Journal of Biomedical Science and Engineering
Vol. 5 No. 12 (2012) , Article ID: 25763 , 6 pages DOI:10.4236/jbise.2012.512087
The attachment of Staphylococcus epidermidis on the surface of a carbon paste electrode at various positive potentials: The effect of pH, incubation time, and solid-medium type
![]()
Department of Analytical Chemistry, University of Pardubice, Pardubice, Czech Republic
Email: libor.cervenka@upce.cz
Received 1 October 2012; revised 1 November 2012; accepted 10 November 2012
Keywords: Carbon Paste Electrode; Attachment; Staphylococcus Epidermidis
ABSTRACT
The attachment of Staphylococcus epidermidis to the surface of carbon paste electrode (CPE) by applying positive potentials (50 - 600 mV) with regard of various buffer pH, cultivation time and solid-medium type was studied. The attachment process was analyzed by measuring the electric current derived from the dye (amido black) adsorbed on the vacant areas of CPE after attachment of microbial cells. The pH was not identified as a single main factor affecting the attachment (p > 0.05), however further insight revealed that the potentials applied had different effects on the microbial cells attachment. Both cultivation time and solid-medium type significantly affected the microbial attachment. Generally, increase of cultivation time up to 168 h resulted in increase of adhesion. Applying potentials 300, 400 and 200 mV resulted in the highest attachment process for bacteria cultivated for 24, 48 and 168 h, respectively. S. epidermidis cultivated on the blood agar and Baird-Parker agar plates showed the higher extent of attachment.
1. INTRODUCTION
The surface characteristics of bacterial cells including hydrophobicity, surface charge and electron donor/acceptor properties play an important role in their attachment to various surfaces. Adherence of microorganisms is accepted as the initial step in the process of colonization of the host tissue or the formation of biofilm [1]. Microorganisms acquire a surface electric charge in aqueous environment due to the ionization of their surface chemical groups, such as amino, carboxyl and phosphoryl groups. The negative charge mostly predominates in bacteria. Since the cell surface is in direct contact with the environment, the charged groups within the surface of cells may interact with charged ions or molecules present in the external medium [2,3]. While hydrophobic interactions between hydrophobic molecules on the surface of the microorganism cells and the inert surface predominate at long distance (cca 10 nm), the electric surface charge acted at shorter distances [4]. In addition, physiological state of the bacterial cells and the pH of the surrounding environment also play a crucial role in attachment process [5-10].
The surface charge of microorganisms is necessary to determine and give us the information whether the cells may attached on the particular surface. Microbial surface charge is often determined using electrostatic interaction chromatography or by measurement of the electrophoretic motilities, and hence the determination of zeta potential using Smoluchowski equation [11,12]. Morisaki et al. [13] proposed a method for study of attachment of bacterial cells to carbon electrodes. The bacterial attachment process was analyzed indirectly by measuring the electric current derived from the organic dye adsorbed on the surface of carbon paste electrodes (CPE). They study the attachment of Pseudomonas syringe using anodic stripping method which allowed accumulating bacterial cells to the surface of CPE enhanced by the particular potential previously determined by the measurement of electrophoretic mobility. In our previous report, the attachment process of microbial cells to the surface of carbon paste electrode (CPE) was analyzed by measuring the electric current derived from the dye (amido black) adsorbed on the vacant areas of CPE after their attachment [14]. We demonstrated that various applied potentials influenced the attachment of Staphylococcus epidermidis onto the surface of CPE. The results showed that positive potentials applied during accumulation of bacteria S. epidermidis enhanced their adsorption to the surface of CPE in comparison with that occurred during free adsorption process and in negative potentials.
The objective of the study was to examine the effect of the solid-type mediums, pH of buffer solutions and the physiological state of S. epidermidis on its attachment to the surface of carbon paste electrode.
2. METHODS
2.1. Organisms and Culture Conditions
Staphylococcus epidermidis CCM 4418 (Czech Collection of Microorganisms, Masaryk University, Brno) stock culture was prepared as follows: the freeze-dried cells of S. epidermidis were activated by transfer into Nutrient agar no. 2 (MPA, HiMedia, Bombay, India) at 37˚C for 24 h followed by an appropriate incubation in MPA agar plate. Stock culture was kept in refrigerator and refreshed each 2 weeks. For the purpose of this study, a fresh culture was prepared from the stock culture by incubating inoculum in freshly prepared MPA (37˚C, 24 h) agar plate.
2.2. The Effect of pH, Cultivation Time and Culture Media on the Bacterial Attachment
From the freshly grown culture (37˚C, 24 h), a cell suspension was prepared in various Britton-Robinson buffers (pH 5.0, 7.2 and 9.0). The concentration of the cell suspension was adjusted to 1.5 according to McFarland turbidimetric standard.
A fresh culture was inoculated on the surface of MPA agar plate and incubated at 37˚C for 24, 48, 96 and 168 h. Thereafter, the cell suspension was prepared in Britton-Robinson buffer (pH 7.2) adjusted to 1.5 according to McFarland turbidimetric standard.
For the purpose of the determination of solid-medium type effect, other types of media were used: Trypton soya agar, blood agar supplemented with 7% of defibrinated sheep blood (BA) and Baird-Parker agar (B-P) (TSA, HiMedia, Bomaby, India). A fresh culture (37˚C, 24 h) was inoculated on the surface of each agar plate and incubated at 37˚C for 24 h. A suspension of bacterial cells was prepared as described above.
2.3. The Preparation of Carbon Paste Electrodes
A mixture of 0.5 g of graphite powder 5.5 - 7.0 μm (CR-5, Maziva Týn n. L., s.r.o., Czech Republic) and 130 μL of mineral oil (M5904, Sigma-Aldrich, Germany) was prepared in ceramic mortar. Freshly prepared carbon paste was packed into the teflon piston holder with inner diameter of 2.0 mm [15]. The resistance of such prepared electrode was 11.7 ± 2.1 Ω.
2.4. Chemicals
All the reagents were purchased in Sigma-Aldrich (Darmstadt, Germany). Amido black dye solution (1.0 µmol·L−1) was prepared by dilution of appropriate amount of the dye in phosphate buffer (pH 7.2). Distilled water purified by deionized water system was used in this study (G ≤ 0.055 µS). The soluble oxygen was removed from all the solutions by purging with argon for 15 min (purity 99.99%, Linde Technoplyn, Prague, Czech Republic).
2.5. Electrochemical Measurement
Three electrodes system consisting of carbon paste electrode (working), Ag/AgCl/3.0M KCl (reference) and platinum wire (counter electrode) connected to PalmSens (Ivium Technologies, Netherland) was used for electrochemical measurement. The surface of CPE was regenerated by renewing and polishing it on wet filter paper before each measurement.
2.6. The Free Adsorption of Organic Dye and Microorganisms to the Surface of CPE
The experimental procedure was adopted from our previous study. Briefly, CPE was immersed into 4 ml of 1.0 µmol·L−1 amido black solution (pH 7.2). The dye accumulation was performed in 70 s stirring at 400 rpm. The CPE with adsorbed organic dye was immersed in fresh phosphate buffer (pH 7.2) and the oxidation current of amido black (I0) was determined using square wave voltammetry (SWV, frequency 25 Hz, potential range from 0 to +1.0 V, potential step 0.025 V and potential pulse 0.025 V). These I0 values served as blank throughout the whole experiment.
Working electrode was immersed into 4 ml of cell suspension (1.5 × 108 cfu·mL−1) in buffer (pH 7.2) for 130 s at 400 rpm. In the case of the pH effect, buffer solutions of pH 5.0 and 9.2 were also used. After the attachment process, amido black solution was allowed to adsorb for 130 s at 400 rpm with the electrode system switched off (7.2 pH). CPE with adsorbed cells and organic dye was dipped into fresh buffer solution (pH 7.2) and the oxidation current of amido black (I0(cells)) was examined using SWV with the condition described above.
The extent of free cell adsorption (I0(extent)) to the surface of CPE was calculated using equation:
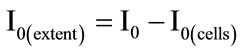 (1)
(1)
where I0 is the oxidation current of the adsorbed dye (µA), I0(cells) is the oxidation current of the dye adsorbed after accumulation of the cells of microorganisms (µA). The more cells attached to the surface of the electrode the greater the value of I0(extent).
2.7. The Adsorption of Microorganisms to the Surface of CPE Using Various Potential
CPE was immersed into 4 ml of cell suspension (1.5 × 108 cfu·mL−1) in phosphate buffer (pH 7.2) for 130 s at 400 rpm and various potentials from +50 mV to +600 mV (50 mV step) were applied. The cell suspension was then changed for amido black solution (1.0 µmol·L−1) and the adsorption process continued for 130 s at 400 rpm with the electrode system switched off. The oxidation current (IE(cells)) of amido black adsorbed on the surface of CPE was then determined using SVW in phosphate buffer (pH 7.2). The extent of cell adsorption affected by applied potential (IE(extent)) was calculated according to equation:
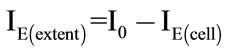 (2)
(2)
The results are interpreted as follow: I0(extent) ≥ IE(extent) means that the adsorption of microorganisms was not enhanced by the application of potential, i.e. the amount of the cells on the surface of CPE was similar or lower in comparison with free adsorption of cells. If I0(extent) < IE(extent), the attachment of cells of microorganisms was enhanced by the application of potential.
2.8. Statistical Analysis
The experiments were carried out in two separate trials and individual measurements were replicated five times at least. The results are expressed as average values with standard deviations. Scheffé’s method was applied for multiply comparison of all the datasets. Analysis of variance (ANOVA) was used as a tool for determination of the effects of applied potentials, cultivation time, pH and solid-type media on the IE(extent) values. Both statistical methods were set on the probability level of p = 0.05.
3. RESULTS AND DISCUSSION
The free adsorption process (without applied potential) of S. epidermidis on the surface of CPE decreased with increasing of pH. As shown in Figure 1, I0(extent) gave 0.97 ± 0.06 µA at the pH 5.0 and 0.78 ± 0.03 µA at the pH 7.2 (p < 0.01). In basic environment, no free attachment of S. epidermidis was observed. It was previously published that alkaline pH (8.5) had inhibiting effects on the attachment of S. epidermidis and S. aureus strains [5]. On the contrary, decrease of gram-positive Listeria monocytogenes adhesion at pH 5.0 was determined in comparison with physiological condition (i.e. pH 7.4) [10]. In acidic environment (pH 5.0), the attachment of bacterial cells to the surface of the CPE was slightly en hanced at 50 and 100 mV in comparison with that occurred during free attachment process (p < 0.05) in our study. The rest of the potentials either did not affect the adsorption of S. epidermidis (200, 500 - 600 mV) cells or caused the deliberation of the cells from the surface of the CPE (150, 250 - 450 mV). According to statistical analysis (Student’s t-test), four potentials (100, 150, 300 and 400 mV) applied to CPE significantly promoted adsorption of bacterial cells at pH 7.2 (p < 0.05). It is clear from Figure 1 that applying 300 mV had a marked effect on the adsorption of S. epidermidis to CPE giving the IE(extent) 2.9 times higher than the extent of the free adsorption (I0(extent)). The rest of applied potentials resulted in lower values of IE(extent) indicating that cells were deliberated from the surface of carbon paste electrode. Interestingly, the attachment of bacteria to the surface of CPE occurred when potentials 100, 350 and 450 - 600 mV were applied in basic environment (pH 9.0). Other potentials did not allow bacteria to be attached similarly as during free adsorption. It has to be noticed that bacteria were still viable at the end of the attachment process in all the buffer solutions and the cells concentration did not differ from the initial count (p < 0.05). However, the overall effect of pH on the bacterial attachment on the surface of CPE at different potentials used in this study was not significant (p > 0.05), i.e. the pH was not identified as the significant main factor in ANOVA procedure. The adhesion forces reached its highest value when the pH of the solution was near the isoelectric point of bacteria as was determined in the study of bacteria-metal attachment [7]. Xie et al. [16] concluded that biofilm development in the electrode at various potentials could be perceived from the point of view of surface charge interaction. They determined the particular potential, at which E. coli biofilm with the highest cell density was
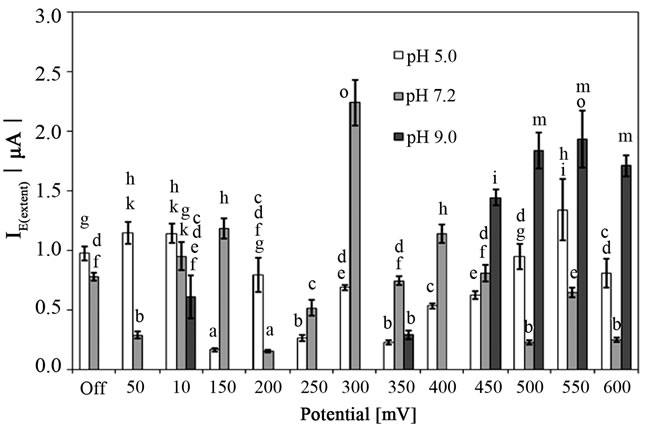
Figure 1. The anodic peak current of the dye (amido black) after attachment of Staphylococcus epidermidis to the surface of carbon paste electrode at various pH affected by electrochemical potentials for 130 s (400 rpm stirring). Off, the free attachment process (with electrode system switched off). Mean values and standard deviations (n = 10 - 12). Different letters above columns indicate statistical difference (p < 0.05).
observed. In our study, the highest IE(extent) value indicating the enhanced attachment of S. epidermidis was determined at 300 mV in 7.2 pH.
It is evident from Figure 2 that attachment process depended on the time of cultivation which was determined as the single main factor affected the adsorption (p < 0.001). Whereas I0(extent) values were similar for 24 h and 48 h cultivation time (p > 0.05), extended cultivation time up to 96 or 168 h at 37˚C resulting in increase of the free attachment of S. epidermidis on the surface of CPE (Figure 3).
The application of the potentials in the range of 50 to 450 mV did not show clear dependence among various incubation times. Three potentials, namely 500, 550 and 600 mV applied to the CPE, showed clear relationship, i.e. adsorption of the cells increased with increase of the incubation time. Comparing the IE(extent) values with that obtained during free adsorption revealed that the adsorp-
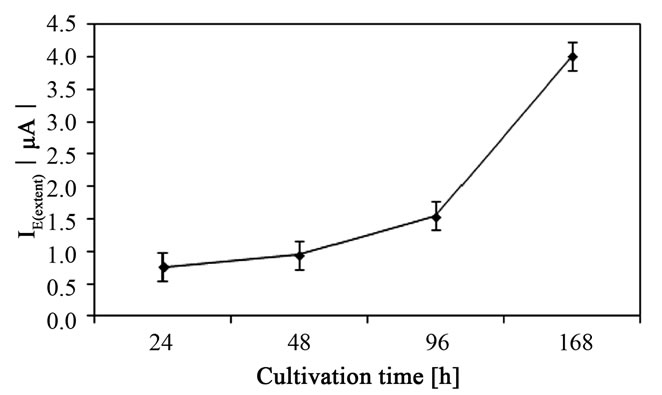
Figure 2. The cultivation time as the main factor influencing the attachment of Staphylococcus epidermidis on the surface of carbon paste electrode. Mean values with 95% confidential interval.
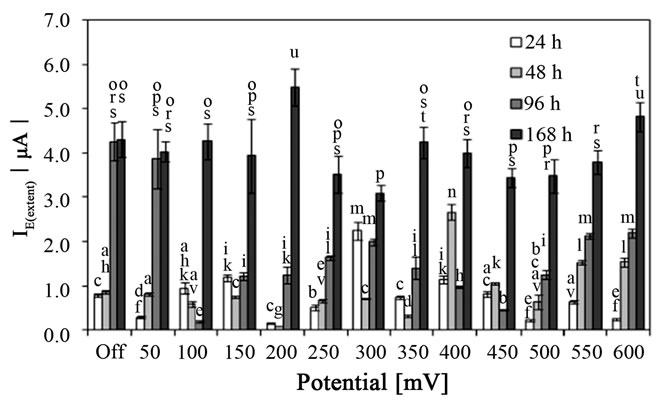
Figure 3. The anodic peak current of the dye (amido black) after attachment of Staphylococcus epidermidis, grown for various time on MPA at 37˚C to the surface of carbon paste electrode (7.2 pH) affected by electrochemical potentials for 130 s (400 rpm stirring). Off, the free attachment process (with electrode system switched off). Mean values and standard deviations (n = 10 - 14). Different letters above columns indicate statistical difference (p < 0.05).
tion process was enhanced by different potentials with regards to the cultivation time. Whereas 24 h of cultivation resulting in higher IE(extent) values at potentials 100, 150, 300 and 400 mV (p < 0.01), the adsorption of bacterial cells cultivated for 48 h was significantly higher (p < 0.001) when applying potentials 400, 450, 550 and 600 mV. Interestingly, the cells incubated for 96 h at 37˚C attached to the surface of CPE in lower extent in comparison with that during free adsorption (without applying potential) in the range from 100 to 600 mV. The potentials had no effect on the adsorption of 168 h culture of S. epidermidis to the surface of CPE with two exceptions. Firstly, the application of 200 mV significantly enhanced the adsorption (p < 0.05), and 300 mV reduced the attachment (p < 0.01) in comparison with that during free attachment process, in second.
The type of solid media exhibited different results on the extent of attachment process of S. epidermidis to the surface of CPE. According to ANOVA procedure, the adsorption process was affected by the type of solid media used for cultivation of S. epidermidis (p < 0.05) as depicted in Figure 4. Regarding the free adsorption of bacterial cells, the cultivation on MPA and B-P plates gave the similar values of I0(extent) 0.78 ± 0.03 and 0.76 ± 0.09 µA, respectively (p > 0.05). Enhanced attachment was discovered when cells were able to grow on blood agar resulting in twofold higher value of I0(extent). On the other hand, the cells cultivated on TSA plates did not freely attach to the surface of CPE. The application of potentials 300, 400 and 500 mV caused the attachment of S. epidermidis grown in TSA plates with the highest IE(extent) value at the potential 300 mV (1.37 ± 0.04 µA) (Figure 5).
A few applied potentials (100, 200 - 300, 500, 550 mV) did not permit the bacteria grown in B-P plates to adhere to the surface of CPE similarly as during free adsorption process. On the other hand, bacteria cultivated in BA at 37˚C resulted in significantly higher IE(extent) in comparison
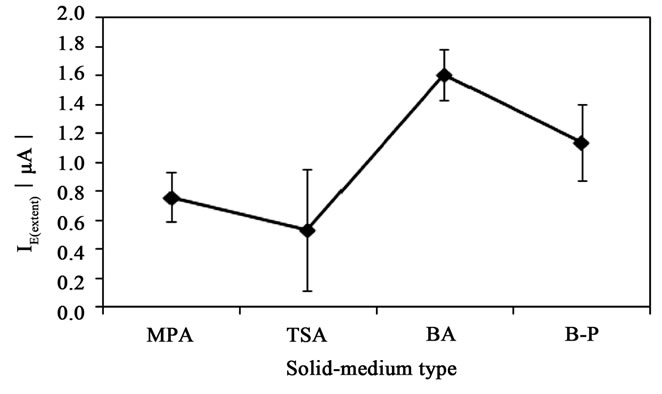
Figure 4. Solid-medium type as the main factor influencing the attachment of Staphylococcus epidermidis on the surface of carbon paste electrode. Mean values with 95% confidential interval. MPA-Nutrient agar No. 2, BA-blood agar, TSA-Trypton soya agar, B-P-Baird-Parker agar.
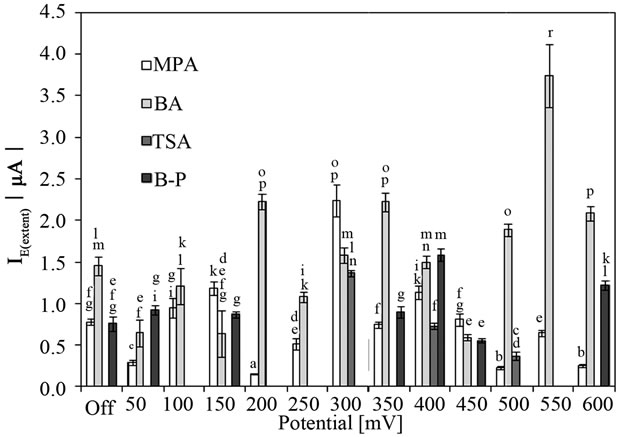
Figure 5 The anodic peak current of the dye (amido black) after attachment of Staphylococcus epidermidis, grown for various solid-medium (24 h, 37˚C) to the surface of carbon paste electrode (7.2 pH) affected by electrochemical potentials for 130 s (400 rpm stirring). Off, the free attachment process (with electrode system switched off). Mean values and standard deviations (n = 11 - 14). MPA-Nutrient agar No. 2, BA-blood agar, TSATrypton soya agar, B-P-Baird-Parker agar. Different letters above columns indicate statistical difference (p < 0.05).
with that in free attachment process (p < 0.01).
It is well known that surface charge of bacteria is dependent on their physiological state which reflects the molecular composition of the cell wall, particularly the number and the type of functional groups [7,17,18]. Various intrinsic and extrinsic factors including growth phase, medium type and nutritional sources had impact on the cell wall composition. The concentration of sugars and amino acids in cell walls of staphylococci varied depending on the growth conditions [19] and the cultivation method and the growth phase also affected the lipopolysaccharide composition of bacterial cells [20]. In stationary phase of growth of S. aureus, increase of genes expression encoding the proteins formation responsible for surface charge was determined [18]. Therefore, the stationary-phased S. aureus had strong negative surface charge in comparison with the cells in exponential growth phase. Higher values of both I0(extent) and IE(extent) for 96 h and 168 h cultures of S. epidermidis determined in our study may indicate the increase of negative surface charge of bacterial cells. Although we did not provide the enumeration of dead cells of S. epidermidis, we found that thermally inactivated yeast cells significantly increased I0(extent) and IE(extent) values compare with those for viable cells [14]. These findings also corresponded with the study of the postmortem changes in the surface charge of the membranes of the human erythrocytes [21]. Although not fully comparable with our research, they demonstrated the increase of charges (depending on pH) after sudden unexpected death of cells. We may just hypothesize that increase of dead cells could result in increase of their attachment to the surface of CPE. The significantly higher IE(extent) and I0(extent) values for S. epidermidis grown in BA plates for 24 h at 37˚C in comparison with other solid-type media can be explained by different nutrient composition. It was described that initial attachment and further biofilm formation depended on the availability of key nutrients in growth media. While the biofilm development of some gram-negative bacteria was enhanced in poor media [6,8], grampositive S. aureus formed biofilm in nutritionally rich medium [9]. In addition, the effect of solid-medium type also influenced the identification of bacterial isolates using advanced analytical technique [22].
4. CONCLUSION
The attachment of S. epidermidis strain on the surface of carbon paste electrode at the potentials ranged from 50 mV to 600 mV was studied. Various conditions such as pH of buffer solution, cultivation time and solid-medium type were investigated as variables influencing the attachment to the surface of CPE. Generally, the attachment of S. epidermidis to the CPE depended on all the tested conditions. Although the effect of pH was not supported by ANOVA procedure, interesting results were obtained. In acidic environment, the applied potentials resulted in similar or lower extent of attachment in comparison with that determined during free attachment process. On the other hand, alkali condition impeded free attachment of bacteria but high IE(extent) values were determined at certain potentials. Both cultivation time and solid-medium type were identified as the single main factors affected the attachment of bacteria to the surface of CPE. The values of IE(extent) indicating the extent of attachment process increased with increasing of cultivation time. The solid-medium used for cultivation also affected the attachment process with higher IE(extent) values for blood agar followed by Baird-Parker agar plates. Interestingly, bacteria grown on TSA were not able to attach to the surface at the majority of applied potentials. Since the solid media differed in nutrient composition, this could probably result in different composition of cell wall and thus, different surface charge properties of the cells. However, another substantial investigation is necessary to clarify this behavior.
REFERENCES
- Araújo, E.A., de Andrade, N.J., da Silva, L.H.M., et al. (2010) Control of microbial adhesion as a strategy for food and bioprocess technology. Food and Bioprocess Technology, 3, 321-332. doi:10.1007/s11947-009-0290-z
- Kordialik-Bogacka, E. (2011) Surface properties of yeast cells during heavy metal biosorption. Central European Journal of Chemistry, 9, 348-351. doi:10.2478/s11532-011-0008-8
- Shi, X. and Zhu, X. (2009) Biofilm formation and food safety in food industries. Trends in Food Science and Technology, 20, 407-413. doi:10.1016/j.tifs.2009.01.054
- Busscher, H.J. and Weerkamp, A.H. (2009) Specific and non-specific interactions in bacterial adhesion to solid substrata. FEMS Microbiology, 46, 165-173. doi:10.1111/j.1574-6968.1987.tb02457.x
- Nostro, A., Cellini, L., di Giulio, M., et al. (2012) Effect of alcaline pH on staphylococcal biofilm formation. Acta Pathologica, Microbiologica, et Immunologica Scandinavica, 120, 733-742.
- Oh, Y.J., Jo, W., Yang, Y., et al. (2007) Influence of culture conditions on Escherichia coli O157:H7 biofilm formation by atomic force microscopy. Ultramicroscopy, 107, 869-874. doi:10.1016/j.ultramic.2007.01.021
- Sheng, X., Ting, Y.P. and Pehkonen, S.O. (2008) The influence of ionic strenght, nutrients and pH on bacterial adhesion to metals. Journal of Colloid and Interface Science, 321, 256-264. doi:10.1016/j.jcis.2008.02.038
- Speranza, B., Corbo, M.R. and Sinigaglia, M. (2011) Effects of nutritional and environmental conditions on Salmonella spp. biofilm formation. Journal of Food Science, 76, M12-M16. doi:10.1111/j.1750-3841.2010.01936.x
- Tang, J., Chen, J., Liu, J., et al. (2012) Effects of different cultivation conditions on Staphylococcus aureus biofilm formation and diversity of adhesion genes. Journal of Food Safety, 32, 210-218. doi:10.1111/j.1745-4565.2012.00370.x
- Tresse, O., Lebret, V., Benezech, T., et al. (2006) Comparative evaluation of adhesion, surface properties and surface protein composition of Listeria monocytogenes strains after cultivation at constant pH of 5 and 7. Journal of Applied Microbiology, 101, 53-62. doi:10.1111/j.1365-2672.2006.02968.x
- Jones, D.S., Adair, C.G., Mawhinney, W.M., et al. (1996) Standardization and comparison of methods employed for microbial cell surface hydrophobicity and charge determination. International Journal of Pharmaceutics, 131, 83-89. doi:10.1016/0378-5173(95)04368-3
- Torimura, M., Itoet, S., Kano, K., et al. (1999) Surface characterization and on-line activity measurements of microorganisms by capillary zone electrophoresis. Journal of Chromatography B, 721, 31-37. doi:10.1016/S0378-4347(98)00490-3
- Morisaki, H., Sugimoto, M. and Shiraishi H. (2000) Attachment of bacterial cells to carbon electrodes. Bioelectrochemistry, 51, 21-25. doi:10.1016/S0302-4598(99)00082-3
- Vu, D.L., Sýs, M. and Červenka, L. (2012) The effect of various potentials on the attachment of Saccharomyces cerevisiae and Staphylococcus epidermidis to carbon paste electrodes. International Journal of Electrochemical Science, 6, 5265-5274. http://www.electrochemsci.org/papers/vol6/6115265.pdf
- Švancara, I. and Metelka, R. (2000) Piston-driven carbon paste holders for electrochemical measurements. In: Vytřas, K. and Kalcher, K. Eds., Sensing in Electroanalysis, University of Pardubice, Pardubice, 7-18.
- Xie, X.H., Li, E.L. and Tang, Z.K. (2010) EQCM and EIS study of the effect of potential of zero charge on Escherichia coli biofilm development. International Journal of Electrochemical Science, 5, 1018-1025. http://www.electrochemsci.org/papers/vol5/5071018.pdf
- Kłodzińska, E., Szumski, M., Dziubakiewicz, E., et al. (2010) Effect of zeta potential value on bacterial behavior during electrophoretic separation. Electrophoresis, 31, 1590-1596. doi:10.1002/elps.200900559
- Matsuo, M., Oogai, Y., Kato, F., et al. (2011) Growthphase dependence of susceptibility to antimicrobial peptides in Staphylococcus aureus. Microbiology, 157, 1786-1797. doi:10.1099/mic.0.044727-0
- Vanderijn, I. (1986) Quantitative analysis of cell walls of nutritionally variant staphylococci grown under various growth conditions. Infection and Immunity, 49, 518-522.
- Bakholdina, S.I., Krasikova, I.N. and Soloveva, T.F. (2001) The effect of the cultivation method and the growth phase on the lipopolysachcaride composition of Yersinia pseudotuberculosis. Russian Journal of Bioorganic Chemistry, 27, 130-134. doi:10.1023/A:1011341405310
- Kotyńska, J., Petelska, A.D., Szeremeta, M., et al. (2012) Changes in surface-charged density of blood cells after sudden unexpected death. Journal of Membrane Biology, 245, 185-190. doi:10.1007/s00232-012-9428-4
- Anderson, N.W., Buchan, B.W., Riebe, K.M., et al. (2012) Effects of solid-medium type on routine identification of bacterial isolates by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. Journal of Clinical Microbiology, 50, 1008-1013. doi:10.1128/JCM.05209-11

