dehydrogenase: Biochemical basis of the enzyme’s isomerization. Journal Agricultural Food Chemistry, 47, 3345- 3351. http://dx.doi.org/10.1021/jf980531v
- Segel, I.H. (1976) Biochemical calculations. 2nd Edition, Wiley & Sons, New York, Chichester, Brisbane, Toronto, Singapore.
- Schuller, D.J., Ban, N., van Huystee, R.B., McPherson, A. and Poulos, T.L. (1996) The crystal structure of peanut peroxidase. Current Biology, 4, 311-321.
- Gorton, L. (1995) Carbon paste electrodes modified with enzymes, tissues, and cells. Electroanalysis, 7, 23-45. http://dx.doi.org/10.1002/elan.1140070104
NOTES
*Corresponding author.

