Journal of Biomedical Science and Engineering
Vol.6 No.7A4(2013), Article ID:34540,5 pages DOI:10.4236/jbise.2013.67A4001
Lithium carbonate plus 131I in the treatment of Graves’ hyperthyroidism
![]()
1Department of Nuclear Medicine, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
2Department of Library, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, China
3Department of Pediatrics, Jinjiang Maternal and Child Health Hospital, Quanzhou, China
Email: *zjs630805@126.com
Copyright © 2013 Jinshun Zha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 16 May 2013; revised 25 June 2013; accepted 5 July 2013
Keywords: Lithium Carbonate; Iodine Radioisotopes; Graves’ Hyperthyroidism; Chemotherapy; Adjuvant
ABSTRACT
Aims: Effectiveness of radioiodine for Graves’ hyperthyroidism (GD) depends on its intrathyroidal persistence, which could be enhanced by lithium by blocking the release of organic iodine and thyroid hormone from the thyroid gland. The present aim focused on the effect of the addition of lithium carbonate to 131I therapy in patients with GD. Methods: 100 consecutive patients with GD were randomly assigned to two groups: group (A) patients treated with 131I and group (B) patients treated with 131I plus lithium carbonate. Patients in B group were treated with a dose of 0.5 g per day (2 × 0.25 g) of lithium carbonate for half a month before and after the administration of 131I. Thyroid weight was estimated by ultrasonography and careful palpation of the thyroid. Radiation absorbed dose rate in the front of the neck was measured on days 1530 and 45 after the administration of 131I. Serum concentrations of thyroidstimulation hormone (TSH), fee tri-iodothyrosine (T3) and free thyroxine (T4) were tested on days 30, 45, 90, 180 before and after treatment. Results: After RIT, radiation absorbed dose rate in the front of neck gradually decreased as time went on (p < 0.01), all of which were significantly higher in B group than those in A group (p < 0.01). Of all different time periods before and after treatment, the mean serum TSH of the two groups was below the normal range without significant difference (p > 0.05), free T3 and free T4 values in both groups rose significantly one month after treatment (all p < 0.01) , then decreased into the normal ranges. Over 30 d of treatment, the values in B group patients were much lower than those in A group (all p < 0.01). No significance was found in other time periods (all p > 0.05). Cure rate of hyperthyroidism was achieved in 36 of the 50 patients (72%) treated with 131I alone and in 38 of the 50 patients (76%) treated with 131I plus lithium. Conclusion: We suggest that for patients withdrawing of ATD and those with short effective half-time, as well as those intolerant or invalid, the short term addition of lithium to 131I allows for a better control of thyrotoxia and the completeness of treatment.
1. INTRODUCTION
131I therapy (RIT) has been accepted as a well-established and effective treatment for Graves’ hyperthyroidism (GD). Effectiveness of radioiodine depends on several factors, including course of disease, dosages of 131I, radiosensitivity and goiter volume, as well as retention volume of 131I in the thyroid gland, its effective half-life and previous treatment with antithyroid drugs (ATD). Lithium carbonate blocks the release of organic iodine and thyroid hormone from the thyroid gland without affecting thyroidal active iodine uptake. Accordingly, it is a doubt whether the short-term addition of lithium carbonate, before and after RIT, could prevent the short deterioration of hyperthyroidism and improve therapeutic effect in patients with GD. To address this question, we performed a randomized controlled study to evaluate the addition of lithium carbonate to RIT therapy.
2. SUBJECTS AND METHODS
2.1. Study Groups
During the period from October 2010 to July 2012, we enrolled 100 sequential GD patients with RIT treated for the first time in our department. The age of 29 females ranged from 17 to 62 years (mean 37.5 years), and the age of 71 females ranged from 10 to 53 years (mean 29.7 years). Random allocation was taken to averagely 50 patients into two groups: group (A) patients treated with 131I and group (B) patients treated with 131I plus lithium carbonate. There were no significant difference between the age of two groups (t = 1.019, p > 0.05). Patients in B group were treated with lithium carbonate half one month before and after 131I treatment at a dose of 0.5 g per day (2 × 0.25 g). Each patient received a baseline evaluation, including routine tests of blood, urine and stool, liver and renal functions, thyroid ultrasonography and electrocardiogram. Any patient who had liver or renal disease was excluded from the study.
The study was approved by the investigational review boards and human use committees in Fujian Medical University 2nd Affiliated Hospital.
2.2. Laboratory Study
Thyroid weight was estimated by ultrasonography and careful palpation, and no difference was found between the two groups (t = 0.987, p > 0.05). The dose of 131I was: thyroid weight (g) × (2.59 - 4.44 MBq) per gram of estimated thyroid tissue/24-h radioactive iodide uptake (RAIU) in the thyroid (%), and adjusted according to patients’ age, course of disease, complications, thyroid textures and weight. The average dose of 131I was 240.5 (148 - 444) MBq. And if the therapeutic dose of 131I was more than 370 MBq, two-dose baiting was given at the first dosage of 314.5 MBq and the remains were given after 48 hours. There was no significant difference on the average dose of 131I between two groups (t = 1.049, p > 0.05).
For patients in both groups, radiation absorbed dose rate the front of neck was measured on days 1530 and 45 after the administration of 131I. Serum creatinine and blood urea nitrogen was measured after 15 d of treatment in B group patients, and free T3, free T4 and TSH were tested before and on days 30, 45, 90, 180 after treatment.
2.3. Evaluation
The therapeutic effects of patients were defined during half year visit as follows: 1) Clinical cure. Symptoms and signs of hyperthyroidism totally disappeared after half year visit, with normal rang of serum FT3 and FT4. 2) Improvement. The symptoms alleviated and signs disappeared partially; Serum FT3 and FT4 decreased significantly, but they were still above normal range. 3) Invalid. The symptoms and signs did not ameliorate, even aggravated; Serum FT3 and FT4 did not decrease significantly. 4) Recurrence. The symptoms and signs of hyperthyroidism reappeared, and thyroid hormones rise again after achieving the criteria of clinical cure. 5) Hypothyroidism. The symptoms and signs of hypothyroidism appeared in patients with low thyroid hormones but high TSH.
2.4. Statistical Analysis
Data were analyzed using SPSS 16.0 for Windows. Results were presented as means ± SD for normal distribution and median (M) for skewed distribution. The therapeutic effects of two groups were compared by x-square test. ANOVA with post hoc Dunnett’s T3 test was used to compare serum thyroid hormones and radiation absorbed dose rate the front of neck between two groups before and after treatment. Differences between the two groups of the same period in serum thyroid hormones were evaluated by t test. All p values < 0.05 (two-tailed) were considered to be significant.
3. RESULTS
Our study, after treatment with 31I plus lithium carbonate, showed that serum urea nitrogen and creatinine were in normal range on days 15.
3.1. Dosimetry
Table 1 showed changes in radiation absorbed dose rate the front of neck in A group and B group. The mean 24 h-RAIU before RIT was 87.2% in A and 89.5% in B group, without significant difference (t = 0.887, p > 0.05) between them. After RIT, radiation absorbed dose rate the front of neck decreased gradually as time went on (F = 17.65, <0.01), all of which were significantly higher in B group than that in A group (F = 98.12, <0.01).
3.2. Thyroid Hormone Levels
Of all different time periods before and after treatment, the mean serum TSH of the two groups were below the normal level without significant difference (F = 1.235, p > 0.05), and serum free T3 and free T4 had significant differences (F = 9.651, F = 22.45, respectively. p < 0.01) (Table 2). Neither free T3 nor free T4 values of the pretreated patients in both groups showed any difference (t = 0.987, t = 1.011, respectively, all p > 0.05); but free T3 and free T4 values in different time period after RIT showed significant difference between the two groups (all p < 0.01). We found that free T3 and free T4 values in both groups rose significantly one month after treatment (Dunnett-t = 5.23, 10.14; 5.12, 9.98; all p < 0.01), then decreased to the normal ranges. In pretreated patients, both free T3 and free T4 values experienced decrease on days 45, 90 and 180 after treatment (Dunnett-t: A group: 9.89, 16.45; 12.34, 21.54; 13.12, 23.45, all p < 0.01; B group: 10.11, 13.65; 11.24, 19.45; 9.78, 18.24, all p < 0.01). And the values were higher on 45 day than that on 90 day and 180 day (Dunnett-t: A group 7.95, 15.21;
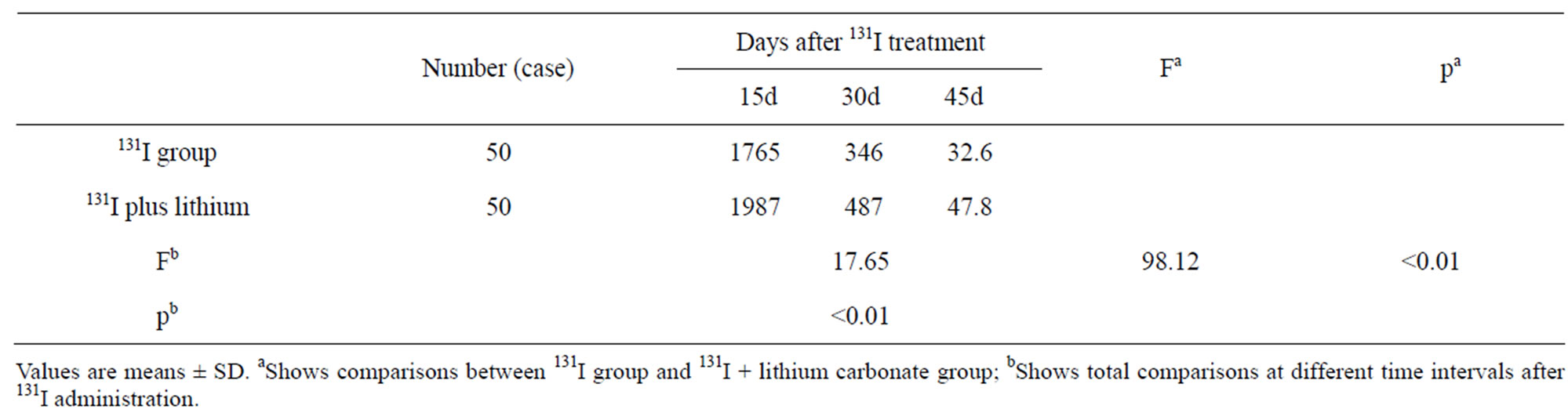
Table 1. Radiation absorbed dose rate the front of neck (μGy/h).
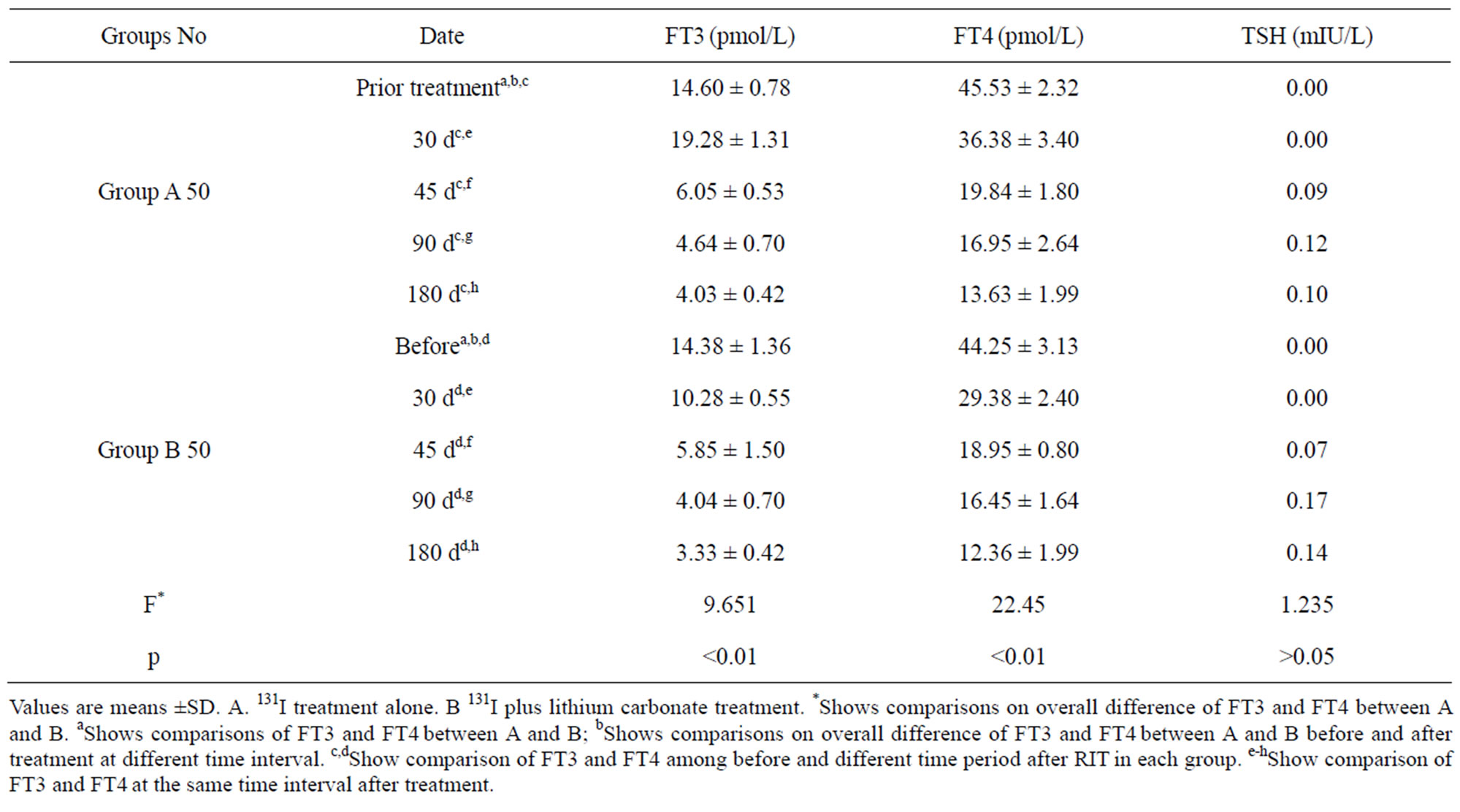
Table 2. Serum thyroid hormones before and after treatment.
9.21, 8.45, all p < 0.01, B group 13.26, 20.12, 10.22, 7.85, all p < 0.01). But the values in both groups on 90d and 180d did not achieve significance. We also compared free T3 and free T4 values in the same time period after treatment. Over 30 d of treatment, the values in B group patients were much lower than those in A group (t = 8.22, 19.18, respectively, all p < 0.01). No significance was found in other time period (all p > 0.05) (Table 3).
3.3. The Efficiency of Treatment As expected, cure of hyperthyroidism was achieved in 36 of the 50 patients (72%) treated with 131I alone and in 38 of the 50 patients (76%) treated with 131I plus lithium. Patients treated with 131I plus lithium had a higher probability of being cured than patients treated with 131I alone, although the two groups did not significantly differ at the end of the study (p > 0.05). In addition, 11 (22%) treated with 10 (20%) treated with 131I plus lithium had improving hyperthyroidism. Table 3 showed the details.
4. DISCUSSION
With radiation damage of thyroid follicle, temporary thyrotoxic symptoms and signs are the most common thyroid dysfunction in patients after receiving treatment due to the released thyroid hormones. The results showed that serum thyroid hormones elevated significantly after treatment of 131I, accounting for the symptoms above. In addition, we also found that one month later, serum thyroid hormones were elevated much lower in patients treated with lithium carbonate as an adjunct to 131I than that in patients treated with 131I alone, indicating the effectiveness of lithium carbonate in blocking the release of lithium carbonate from thyroid gland.
Lithium carbonate blocks the release of organic iodine
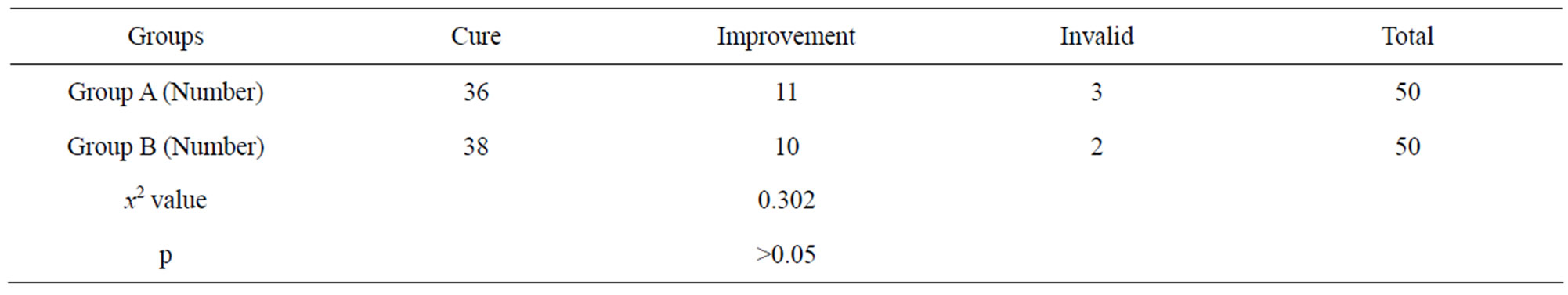
Table 3. Comparison of effective rate by different treatment.
and thyroid hormones from thyroid gland without affecting thyroidal RAIU. According to the radioactive decay and metabolic rule of organic iodine, radiation absorbed dose rate in the front of the neck reduced gradually at different time interval after treatment by 131I in the present study. Interestingly, in patients treated with lithium carbonate as an adjunct to 131I, mean radiation absorbed dose rate in the front of the neck was significantly higher than that with 131I alone. Without overt difference on RAIU and iodine doses, the present results indicated that the retention of 131I in thyroid gland increased after treatment with lithium carbonate as an adjunct to 131I, which might account for its prolonging effective halftime.
Lithium carbonate could decrease the ascending range of serum thyroid hormones and prolong the effective half-time of 131I. Does it potentiate the long-term therapeutic effect of 131I therapy? The present results showed, after half year treated by 131I, there was no significant difference between the two groups on cure rate, improvement rate and inefficiency. Whether it illustrated that lithium carbonate did not produce a final outcome of 131I therapy on hyperthyroidism? However, the results could not draw an appropriate conclusion based on the half-year study, and one or more years’ follow-up is needed.
Results which were available from previous study showed that RIT was a well-established method to treat Graves’ hyperthyroidism, the efficacy of which could be affected by several factors, such as the short persistence of radioiodine in the thyroid gland, effective half-life, previous treatment with antithyroid drugs (ATD), goiter volume and RAIU. The condition of patients will be transiently aggravated in the presence of radioactive thyroiditis, as well as ATD withdrawal before 131I administration [1-4]. Based on the available studies, with no significant alleviation or aggravation, or worrying about the short-term deterioration of thyrotoxicosis after 131I treatment, patients failed to discharge ATD administration before RIT treatment. Due to its radio resistance, pretreatment of PTU could diminish the therapeutic effectiveness or produce a higher failure rate of RIT. Based on the available literature, discontinuation of ATD for at least 4 days (propylthiouracil for 2 weeks) is required prior to RIT [1,5-9]. Fundamental and clinical investigations demonstrated that lithium carbonate, antipsychotic drug, could rapidly resist hyperthyroidism and elevate RAIU, and prolong effective half-time of iodine in thyroid gland. However, side effects of lithium therapy on central nervous system and renal, as well as the effectiveness of ATD administration, stemmed from its no regular application in clinical [5,10-12]. The present study showed that there were no abnormal results of biochemical test in patients after 15-d treatment by 131I, indicating that shortterm use of lithium carbonate was harmless on renal organs.
In summary, lithium carbonate could prolong effective half-life of 131I, and avoid the increase of serum free thyroid hormones one month after 131I treatment to potentiate the control of thyrotoxia. We suggest that for patients withdrawing of ATD and patients with short effective half-time, as well as those intolerant or invalid, the short-term addition of lithium to 131I allows for a better control of thyrotoxia and the completeness of treatment. The information of lithium on enhancing half-year cure rate is limited.
REFERENCES
- Akin, F., Yaylali, G.F. and Bastemir, M. (2008) The use of lithium carbonate in the preparation for definitive therapy in hyperthyroid patients. Medical Principles and Practice, 2, 167-170. doi:10.1159/000112975
- Bogazzi, F., Bartalena, L., Brogioni, S., et al. (1999) Comparison of radioiodine with radioiodine plus lithium in the treatment of Graves’ hyperthyroidism. The Journal of Clinical Endocrinology & Metabolism, 2, 499-503. doi:10.1210/jc.84.2.499
- Bal, C.S., Kumar, A. and Pandey, R.M. (2002) A randomized controlled trial to evaluate the adjuvant effect of lithium on radioiodine treatment of hyperthyroidism. Thyroid, 5, 399-405. doi:10.1089/105072502760043486
- Dunkelmann, S., Künstner, H., Nabavi, E., et al. (2006) Lithium as an adjunct to radioiodine therapy in Graves’ disease for prolonging the intrathyroidal effective halflife of radioiodine. Useful or not? Nuklearmedizin, 5, 213-218.
- Bogazzi, F., Bartalena, L., Campomori, A., et al. (2002) Treatment with lithium prevents serum thyroid hormone increase after thionamide withdrawal and radioiodine therapy in patients with Graves’ disease. The Journal of Clinical Endocrinology & Metabolism, 10, 4490-4495. doi:10.1210/jc.2002-020580
- Kirov, G., Tredget, J., John, R., et al. (2005) A cross-sectional and a prospective study of thyroid disorders in lithium-treated patients. Journal of Affective Disorders, 87, 313-317.
- Mijnhout, G.S. and Franken, A.A. (2008) Antithyroid drug regimens before and after 131I-therapy for hyperthyroidism: Evidence-based? Netherlands Journal of Medicine, 6, 238-241.
- Dunkelmann, S., Kuenstner, H., Nabavi, E., et al. (2007) Change in the intrathyroidal kinetics of radioiodine under continued and discontinued antithyroid medication in Graves’ disease. European Journal of Nuclear Medicine and Molecular Imaging, 2, 228-236. doi:10.1007/s00259-006-0234-z
- Burch, H.B., Solomon, L., Cooper, D.S., et al. (2001) The effect of antithyroid drug pretreatment on acute changes in thyroid hormone levels after (131)I ablation for Graves’ disease. The Journal of Clinical Endocrinology & Metabolism, 7, 3016-3021. doi:10.1210/jc.86.7.3016
- Ng, Y.W., Tiu, S.C., Choi, K.L., et al. (2006) Use of lithium in the treatment of thyrotoxicosis. Hong Kong Medical Journal, 4, 254-259.
- Hoogenberg, K., Beentjes, J.A., Piers, D.A. (1998) Lithium as an adjunct to radioactive iodine in treatment-resistant Graves thyrotoxicosis. Annals of Internal Medicine, 8, 670. doi:10.7326/0003-4819-129-8-199810150-00025
- Barbaro, D., Grosso, M., Boni, G., et al. (2010) Recombinant hurman TSH and ablation of post-surgical thyroid remnants in differentiated thyroid cancer the effect of pre-treatment with furosemide and furosemide plus lithium. European Journal of Nuclear Medicine and Molecular Imaging, 2, 242-249. doi:10.1007/s00259-009-1254-2
NOTES
*Corresponding author.

