Journal of Biomedical Science and Engineering
Vol.07 No.12(2014), Article ID:50457,10 pages
10.4236/jbise.2014.712093
An Efficient Method for Epileptic Seizure Detection in Long-Term EEG Recordings
Alaa Eldeen Mahmoud Helal1, Ahmed Farag Seddik1, Mohammed Ali Eldosoky1, Ayat Allah Farouk Hussein2
1Biomedical Engineering Department, Faculty of Engineering, Helwan University, Cairo, Egypt
2Clinical Neurophysiology Department, Faculty of Medicine, Cairo University, Cairo, Egypt
Email: smllsisi@yahoo.com, ahmed_sadik@h-eng.helwan.edu.eg, hm1_eldosoky@hotmail.com, aia.hussein@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 July 2014; revised 12 September 2014; accepted 28 September 2014;
ABSTRACT
Epilepsy is one of the most prevalent neurological disorders with no age, racial, social class, and neither national nor geographic boundaries. There are 50 million sufferers in the world today with 2.4 million new cases occur each year. Electroencephalogram (EEG) has become a traditional procedure to investigate abnormal functioning of brain activity. Epileptic EEG is usually characterized by short transients and sharp waves as spikes. Identification of such event splays a crucial role in epilepsy diagnosis and treatment. The present study proposes a method to detect three epileptic spike types in EEG recordings based mainly on Template Matching Algorithm including multiple signal-processing approaches. The method was applied to real clinical EEG data of epileptic patients and evaluated according to sensitivity, specificity, selectivity and average detection rate. The promising results illuminate that hybrid processing approaches in temporal, frequency and spatial domains can be a real solution to identify fast EEG transients.
Keywords:
Electroencephalogram (EEG), Seizure Detection, Epilepsy Diagnosis

1. Introduction
1.1. Medical Background and Medications
An epileptic seizure is a transient occurrence of electrical hyperactivity in different parts of the brain produces physical symptoms such as short periods of inattention and loss of memory, a sensory hallucination, or a whole body convulsion. Epileptic patients are suffering from unpredictable, persistent and frequent seizures that limit the independence of them, increase the risk of serious injury, resulting in social isolation and economic hardship. Causes of epilepsy vary from a brain injury, tumor, stroke, genetic mutations and any problems in the brain from a previous infection [1] [2] . Electroencephalogram (EEG) is the recording of the brain’s electrical activity by electrodes attached to the scalp (scalp EEG) or through special electrodes implanted in the brain (intracranial EEG). Scalp EEG is the most common and widely used clinical procedure for diagnosing, monitoring and managing the neurological disorders related to epilepsy [3] . Brain functioning affects the morphology of EEG [4] . Spikes in EEG are usually an indication of abnormal functioning of the brain and highly correlated with epilepsy [5] . For identification of these rapid epileptiform waveforms, neurophysiologists must go through (quantitative) EEG data via his/her naked eye to identify such abnormalities. Visual examination of long EEG recordings is challenging as well as tiresome, tedious, laborious and too time consuming. Thus, automatic spike detection attempts have received intense attention, urgently required to limit such difficulties of the traditional EEG examination. This study presents a method to detect three spike types that usually appeared in epileptic EEG.
1.2. Related Work
Over the years, several algorithms have been developed for detection of spikes, sharp waves and other transient signals in scalp EEG. A great and venerable contribution has been given by Dr. Rajeev Yadav and his team, presenting new methods for automatic seizure detection and spike sorting, helping us to understand the nature of EEG, particularly its rapid changes at the beginning and during seizures [6] [7] . The following paragraph highlights their prominent work in this field:
In [8] , R. Yadav, et al., proposed a new algorithm for seizure detection using frequency-weighted energy. The method performance was evaluated using 100 hours of a single channel stereo encephalograms (SEEG) obtained from five different patients, resulting 96.6% and 0.21/h of sensitivity and false detection rate respectively. An overall improvement has been achieved in terms of sensitivity, specificity and FDR in [9] , by using a dual-stage classifier applied for 300 hours of SEEG recordings obtained from fifteen patients. In [10] , a patient-specific model for seizure detection method has been proposed using statistically optimal null filters. This method was relied on a priori known seizure (template patterns) for subsequent detection of similar seizures in EEG data for seven patients. In [11] , a novel morphology-based classifier (with simple computations appropriate to real-time applications) to identify sharp waves in intracranial EEG was presented. The method detects various types of seizures (rhythmic, non-rhythmic, short and long-seizures) with a sensitivity of 100%, a false detection rate of 0.1/h and an average onset delay of 9.1 s. In terms of spike detection and sorting, a spike classification algorithm combining template matching and principal component analysis (PCA) was proposed in [12] . Using intracranial EEG from 5 patients, the method resulted in 82.1% of the detected spikes in non-overlapping and disjoint clusters. In the literature review, Oikonomou, et al., in [13] and Alexandros T. Tzallas, et al., in [14] summarized the most techniques used to detect spikes in EEG signal. The EEG signal is characterized by rapid dynamics that instantly change through distinctive stages before (preictal), during (ictal), and after (post ictal) a seizure [15] . This nonlinearity nature and fast transitions between non-seizure, pre-seizure, and seizure states, guide researchers to a promising solution by combining two or more techniques to capture the EEG features in multiple domains, where epileptic spikes could be recognized by its characteristics in wide feature spaces. Therefore, two techniques that analyze the EEG signal in temporal and spatial or temporal and frequency domains were combined in previous detection methods [16] . Our method presented in this work, is extensive so that; it recognizes three types of epileptic spikes in a broad framework including more than one domain. Our algorithm was designed to proceed sequentially in short windowing epochs of the tested data, to suspend its rapid dynamics and capture any morphological fluctuation through distinctive states before, during, and after seizures.
1.3. Epileptic Spikes
Two significant states are involved during the seizure evolution: ictal (during seizure) and inter-ictal (between seizures). In inter ictal period, the signal is characterized by certain morphologies, including isolated spikes, sharp waves, poly spikes and wave complexes (spikes followed by slow waves forming spike-and wave complex) last from 20 to 200 ms, while these abnormalities extend to 150 - 350 ms during ictal states [3] [13] [14] . Wave complexes, isolated and poly spikes are the typically observed patterns in epileptic EEG [17] [18] . So, in this work, we focused on these three spike types that generally seen in the majority of epileptic patients. The wave complexes are a spike followed by a slow wave occurring at rates below 3 Hz [19] , with duration from 150 to 350 ms [3] [13] . Isolated spikes usually appear alone and clearly distinct from the background activity by a sudden rise in amplitude with a duration varying from 20 to 70 ms [13] . The third epileptiform pattern is the poly spike which consists of several sequenced spikes followed by slow waves [20] , at a frequency of 3 - 6 Hz [17] .
1.4. Proposed Approach
Our approach comprises two steps:
1) Derivation of template models for three epileptic spikes; Epileptic complex spike (ECS), Epileptic isolated spike (EIS) and Epileptic poly spike (EPS).
2) Analogizing (comparing) various features of the predefined templates against the same features extracted from a sequenced window of the raw EEG data under analysis.
Mainly, spectral, spatial and frequency-temporal features were analyzed to detect similar windows of the raw data to any epileptic spike templates (ECS, EIS and EPS). Lomb-Scargle Transform (LST), Independent Component Analysis (ICA) and Daubechies Wavelet (Db4) are provided to extract features that will be analogized via a simple technique called “Cosine Similarity”. Firstly, the Cosine Similarity measure is used as a pre-classifier in a manner that; if there are similarities (according to specified threshold for each characteristic) between the tested window and any template, the tested window is suspected to contain an epileptic spike. Secondly, to avoid spike deception (reducing the false detection rate), a variable threshold determined by Mean Squared Error (MSE) is applied, to decide either the tested window is an epileptic spike or a noisy background.
2. Materials and Methods
This study was conducted in the Clinical Neurophysiology Unit, Kasr El Aini Hospital, Faculty of medicine, Cairo University. The data used for this research were obtained from epileptic patients who had been under evaluation and treatment in the Clinical Neurophysiology Unit. The patients were diagnosed as they suffered from different epilepsy types. Through this study, all international ethical committees was taken into account, the patients were notified and agreed to conduct this study on their EEGs. The EEG was recorded using scalp electrodes according to the International 10 - 20 system.
2.1. Data Acquisition
Two real EEG data sets were used during development procedures and performance evaluation of the method. These data are the following:
1) Dataset 1 (for extracting epileptic spikes)
By the close investigation of many epileptic EEG recordings (containing various morphologies of spikes, sharp waves, spike and-wave complexes, poly spike-and-wave complexes), neurophysiologists were requested to identify only three epileptic spike types. Each spike type was marked along its duration. Several patterns for each spike type was picked up and separated into discrete segments of 350 msec. A template model for each spike type is estimated based on these extracted segments.
2) Dataset 2 (for testing)
The data were collected from 15 epileptic patients and allocated for training and performance evaluation of the method.
2.2. Pre-Processing and Artifact Rejection
The noises in EEG are called artifacts that should be removed for proper analysis and minimize their influence in the feature extraction stage. These artifacts are generated from the body itself (bio artifacts) or caused by external interferences. Bio artifacts are originating from non-cerebral sources as EMG, EOG and ECG but may be detected by an EEG machine. External artifacts are occurring during recording due to bad electrodes, baseline movement, electrical power line (50/60 Hz), mobile interference…To minimize the influence of these artifacts, an analog anti-aliasing filter and a band-pass Butterworth filter (with sampling frequency 200 Hz and cut-off frequencies of 0.1 - 100 Hz) were used to remove linear trends, attenuate channel drifts and improve the reliability of ICA decomposition.
2.3. Template Derivation
To perform a certain template, suitable epochs corresponding to each spike type were picked out by qualified neurophysiologists from epileptic EEG recordings. The width of each segment was 350 msec (70 samples) to include a whole epileptiform discharge (clear spike) and its instantaneous background. The template for each epileptic spike (ECS, EIS, and EPS) is estimated as the median of its separated segments, (mostly, 115 - 120 segments were selected for each spike type).
2.4. Feature Extraction
2.4.1. Independent Component Analysis (ICA) and Wavelet Transform (WT)
Independent Component Analysis (ICA) is a recent technique to separate statistically independent components from a mixture of data [21] . ICA can be used to decompose the signal into spatio-temporal independent components, giving important information about the spatial and temporal features of the signal [22] [23] . Kobayashi et al., in [24] [25] [26] has illustrated that, the scalp EEG can be decomposed by ICA into components with fixed potential field distributions (spatial components) and maximally independent time waveforms (temporal components), indicating that, ICA performs better than Principal Component Analysis (PCA) in this task. Moreover, the epileptic transients (as spikes) can be separated from the background, (since each transient having a slightly different generator geometry, very small time delay and jitter), concluding that the scalp distribution of the epileptic components obtained by ICA contains important spatial information about the generator sources of epileptiform discharges. The results in [25] indicate that the spatial components of epileptic discharges (extracted by ICA) could be powerful to identify the location of epileptic generator sources of the scalp. Details of algorithms to extract the spatial components by ICA are described in [24] [27] . Briefly, let x(t) denote a vector of n observed signals x at time t;
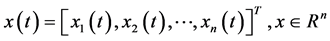 (1)
(1)
where, x(t) is a mixture of m unknown sources s;
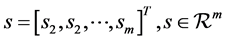 (2)
(2)
To obtain the temporal independent components Y, produced by m sources, ICA estimates a separating (unmixing) matrix W such that;
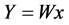 (3)
(3)
Equation (3) implies that;
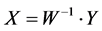 (4)
(4)
Onton, et al., in [27] has shown that; the resulting (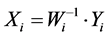 ), i.e. the product of the ith column of inverse matrix (
), i.e. the product of the ith column of inverse matrix (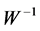 ) and the ith row of Y, is a portion of the original data (X) that forms the spatial independent components (
) and the ith row of Y, is a portion of the original data (X) that forms the spatial independent components (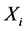 ), and the whole data (X) are the sum of these ICs (
), and the whole data (X) are the sum of these ICs (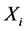 ),
),
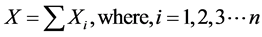 (5)
(5)
Thus, the columns of the inverse matrix (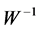 ) contain relative weights (spatial features) by which each independent component (
) contain relative weights (spatial features) by which each independent component (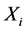 ), could be projected back into the scalp, forming what is called IC scalp map. In other words, each column of the inverse matrix (
), could be projected back into the scalp, forming what is called IC scalp map. In other words, each column of the inverse matrix (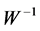 ) represents the relative projection weight where the independent components (ICs) denote its source locations on the scalp at each electrode of a single component source [27] . Consequently, in our method, it was suggested that; by comparing the spatial components (
) represents the relative projection weight where the independent components (ICs) denote its source locations on the scalp at each electrode of a single component source [27] . Consequently, in our method, it was suggested that; by comparing the spatial components (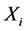 ) for both the tested EEG window (segments of the signal under analysis) and each spike template (according to an appropriate threshold), waveforms that resemble any of spike templates (ECS, EIS and EPS) along the tested EEG signal could be recognized. However, the performance can be improved by extracting more features in other spaces beside that extracted by ICA. Recently, researchers have advised combining both Wavelet (WT) and ICA to exploit their benefits which complement each other [28] . Wavelet Transform (WT) gives a macroscopic vision by decomposing the signal into its constituent frequency bands at different time localizations, according to the desired scale’s resolution [16] . For a conventional mother wavelet
) for both the tested EEG window (segments of the signal under analysis) and each spike template (according to an appropriate threshold), waveforms that resemble any of spike templates (ECS, EIS and EPS) along the tested EEG signal could be recognized. However, the performance can be improved by extracting more features in other spaces beside that extracted by ICA. Recently, researchers have advised combining both Wavelet (WT) and ICA to exploit their benefits which complement each other [28] . Wavelet Transform (WT) gives a macroscopic vision by decomposing the signal into its constituent frequency bands at different time localizations, according to the desired scale’s resolution [16] . For a conventional mother wavelet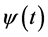 , the Discrete Wavelet Transform (DWT) at scale “a” and position “b”, is defined as;
, the Discrete Wavelet Transform (DWT) at scale “a” and position “b”, is defined as;
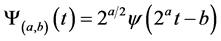 (6)
(6)
where, a and b are discrete values based on powers of two, 


By varying “b”, the wavelet function 
2.4.2. Lomb-Scargle Periodogram (LSP)
A significant characteristic that discriminates epileptic spikes from other EEG waveforms is its increase in instantaneous energy [32] . Due to the nonstationary nature of EEG signals, (meaning that characteristics as mean, variance or power-spectra, change with time [37] ), detection of spikes by inspecting its spectrum in time-domain, is not preferable. Since the amplitude and spectrum of spikes vary from signal to signal, subject to subject, it even varies from time to time for the same subject [32] . On the other hand, the “rhythmic” behavior of epileptic seizures that characterized by a peak in power spectrum at a specific frequencies [8] , could be used to reveal such epileptic spikes. Saini et al. [38] showed the efficiency of applying Lomb-Scargle transform (LST) for estimating the power spectrum, which could be convenient for our application. For xn, provides a vector of n observed signals x at time n, the Lomb periodogram at frequency f is defined by;

where


2.5. Cosine Similarity Measure
Mathematically, one to one function is that produces one specific output for each one input. The cosine function is from these functions, because each tiny angle change is accompanied by precise increment or decrement in its cosine value. This significant property can be employed to detect similarity (coincidence) between two vectors by determining the cosine of the angle between them, at different time points. Using a cosine function for such purposes is called Cosine Similarity measure. It is currently implemented in speech and face recognition, fingerprints, text, and signature investigation. Compared to other tools, simplicity, accuracy and higher resolution of Cosine Similarity (since its outcome is neatly bounded between −1 to 1), give it many preferences to detect similarity between patterns. The Cosine Similarity measure has been used in many EEG applications. For instance, in [39] , this measure is used to reject ocular artifacts (EOG) by discarding similar waveforms to EOG patterns in the tested EEG signal along different channels. By using this approach, similarity between patterns is calculated according to its vectors’ directions, making this technique appropriate for fast transients and chaotic signals as EEG. This is detailed in [40] where a new technique based on this measure was used to identify similarity between two time series, at different time locations. Powered with good results in [40] , the author recommended applying this technique for audio recognition and detecting variability of beat to beat in ECG signals. In current work, for each initial epileptic spike template (ECS, EIS and EPS), the spatial, wavelet and spectral features were extracted and ranked as N rows of an N × M matrix, then it is reshaped into an N∙M size vector, (since the length is not the same in all feature types). Thus, a specific vector feature for each template involves adequate attributes that depict its distribution over the features-plane space. Accordingly, identical procedures will be performed for each consecutive window of raw EEG data under test, to compose its vector of features. The Cosine Similarity measure decides whether the data under analysis contains similar waveforms to any spike template or not, (by identifying similarity between their vector of features, according to a specific threshold for each template).
Let the vector feature of the i-th window (of the raw EEG data under analysis), is denoted by;

and

is the vector feature of epileptic spike templates; ECS, EIS, and EPS, for
The cosine value of the angle 



To avoid the periodic nature of the cosine function, the absolute value of








If MSE of the i-th window 



then, 







where σ, is the standard deviation of the previous MSE thresholds. At that stage, the procedure will be started again with the default template 


3. Results
The described method was tested using EEG data set 2 mentioned above. The set contains 20 single channel EEG data files (with different lengths), were gathered by scalp EEG electrodes according to the 10 - 20 international system. These data files were collected during various stages of sleep-wake cycles, including a wide variety of epileptiform activities from 15 epileptic patients (11 males, 4 females), aged 14 - 65 years. From this data, 10 files were intermixed and randomly selected as a tested set. The performance has been evaluated according to the conventional examination by experienced neurophysiologists from Clinical Neuro Physiology Unit, Kasr El Aini Hospital, Faculty of Medicine, Cairo University, where their decision was used as the “gold standard”. In detail, during the visual investigation of the tested data, neurophysiologists were asked to mark each spike, by labeling the type, onset time and its duration. Later, the results obtained by the method were assessed based on the report made by the experts. However, the performance was also determined in terms of Sensitivity, Specificity, Selectivity and Average Detection Rate. According to this test, our method correctly identified 663 of 710 as isolated spikes, providing a sensitivity of 93.3%, a specificity of 90.1, a selectivity of 92.3 and average detection rate of 91.7%, which is the best compared to the results of the other two spike types. On the other hand, for complex spike type, the method succeeded to detect 306 of 361 with 84.7%, 88.6%, 82.2% and 86.6% in terms of sensitivity, specificity, selectivity and average detection rate respectively, while 207 of 258 were identified as poly spikes with 80.2%, 88.8%, 78.4% and 84.5% of sensitivity, specificity, selectivity and average detection rate respectively. The overall performance was 88.4% sensitivity, 89.2% specificity, 86.8% selectivity, and 88% for average detection rate when results of the three spike types were combined. The total number of missed spikes was 153. These missed spikes were reexamined by neurophysiologists and classified as 47 isolated, 55 complex and 51 poly spikes. By carefully reviewing all missed spikes, it was found that its duration ranged from 18 to 97 ms with amplitude between 90 - 176 μV. The inability to detect these short spikes is due to the width chosen for the initial templates (ECS, EIS, and EPS). Although the template length can be decreased, but it will be more sensitive to tiny artifacts and spurious spikes resulting unreasonable percentage of false detections (this was concluded after many experimental procedures on training data).
In total 178 false detections were verified and categorized by neurophysiologists as the following;
- 41 false-detections were Wicket spikes (characterized by sharp arched waveform due to temporal alpha activity).
- 24 false-detections were Vertex spikes (quite sharply repetitive waves lasting in 200 m Sec with amplitude up to 150 - 250 μV).
- 37 false-detections were due to different EEG artifacts, and only 76 false-detections were reported as false positives. According to these observations, a pleasurable percentage of false detections are more satisfactory rather than missing a true spike. However, fast computational time of the algorithm shouldn’t be ignored, on average, 2.091 Sec was required to process 600 windows of raw EEG data (3.5 min) on a 2.10 GHz, Intel Core i3 laptop.
4. Conclusion and Future Work
EEG morphology is the primary, meaningful characteristic reflects the brain activity. It can be said, each waveform in EEG is generated due to its own brain’s mechanisms that completely different from others. These mechanisms follow nonlinear and chaotic nature resulting enormous EEG waveforms. Spikes are from such EEG waveforms that play essential roles to diagnose brain disorders. Many spike types are common waveforms accompanied in epileptic EEG. Identification the presence of spikes provides vital diagnostic information in many clinical situations. Spikes are not just a sudden amplitude rise (in the time domain) distinguished from its background, but also containing underlying features in frequency and spatial domains. In this paper, we have presented a methodology to detect only three types of epileptic spikes which are isolated, wave complex and poly spikes in prolonged EEG recordings. According to results, the method achieves 88.4% sensitivity, 89.2% specificity, 86.8% selectivity, and 88% for average detection rate. While we present these results that may be promising, but at the same time, it must be remembered the algorithm performance was evaluated on limited data sets. Also, the measured parameters TP, FN, FP and TN were determined according to a visual investigation by EEG experts, utilizing the True Scan EEG system (DEYMED Diagnostic, Czech Republic), where human error during this manual inspection cannot be ignored. It is very difficult to compare our algorithm against others because the focus of this study is detecting only three spike types as a preliminary stage that will be extended for more epileptic waveforms. Besides, our tested data are different from the data used to evaluate other suggested methods, with various measurements for evaluating the performance of each method, and opinions of experts about spike patterns. From this study, we have obviously concluded that, analyzing EEG signal in a broad framework, including temporal, frequency, and spatial domains may be a good solution to capture fast EEG transients as epileptic spikes. Also, to take advantage of information and activities of adjacent channels, it is recommended that processing EEG data in a multichannel scheme can highly improve the performance rather than processing each channel individually. We will recruit this important note in the future work. In our algorithm, the three waveforms isolated, wave complex and poly spikes were chosen mainly for simplicity to employ the concept of processing EEG in more than one domain can overcome its chaotic and nonlinearity nature. Improvement of our algorithm by including extra epileptic waveforms and validation with larger data sets is our next tasks. From approval conditions of a new tool is its fast and easy use in clinical practice. So fast computational time performs very crucial advantage, especially in EEG field, where large quantity data are required to be inspected. In conclusion, according to the reasonable performance of our method, particularly the fast computation that nominates it as a simple, powerful and fast tool for offline quantitative EEG examinations, saving more time and exertion. However, in the longer run, transforming EEG processing into a comprehensive domain, including its temporal, frequency and spatial underlying features, could provide a promising platform for smart diagnostic tools for many neurological disorders.
Acknowledgements
The authors gratefully appreciate the contributions of Dr. Rajeev Yadav and acknowledge his encouragement and advice.
Cite this paper
Dawson, A. (2017). Why Marx Was a Bad Driver: Alienation to Sensuality in the Anthropology of Automobility. Advances in Anthropology, 7, 1-16. http://dx.doi.org/10.4236/aa.2017.71001
References
- 1. Longo, D.L (2012) 369 Seizures and Epilepsy Harrison’s Principles of Internal Medicine. 18th Edition, McGraw-Hill, New York, 3258.
- 2. Lerche, H., Shah, M., Beck, H., Noebels, J., Johnston, D. and Vincent, A. (2012) Ion Channels in Genetic and Acquired Forms of Epilepsy. The Journal of Physiology, 591, 753-764. Published Online 2012 October 22.
https://doi.org/10.1113/jphysiol.2012.240606 - 3. Acir, N., Oztura, I., Kuntalp, M., Balkan, B. and Guzelis, C. (2005) Automatic Detection of Epileptiform Events in EEG by a Three-Stage Procedure Based on Artificial Neural Networks. IEEE Transactions on Biomedical Engineering, 52, 30-40.
https://doi.org/10.1109/TBME.2004.839630 - 4. Radmehr, M. and Anisheh, S.M. (2013) EEG Spike Detection Using Stationary Wavelet Transform and Time-Varying Autoregressive Model. International Journal of Computer Applications, 83, 3 p.
https://doi.org/10.5120/14505-2117 - 5. Salam, M.T., Sawan, M. and Nguyen, D.K. (2011) A Novel Low-Power-Implantable Epileptic Seizure-Onset Detector. IEEE Transactions on Biomedical Circuits and Systems, 5, 568-578.
- 6. Yadav, R., Swamy, M.N.S. and Agarwal, R. (2012) Model-Based Seizure Detection for Intracranial EEG Recordings. IEEE Transactions on Biomedical Engineering, 59, 1419-1428.
- 7. Yadav, R., Shah, A.K., Loeb, J.A., Swamy, M.N.S. and Agarwal, R. (2012) Morphology-Based Automatic Seizure Detector for Intracerebral EEG Recordings. IEEE Transactions on Biomedical Engineering, 59, 1871-1881.
https://doi.org/10.1109/TBME.2012.2190601 - 8. Yadav, R., Agarwal, R. and Swamy, M.N.S. (2007) Detection of Epileptic Seizures in Stereo-EEG Using Frequency-Weighted Energy. Circuits and Systems, 50th Midwest Symposium, MWSCAS 2007, Montreal, 5 -8 August 2007, 77-80.
https://doi.org/10.1109/mwscas.2007.4488544 - 9. Yadav, R., Agarwal, R. and Swamy, M.N.S. (2008) A Novel Dual-Stage Classifier for Automatic Detection of Epileptic Seizures. 30th Annual International Conference of the Engineering in Medicine and Biology Society, Vancouver, 20-25 August 2008, 911-914.
https://doi.org/10.1109/iembs.2008.4649302 - 10. Yadav, R., Agarwal, R. and Swamy, M.N.S. (2009) A New Improved Model-Based Seizure Detection Using Statistically Optimal Null Filter. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2009, 1318-1322.
https://doi.org/10.1109/iembs.2009.5334138 - 11. Yadav, R., Agarwal, R. and Swamy, M.N.S. (2010) A Novel Morphology-Based Classifier for Automatic Detection of Epileptic Seizures. International Conference of the IEEE Engineering in Medicine and Biology Society, Buenos Aires, 31 August-4 September 2010, 5545-5548.
https://doi.org/10.1109/iembs.2010.5626781 - 12. Yadav, R., Shah, A.K., Loeb, J.A., Swamy, M.N.S. and Agarwal, R. (2011) A Novel Unsupervised Spike Sorting Algorithm for Intracranial EEG. 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, 30 August-3 September 2011, 7545-7548.
- 13. Oikonomou, V.P., Tzallas, A.T. and Fotiadis, D.I. (2007) A Kalman Filter Based Methodology for EEG Spike Enhancement. Computer Methods and Programs in Biomedicine, 85, 101-108.
https://doi.org/10.1016/j.cmpb.2006.10.003 - 14. Tzallas, A.T., Tsipouras, M.G., Tsalikakis, D.G., Karvounis, E.C., Astrakas, L., Konitsiotis, S. and Tzaphlidou, M. (2012) Automated Epileptic Seizure Detection Methods: A Review Study. In: Stevanovic, D., Ed., Epilepsy-Histological, Electroencephalographic and Psychological Aspects, InTech.
http://www.intechopen.com/books/epilepsy-histological-electroencephalographic-and-psychological-aspects/automated-epileptic-seizure-detection-methods-a-review-study - 15. Kamath, C. (2015). Analysis of EEG Dynamics in Epileptic Patients and Healthy Subjects Using Hilbert Transform Scatter Plots. Open Access Library Journal, 2, e745.
https://doi.org/10.4236/oalib.1100745 - 16. Orosco, L., Correa, A. and Laciar, E. (2013). Review: A Survey of Performance and Techniques for Automatic Epilepsy Detection. Journal of Medical and Biological Engineering, 33, 526-537.
https://doi.org/10.5405/jmbe.1463 - 17. Seneviratne, U., Cook, M. and D’Souza, W. (2012) The Electroencephalogram of Idiopathic Generalized Epilepsy. Epilepsia, 53, 234-248.
https://doi.org/10.1111/j.1528-1167.2011.03344.x - 18. GopikaGopan, K., Harsha, A., Joseph, L.A. and Kollialil, E.S. (2013) Adaptive Neuro-Fuzzy Classifier for “Petit Mal” Epilepsy Detection Using Mean Teager Energy. International Conference on Advances in Computing, Communications and Informatics, Mysore, 22-25 August 2013, 752-757.
- 19. Markand, O.N. (2003) Pearls, Perils, and Pitfalls in the Use of the Electroencephalogram. Seminars in Neurology, 23, 7-46.
https://doi.org/10.1055/s-2003-40750 - 20. Pang, C.C.C., Upton, A., Shine, C. and Kamath, M. (2003) A Comparison of Algorithms for Detection of Spikes in the Electroencephalogram. IEEE Transactions on Biomedical Engineering, 50, 521-525.
https://doi.org/10.1109/TBME.2003.809479 - 21. Hyvarinen, A., Karhunen, J. and Oja, P. (2001) Independent Component Analysis. John Wiley and Sons, Hoboken.
https://doi.org/10.1002/0471221317 - 22. Comon, P. and Jutten, C. (2009) Handbook of Blind Source Separation of Independent Component Analysis and Applications. Academic Press, Cambridge.
- 23. Ossadtchi, A., Baillet, S., Mosher, J.C., Thyerlei, D., Sutherling, W. and Leahy, R.M. (2004) Automated Interictal Spike Detection and Source Localization in Magnetoencephalography Using Independent Components Analysis and Spatiotemporal Clustering. Clinical Neurophysiology, 115, 508-522.
https://doi.org/10.1016/j.clinph.2003.10.036 - 24. Kobayashi, K., James, C.J., Nakahori, T., Akiyama, T. and Gotman, J. (1999) Isolation of Epileptiform Discharges from Unaveraged EEG by Independent Component Analysis. Clinical Neurophysiology, 110, 1755-1763.
https://doi.org/10.1016/S1388-2457(99)00134-0 - 25. Kobayashi, K., Merlet, I. and Gotman, J. (2001) Separation of Spikes from Background by Independent Component Analysis with Dipole Modeling and Comparison to Intracranial Recording. Clinical Neurophysiology, 112, 405-413.
https://doi.org/10.1016/S1388-2457(01)00457-6 - 26. Kobayashi, K., Akiyama, T., Nakahori, T., Yoshinaga, H. and Gotman, J. (2002) Systematic Source Estimation of Spikes by a Combination of Independent Component Analysis and RAP-MUSIC. II: Preliminary Clinical Application. Clinical Neurophysiology, 113, 725-734.
https://doi.org/10.1016/S1388-2457(02)00047-0 - 27. Onton, J., Westerfield, M., Townsend, J. and Makeig, S. (2006) Review Imaging Human EEG Dynamics Using Independent Component Analysis. Neuroscience and Biobehavioral Reviews, 30, 808-822.
https://doi.org/10.1016/j.neubiorev.2006.06.007 - 28. Walters-Williams, J. and Li, Y. (2011) A New Approach to Denoising EEG Signals—Merger of Translation Invariant Wavelet and ICA. International Journal of Biometric and Bioinformatics, 5, 130-148.
- 29. Song, Y. and Zhang, J. (2013) Automatic Recognition of Epileptic EEG Patterns via Extreme Learning Machine and Multiresolution Feature Extraction. Expert Systems with Applications, 40, 5477-5489.
https://doi.org/10.1016/j.eswa.2013.04.025 - 30. Indiradevi, K.P., Elias, E., Sathidevi, P.S., Dinesh Nayak, S. and Radhakrishnan, K. (2008) A Multi-Level Wavelet Approach for Automatic Detection of Epileptic Spikes in the Electroencephalogram. Computers in Biology and Medicine, 38, 805-816.
https://doi.org/10.1016/j.compbiomed.2008.04.010 - 31. Halford, J., Schalkoff, R. and Zhou, J. (2013) Standardized Database Development for EEG Epileptiform Transient Detection: EEGnet Scoring System and Machine Learning Analysis. Journal of Neuroscience, 212, 308-316.
https://doi.org/10.1016/j.jneumeth.2012.11.005 - 32. Suresh, H.N. and Balasubramanyam, V. (2012) Wavelet Transforms and Neural Network Approach for Epileptical EEG. 12-17.
- 33. Jahankhani, P., Kodogiannis, V. and Revett, K. (2006) EEG Signal Classification Using Wavelet Feature Extraction and Neural Networks. IEEE John Vincent Atanasoff 2006 International Symposium on Modern Computing, Sofia, 3-6 October 2006, 120-124.
https://doi.org/10.1109/JVA.2006.17 - 34. Subasi, A. (2007) EEG Signal Classification Using Wavelet Feature Extraction and a Mixture of Expert Model. Expert Systems with Applications, 32, 1084-1093.
https://doi.org/10.1016/j.eswa.2006.02.005 - 35. Guo, L., Rivero, D., Dorado, J., Rabunal, J.R. and Pazos, A. (2010) Automatic Epileptic Seizure Detection in EEG Based on Line Length Feature and Artificial Neural Network. Journal of Neuroscience Methods, 191, 101-109.
https://doi.org/10.1016/j.jneumeth.2010.05.020 - 36. Esteller, R., Echauz, J. and Tcheng, T. (2004) Comparison of Line Length Feature before and after Brain Electrical Stimulation in Epileptic Patients. 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2, 4710-4713.
https://doi.org/10.1109/IEMBS.2004.1404304 - 37. Quiroga, R.Q. (1998) Quantitative Analysis of EEG Signals: Time-Frequency Methods and Chaos Theory. Institute of Physiology-Medical University Lubeck and Institute of Signal Processing-Medical University Lubeck.
- 38. Saini, B.S., Singh, D., Uddin, M. and Kumar, V. (2008) Improved Power Spectrum Estimation for RR-Interval Time Series. World Academy of Science, Engineering and Technology, 46, 44-48.
- 39. Li, M., Cui, Y. and Yang, J. (2013) Automatic Removal of Ocular Artifact from EEG with DWT and ICA Method. Applied Mathematics & Information Sciences, 7, 809-816.
https://doi.org/10.12785/amis/070252 - 40. Manzo-Martínez, A. and Camarena-Ibarrola, J. (2013) A New and Efficient Alignment Technique by Cosine Distance. International Journal of Combinatorial Optimization Problems and Informatics, 4, 12-24.

