Advances in Biological Chemistry
Vol. 2 No. 3 (2012) , Article ID: 21812 , 6 pages DOI:10.4236/abc.2012.23034
Synthesis, characterization and antimicrobial activities of some metal(II) amino acids’ complexes
![]()
1Department of Chemistry, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria
2Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria
3Department of Chemistry, University of Botswana, Gaborone, Botswana
4Department of Pharmaceutics, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria
5Adis Pharma Inc., 5238 Palmetto Place, Mississauga, ON L5M OC8, Canada
Email: *Htt1haye@yahoo.com
Received 5 June 2012; revised 11 July 2012; accepted 22 July 2012
Keywords: Glycine; Phenylalanine; Coordination Compound; Antimicrobial; Chelation
ABSTRACT
Metal(II) coordination compounds of glycine and phenylalanine were synthesized and characterized using infrared and electronic spectroscopic, and magnetic susceptibility measurements. The complexes were tested for antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Methicillin Resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa, Proteus vulgaris and Candida albicans. The stoichiometric reaction between the metal (II) ions and ligands in molar ratio M:L (1:3) [where M = Mn, Co, Ni, Cu and Cd; L= glycine; phenylalanine] resulted in the formation of five-coordinate square pyramidal dinuclear geometry for both copper complexes and six-coordinate octahedral geometry for the other complexes. The spectroscopic and magnetic moment data suggested that the ligands coordinated via both their amino and carboxylate ion moieties. The complexes demonstrated better activities against one or more of the tested microbes than acriflavine, the standard drug used.
1. INTRODUCTION
Antimicrobial resistance is fast becoming a global concern with rapid increase in multidrug-resistant bacteria and fungi [1,2]. This has mandated continued search for new antimicrobial compounds, including coordination complexes of biologically important molecules [3-5]. The increased lipophilic character of these coordinated compounds, with the resultant enhanced ability to permeate the cell membrane of the microbes, have been suggested as reasons for their improved activity over their parent ligands [6,7]. Chelation, which has been reported to reduce the polarity of the metal ion by partial sharing of its positive charge with the donor group of ligands, also supports this theory [8,9]. Promising antimicrobial activities of some amino acids chelates have been reported, including those of histidine [1,10], arginine and glutamic acid [11]. However, there is dearth of information on coordination compounds of phenylalanine. Coordination compounds of glycine (L1) and phenylalanine (L2) metalligand ratio 1:2 have been reported with marginal antimicrobial activities [12]. It was therefore suggested, based on the chelate theory [6,9], that increasing the number of chelate rings may improve the antimicrobial activities of these complexes. Hence the objective of this work was to synthesize, characterize and determine the antimicrobial activities of coordination compounds of L1 and L2 with metal-ligand ratio 1:3. This was with a view of identifying lead metal(II) compounds for further studies in drug development against multidrug resistant bacteria and fungi.
2. MATERIALS AND METHODS
2.1. General
All reagents and solvents used were of analytical grade. Melting points (M.P) or temperature of decomposition (d) were measured using open capillary tubes on a Gallenkamp (variable heater) melting point apparatus. The UVVis spectra were obtained using a Genesis 10 UV-Vis spectrophotometer, solid reflectance. The magnetic susceptibility measurement for some of the complexes was obtained at room temperature using a MSBAUTO (Sherwood scientific) Gouy balance. The infrared spectra (KBr) were recorded on a Genesis II FT-IR spectrophotometer.
2.2. Synthesis of Complexes
The coordination compounds were prepared according to a modification of previously reported method [13] by the addition of 0.01 M of appropriate metal salt (1.62, 2.17, 2.43, 2.51 and 2.69 g for copper, cadmium, nickel, cobalt and manganese, respectively) to a solution of the ligand L1 0.03 M (2.29 g) and L2 0.03 M (5.12 g) dissolved with stirring in distilled water with the addition of 0.005 M Na2SO4 (0.71 g). The mixture was then heated on a water bath for 2 h. An immediate precipitation was obtained for majority of the complexes, while some required further concentration and cooling. The products obtained were filtered, washed with methanol and dried in vacuo at 60˚C.
2.3. Antimicrobial Activity Using Disc Diffusion Assay
The antimicrobial properties of the complexes were obtained using an adaptation of the filter disc diffusion method [14]. The strains used were Escherichia coli NCTC 8196, Pseudomonas aeruginosa ATCC 19429, Staphylococcus aureus NCTC 6571, Proteus vulgaris NCIB, Bacillus subtilis NCIB 3610 and Methicillin resistant S. aureus (MRSA) clinical isolate for bacteria, and Candida albicans NCYC 6 for fungi while acriflavine was the standard drug.
3. RESULTS AND DISCUSSION
3.1. Physico-Chemical Properties
The colors, percentage yields and melting points (M.P) or temperature of decomposition (d) of the coordination compounds are presented in Table 1. The complexes showed a wide range of colors that were in agreement with those obtained for similar coordination compounds.
Some of the compounds decomposed before melting. All the complexes were sparingly soluble in general organic solvents and therefore efforts at growing their single crystals for X-ray structural studies were unsuccessful.
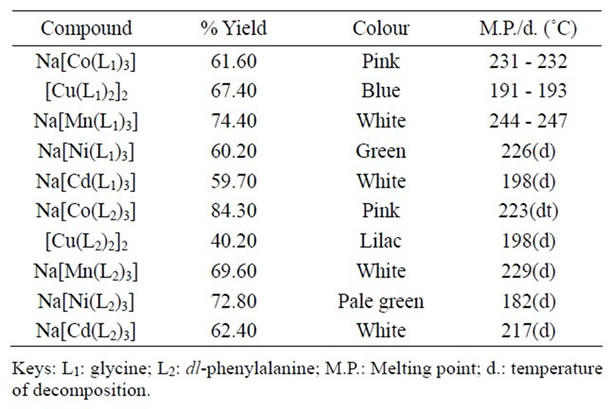
Table 1. Some physical properties of the compounds.
3.2. Electronic Spectra and Magnetic Moment
The absorption bands of the ligands (Table 2) corresponded to the n → σ*, n → π* and π*→ π* transitions of -NH2 and -COO–, the major chromophores, and -C6H5. Shifts in these bands and the observed d-d transitions of the compounds, as presented in Table 2, indicated coordination.
3.2.1. Glycinato Complexes
The visible spectrum for this Cu(II) complex displayed bands at 620 and 632 nm assigned to 2B1g → 2A1g and 2B1g → 2E1g transitions, ascribed to a square pyramidal geometry [15]. Its magnetic moment value of 1.53 B. M, indicative of an antiferromagnetic spin-spin interaction through molecular association with possible Cu-Cu interaction or dimerization, further confirmed this geometry [8,15,16]. Hence, these facts allowed the proposal of a dinuclear square pyramidal geometry for the complex. The spectrum for the Cd(II) complex exhibited bands at 772 and 829 nm, indicative of charge transfer transition (M→L) which is expected of Cd(II) with filled 4d orbital in the ground state [16]. An octahedral geometry is however proposed for the Cd(II) complexes [16]. Nickel(II) complexes are known to exhibit complicated equilibrium between coordination numbers six (octahedral) and four (square planar/tetrahedral) [17]. The absorption bands at 505, 523 and 544 nm (Table 2) were consistent with a six-coordinate octahedral geometry and were assigned to 3A2g(F) → 3T1g (F), 3A2g(F) → 1Tg(F) and 3A2g(F) → 1Eg transitions, respectively [16]. Its magnetic moment of 1.28 B.M was lower than the spin only value of 2.83 B.M and suggested interconversion of stereochemistries or dimerization [18,19]. The Co(II) complex gave two well resolved absorption bands at 520 and 667 nm, which were assigned to 4T1g(F) → 4A2g(F) and 4T1g(F) → 4T1g(P) transitions, respectively consistent with a six coordinate octahedral
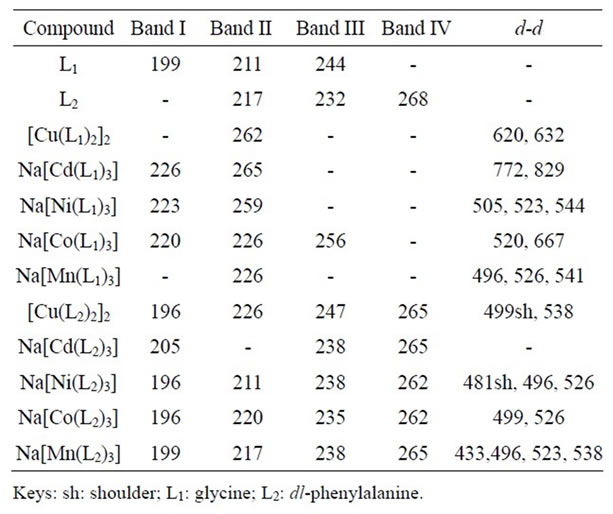
Table 2. Uv-Vis spectra bands (nm) of the compounds.
geometry. Its magnetic moment of 5.20 B.M indicated a high spin d7 system with three unpaired electrons, which corroborates the proposed octahedral geometry [16,20,21]. The Mn(II) complex exhibited low energy bands at 496, 526 and 541 nm that were consistent with a six coordinate octahedral geometry [16] and were assigned 6A1g→4Eg, 6A1g→4T2g(G) and 6A1g→4T1g(G) transitions, respectively. These transitions are a consequence of forbidden transitions to excited states of spin multiplicity other than six [22,23].
3.2.2. Phenylalanito Complexes
For a perfect octahedral complex, transition spectrum for a Cu(II), d9 complex is expected to show only a single band. However, the Cu(II) ion in an octahedral environment is subject to Jahn Teller distortion which lowers the symmetry of the molecule and reduces the degeneracy of the eg orbitals [22,24]. The d-d transitions of the Cu(II) complex at 499sh and 538 nm (Table 2) were assigned to 2B1g → 2A1g and 2B1g → 2E1g transitions, respectively. Its magnetic moment value of 1.73 B. M suggested a dinuclear square pyramidal complex [16]. However, no distinct d-d transition band was observed with the cadmium-phenyalanito complex [22]. Bands at 496, 481sh and 526 nm, assignable to the 3A2g(F) → 3T1g (F), 3A2g(F) → 1Tg(F) and 3A2g(F) → 1Eg transitions, respectively, observed with the Ni(II) complex, suggested a distorted octahedral geometry. The Co(II) complex showed d-d transition bands at 499 and 526 nm and were assigned to 4T1g(F) → 4T1g(P) and 4T1g(F) → 4A2g(F) transitions, respectively typical of an octahedral geometry [24]. Weak transition bands were observed for the manganese complex at 433, 496, 523 and 538 nm and attributed to 6A1g→4A1g(G), 6A1g→4Eg(G), 6A1g→4T2g(G) and 6A1g→ 4T1g(G) transitions, respectively for a distorted octahedral geometry [16].
3.3. Infrared Spectra
The IR spectral assignment of the metal complexes was achieved by comparing their vibration frequencies with those of the free ligands (Table 3) and literature reports of similar compounds [25,26].
3.3.1. Glycinato-Complexes
The N-H stretching vibration at 3119 cm–1 in glycine was shifted to higher frequencies with the complexes, suggesting that the coordination of the metal ions with the ligand was via the nitrogen atom [25-27]. This was corroborated by similar shifts in the weak C-N stretching frequency of the ligand at 1127 cm–1 to higher frequencies in the complexes [28].
Infrared studies on coordination compounds of amino acids have shown that the carboxylate ion is affected by coordination, making it a useful tool in structural studies [26]. Similarly, the asymmetric stretching vibration at 1615 cm–1 for the carboxylate ion was shifted to higher frequencies with the complexes, confirming coordination via this functional group. For the symmetric stretch, sharp extended bands were observed instead of distinct bands. This has been reported to be due to the zwitterionic nature of the ligand in the crystalline form [26]. Sharp extended bands at ~3480 - 3620 cm–1 in the complexes indicated intermolecular hydrogen bonding between the uncoordinated oxygen atom of the carboxyl group and the amino group of neighboring molecule. Broad bands at ~1340 cm–1 however confirmed the intramolecular hydrogen bonding [29]. New bands at 502 - 548 and 621 - 695 cm–1 were assigned to (M-N) and M-O bond stretching band frequencies, respectively and served as further evidence of coordination via the nitrogen and oxygen atoms of the ligand.
3.3.2. Phenylalanito-Complexes
Bathochromic shifts of the phenylalanine N-H stretching frequency at 3454 cm–1 was observed in all the complexes, with the exception of the hypsochromic shift given by the cobalt complex (Table 3). This ligand had weak bands at 1626 and 1566 cm–1 that were assigned to the asymmentric and symmentric stretching bands of the carboxylate ion. They were weak as a result of the zwitterionic nature of the ligand [26,30]. In the complexes, the asymmetric stretching band experienced hypsochromic shifts. However, cadmium and copper complexes gave bathochromic shifts. It has been reported that for aromatic compounds, their N-H bending vibration bands often overlapped the aromatic C=C absorption bands [30]. Consequently, a distinct C=C band was not obtained for most of the complexes.
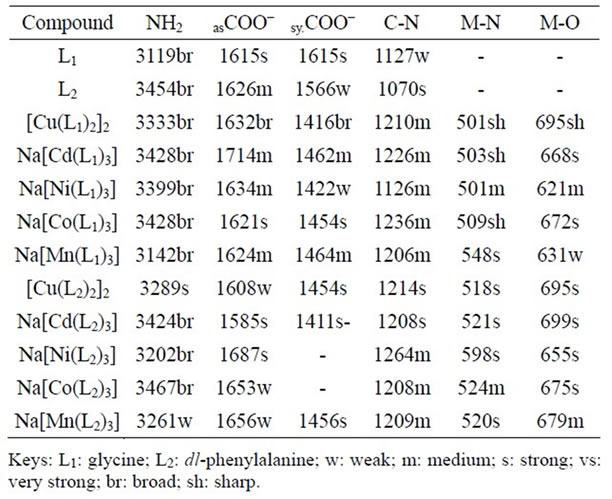
Table 3. Infrared frequencies (cm–1) of the ligands and compounds.
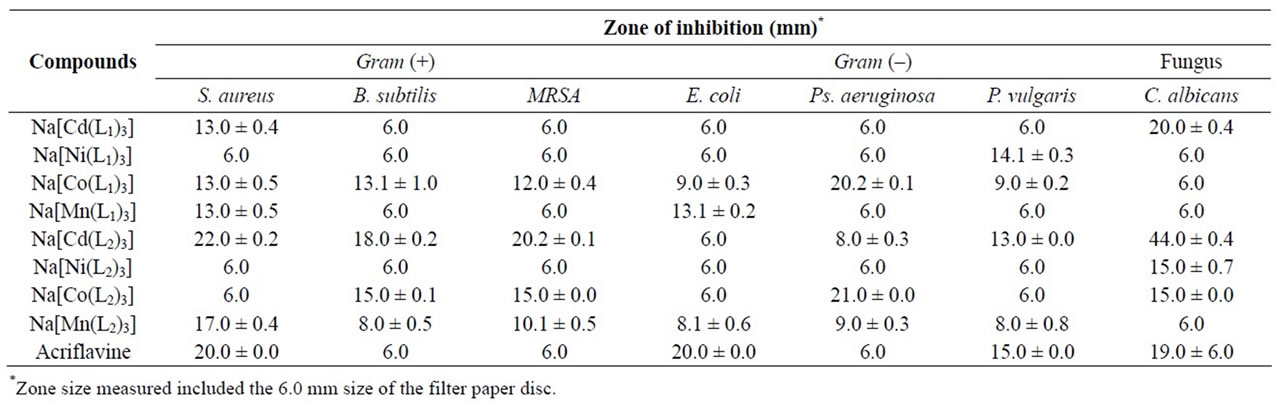
Table 4. Zones of inhibition for the coordinated compounds (20 mg/ml).
Broad bands at 3468 - 3823cm–1 in the complexes indicated N-H∙…O interaction [31]. In the low frequency region, the complexes had new bands at 598 - 518 and 699 - 655cm–1, attributable to (M-N) and (M-O) stretching. This further supported that the coordination of the complexes was via the amino and carboxylate groups. Therefore, based on the above discussion of the results of the magnetic moment, electronic and infrared spectra, a dinuclear bipyramidal geometry is hereby proposed for the glycinatoand phenylalanito-Cu(II) complexes and octahedral geometry for the others. However, the octahedral geometry of the Mn(II) and Ni(II) complexes are suggested to be distorted.
3.4. Antimicrobial Activity
The antimicrobial activities of the compounds are presented in Table 4. The results obtained indicated that glycine, and both copper complexes were inactive to all the tested organisms while phenyalanine and Na[Ni(L2)3] had marginal (zone of inhibition 14.0 mm) antifungal activity (Table 4). The activity of acriflavine against E. coli was significantly (p < 0.05) higher than all the coordinated compounds while the standard drug was inactive against some of the tested bacteria. The antimicrobial activities of some of the complexes against B. subtilis, MRSA, Ps. aeruginosa and C. albicans were significantly higher than the standard drug (Table 4), indicating their potentials as antimicrobial agents against these microbes. The compound with the highest antimicrobial spectrum was Na[Cd(L2)3] followed by Na[Co(L1)3] and Na[Co(L2)3]. Also, Na[Cd(L2)3] had the greatest activity against C. albicans as well as against the Gram +ve bacteria tested. Na[Co(L1)3] and Na[Co(L2)3] demonstrated the highest activity against Ps. aeruginosa, the most susceptible Gram −ve bacterium tested. This may confirm the antimicrobial property of cobalt [6]. However, higher activities given by these cobalt complexes than those of copper, contrary to the antimicrobial potencies of their metals [4,32-35], may indicate the influence of the proposed dinuclear structure.
Similar to previous reports [4,36], the complexes showed better activity against the Gram +ve bacteria, with the exception of Na[Cd(L2)3] and Na[Ni(L1)3] against P. vulgaris, Na[Co(L1)3], Na[Mn(L1)3] against E.coli and Na[Co (L2)3] against Ps. aeruginosa (Table 4). This indicates their usefulness as broad spectrum antibacterial agents. The four-coordinate Cu(L1)2 and Cu(L2)2 had better activities against E. coli and S. aureus [12] than the present dinuclear Cu(II) complexes. The activities of Ni(L1)2, Co(L1)2 and Mn(L1)2 against C. albicans [12] were also better than the current six-coordinate complexes while that of Mn(L1)2 against B. subtilis [12] was significantly higher. Apart from these exceptions, the six-coordinate complexes (Table 4) demonstrated better activities than their corresponding four-coordinate complexes [12]. This enhancement of activity suggests that chelation moderately increased the lipophilic character of the compounds and subsequently their permeation through the lipoid layer of the microbe’s cell membrane [37-40].
4. CONCLUSION
The results of this study indicated that L1 and L2 coordinated in a bi-dentate manner using both nitrogen and oxygen atoms. This assignment is corroborated by spectral and magnetic moment data. The compounds exhibited a broad spectrum of activity against the tested microbes. Some of the compounds however exhibited increased antimicrobial activity with increased number of chelate rings. Hence, chelation may serve as a useful tool in the design of potential antimicrobial agents.
![]()
![]()
REFERENCES
- Saha, S., Dhanasekaran, D., Chandraleka, S., Thajuddin, N. and Panneerselvam, A. (2010) Synthesis, characterization and antimicrobial activity of cobalt metal complexes against drug resistant bacterial and fungal pathogens. Advances in Biological Research, 4, 224-229.
- Mulligan, M.E. and Murray-Leisure, K. (1993) Methicillin-resistant Staphylococcus aureus: A consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. American Journal of Medicine, 94, 313-328. doi:10.1016/0002-9343(93)90063-U
- Farrell, N. (2003) Metal complexes as drugs and chemotherapeutic agents. Comprehensive Coordination Chemistry, 9, 809-840.
- Faúndez, G., Troncoso, M., Navarrete, P. and Figueroa, G. (2004) Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. Bio-Medical Central Microbiology, 4, 19-26.
- Johari, R., Kumar, G., Kumar, D. and Singh, S. (2009) Synthesis and antibacterial activity of M(II) schiff-base complex. Journal of the Indian Chemistry Council, 26, 23-27.
- Chang, E., Simmers, C. and Knight, A., (2010) Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals, 3, 1711-1728. doi:10.3390/ph3061711
- Husseiny, A.F., Aazam, E.S. and Al Shebary, J. (2008) Synthesis, characterization and antibacterial activity of schiff-base ligand incorporating coumarin moiety and it metal complexes. Inorganic Chemistry, 3, 64-68.
- Chohan, Z.H., Arif, M., Akhtar, M.A. and Supuran, C.T. (2006) Metal based antibacterial and antifungal agents: Synthesis, characterization and in-vitro biological evaluation of Co(II), Cu(II), Ni(II), and Zn(II) complexes with amino acid-derived compounds. Bioinorganic Chemistry and Applications, 2006, 1-11. doi:10.1155/BCA/2006/83131
- Rehman, S., Ikram, M., Rehman, S., Faiz, A. and Shahnawaz, A. (2010) Synthesis, characterization and antimicrobial studies of transition metal complexes of imidazole derivative. Bulletin of Chemical Society of Ethiopia, 24, 201-207.
- Nomiya, K., Takahashi, S., Noguchi, R., Nemoto, S., Takayama, T. and Oda, M. (2000) Synthesis and characterization of water-soluble silver(I) complexes with Lhistidine (H2his) and (S)-(-)-2-pyrrolidone-5-carboxylic acid (H2pyrrld) showing a wide spectrum of effective antibacterial and antifungal activities. Crystal structures of chiral helical polymers [Ag(Hhis)]n and {[Ag(Hpyrrld)]2}n in the solid state. Inorganic Chemistry, 39, 3301-3311. doi:10.1021/ic990526o
- Legler, A., Kazachenko, A., Kazbanov, V. and Per’ yanova, O. (2001) Synthesis and antimicrobial activity of silver complexes with arginine and glutamic acid. Pharmaceutical Chemistry, 35, 35-36. doi:10.1023/A:1014098810078
- Aiyelabola, T., Ojo, I. and Akinkunmi, O. (2012) Structural and antimicrobial studies of coordination compounds of phenylalanine and glycine. International Journal of Chemistry, 4, 49-59. doi:10.5539/ijc.v4n2p49
- Nomiya, K. and Yokoyama, H. (2002) Synthesis, crystal structures anti antimicrobial activities of polymeric silver(I) complexes with three amino-acids [aspartic acid (H2asp), glycine (Hgly) and asparagines (Hasn)]. Journal of Chemical Society, Dalton Transaction, 2483-2490. doi:10.1039/b200684g
- Murray, P.R., Baroon, E.J., Pfaller, M.A., Tenover, F.C. and Yolke, R.H. (1995) Manual of clinical microbiology. 6th Edition, American Society for Microbiology, Washington DC, 56-77.
- Youssef, N.S. and Hegab, K.H. (2005) Synthesis and Characterization of Some Transition Metal complexes of thiosemicarbazones derived from 2-acetylpyrrole and 2- acetylfuran. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 35, 391-399. doi:10.1081/SIM-200059215
- Greenwood, N.N. and Earnshaw, A. (1997) Coordination compounds. Butterworth-Heinemann, Hong Kong, 1060- 1090.
- Osowole, A. (2011) Synthesis, characterization and magnetic and thermal studies on some metal(II) thiophenyl schiff base complexes. International Journal of Inorganic Chemistry, 2011, 1-7. doi:10.1155/2011/650186
- Dawood, Z.F., Mohammed, T.J. and Sharif, M.R. (2009) New nickel(II) complexes with benzilbis (semicarbazone) and dithiocarbamate ligands. Iraqi Journal of Veterinary Sciences, 23, 135-141.
- Osowole, A.A., Woods, J.A.O. and Odunola, O.A. (2000). Synthesis and characterization of synthesis and physico-chemical properties of some copper(ii) β-ketoamines and their adducts with 2,2-bipyridine and 1,10-phenanthroline.some nickel(II) β-ketoamines and their adducts with 2,2-bipyridine and 1,10-phenanthroline. Synthesis and Reactivity of Inorganic and Metal Organic Chemistry, 32, 783-799. doi:10.1081/SIM-120004446
- Ballhausen, C.J. (1962) An introduction to ligand field theory. McGraw Hill, New York.
- Sathisha, M., Revankar, V. and Pai, K. (2008) Synthesis, structure, electrochemistry and spectral characterization of bis-isatin thiocarbohydrazone metal complexes and their antitumor activity against Ehrlich ascites carcinoma in Swiss albino mice. Metal Based Drugs, 2008, 362-375.
- Miessler, G.L. and Tarr, D.A. (1999) Coordination compounds. Pearson Prentice Hall, New York, 315-316.
- Cotton, F.A. and Wilkinson, G. (1981) Advanced inorganic chemistry. John Wiley and Sons, New York, 628- 816.
- Lever, A.B.P. (1986) Inorganic electronic spectroscopy. Elsevier, London, 481-579.
- Fessenden, R.J. and Fessenden, J.S. (1990) Organic chemistry. Harness and Nabie Inc., 1048.
- Nakamoto, K. (2009) Complexes of amino acids. In: K. Nakamoto, Ed., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley Interscience, New York, 66-74.
- Elzahany, E.A., Hegab, K.H., Safaa, K.H. and Youssef, N.S. (2008) Synthesis, characterization and biological activity of some transition metal complexes with Schiff Bases derived from 2-formylindole, salicyladehyde and N-amino rhodanine. Australian Journal of Basic and Applied Sciences, 2, 210-220.
- Fahmideh, S., Lotf, A.S. and Shahriar, G. (2010) Synthesis, characterization and anti-tumour activity of Fe(III) schiff base complexes with unsymmetric tetradentate ligands. Bulletin of Chemical Society of Ethiopia, 24, 193- 199.
- Konstantinovic, S., Radovanovic, C., Cakic, Z. and Vasic, V. (2003) Synthesis and characterization of Cd(II), Ni(II), Cu(II) and Zn(II) complexes with 3-salicylidenehydrazono-2-indolinone. Journal of the Serbian Chemical Society, 68, 641-647. doi:10.2298/JSC0309641K
- Pavia, D., Lampman G. and Kriz G. (2001) Introduction to spectroscopy, a guide for students of organic chemistry. Brooks and Cole, Florence.
- Saunders, D.L. (2009) Isolation of lead-amino acid and mercury-amino acid complexes with characterization in the solid state, the solution state, and the gas phase. Ph.D. Thesis, Department of Chemistry, Dalhousie University, Halifax.
- Ibrahim, M., Wang, F., Lou, M., Xie, G., Li, B., Bo, Z., Zhang, G., Liu, H. and Wareth, A. (2011) Copper as an antibacterial agent for human pathogenic multidrug resistant Burkholderia cepacia complex bacteria. Journal of Bioscience and Bioengineering, 112, 570-576. doi:10.1016/j.jbiosc.2011.08.017
- Abushelaibi, A. (2005) antimicrobial effects of copper and brass ions on the growth of Listeria Monocytogenes at different temperatures, pH and nutrients. Ph.D. Thesis, Department of Food Science, Agricultural and Mechanical College, Louisiana State University, Louisiana.
- Centre for Applied Microbiology and Research (2000) http://www.copper.org/about/pressreleases/2000/DemonstratePotential.html
- Copper Development Centre (2004) Antimicrobial: Copper. http://www.copper.org.sg/publications/cca/0309/pg06_japan_research.html
- Kabbani, A.T., Hammud, H.H. and Ghannoum, A.M. (2007) Preparation and antibacterial activity of copper and cobalt complexes of 4-chloro-3-nitrobenzoate with a nitrogen donor ligand. Pharmaceutical Chemistry Bulletin, 55, 446-450. doi:10.1248/cpb.55.446
- Chohan, Z.H., Scozzafava, A. and Supuran, C.T. (2006) Synthesis of biologically active Co(II), Cu(II), Ni(II) and Zn(II) complexes of symmetrically 1,1’-disubstituted ferrocene derived compounds. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 33, 241-257. doi:10.1081/SIM-120017783
- Chohan, Z.H., Farooq, M.A., Scozzafava, A. and Supuran, C.T. (2002) Antibacterial schiff bases of oxalyl-hydrazine; diamide incorporating pyrrolyl and salicylyl moieties and of their zinc(II). Journal of Enzyme Inhibition and Medicinal Chemistry, 17, 1-7. doi:10.1080/14756360290005598
- Garba, S. and Salihu, L. (2011) Antibacterial activities of 2-O-butyl-1-O-(2’-ethylhexyl) benzene-1,8-dicarboxylate and 1-phenyl-1,4-pentanedione isolated from Vitellaria paradoxa root bark. Asian Journal of Scientific Research, 4, 149-157. doi:10.3923/ajsr.2011.149.157
- Stanila, A., Marcu, A., Rusu, D., Rusu, M. and David, L. (2007) Spectroscopic studies of some copper (II) complexes with amino acids. Journal of Molecular Structure, 834-836, 364-368. doi:10.1016/j.molstruc.2006.11.048
NOTES
*Corresponding author.

