Journal of Cancer Therapy
Vol. 4 No. 6A3 (2013) , Article ID: 34307 , 6 pages DOI:10.4236/jct.2013.46A3001
Intravesical Treatment with Vorinostat Can Prevent Tumor Progression in MNU Induced Bladder Cancer
![]()
Email: *346109106@qq.com, *songyanfeng@lzu.edu.cn
Copyright © 2013 Degui Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 24th, 2013; revised March 29th, 2013; accepted April 6th, 2013
Keywords: Vorinostat; Apoptosis; Combination; Bladder Cancer; Therapy
ABSTRACT
Background: Histone deacetylase inhibitors (HDACI) are promising class of drugs acting as antiproliferative agents by promoting differentiation as well as inducing apoptosis. Vorinostat (suberoylanilide hydroxamic acid, SAHA) is the first among this new class of anticancer drugs to be approved by FDA for the treatment of cancer but only for cutaneous T cell lymphoma (CTCL). The objective of this study is to investigate the inhibitory effect of SAHA on the viability of human bladder cancer cells and its synergetic effect with chemotherapy agents in vitro and in vivo. Methods: The cell viability of human bladder cancer cell lines after treated with SAHA or SAHA combining mitomycin c (MMC), Cisplatin (DDP) and Adriamycin (ADM) were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Hoechst staining was used to observe cell morphology for apoptotic cells. The survivin protein and acetylated histone H3 levels in bladder cancer were quantified by Western blot analysis. In vivo tumor growth inhibition of intravesical inject SAHA was determined in rats with N-methyl-N-nitrosourea (MNU) induced bladder cancer. Results: SAHA significantly inhibited growth of bladder cancer cell lines with concentration and time dependent manner. Furthermore, better results of tumor inhibition would be achieved when it was combined with chemotherapeutic agents in vitro and in vivo. Survivin expression decreased and acetylated histone H3 expression increased in bladder cancer cells lines after SAHA treatment. Intravesically injections of SAHA can inhibit tumor progress when combined with DDP. Conclusions: SAHA can act as HDACI which has direct anti-cancer effect and can enhance the action of several chemotherapy agents markedly. SAHA may sensitize bladder cancer to anti cancer drugs by down regulating survivin expression. Intravesically injections of SAHA can prevent tumor progression in MNU induced bladder cancer.
1. Introduction
Bladder cancer is a commonly occurring cancer. Existing local therapies for transitional cell carcinoma (TCC) of the bladder include local resection for nonmuscle-invasive disease and cystectomy for muscle-invasive disease. These strategies are effective but far from satisfaction. Nearly 50% to 70% of patients treated for superficial disease develop recurrence, and as many as 20% progress to more aggressive disease [1]. Furthermore, since chemotherapy and radiotherapy give disappointing results, new therapeutic approaches are needed. An increasing body of evidence concerning the importance of epigenetic changes in cancer onset and progression has raised interest in the manipulation of transcription as a mode of cancer therapy; altering gene expression through chromatin modification now seems to be a viable target. Consistent with this, histone deacetylase inhibitors (HDACI) have emerged as promising anticancer drugs [2]. Acetylation of core nucleosomal histones is regulated by the opposing activities of histone acetyltransferases (HAT) and histone deaceytltransferase HDAC. HDACs catalyze the removal of acetyl groups on the NH2-terminal lysine residues of core nucleosomal histones, and this activity is generally associated with transcriptional repression. Aberrant recruitment of HDAC activity has been associated with the development of certain human cancers [3]. HDACIs are structurally different, but share the capacity to enhance cell differentiation, induce apoptosis [2], inhibit cancer cell growth [4]. SAHA is the prototype of a family of small synthetic molecules that target and inhibit histone deacetylases (HDACs) which are known to silence certain genes that allow tumor cells to proliferate. SAHA is more than just an alternative to established anticancer agents; it is a novel targeted anticancer drug that is selective in its effects on cancer cells [5]. It is first among this new class of targeted anticancer drugs called HDAC inhibitors to be approved by the FDA for the treatment of cancer but only for cutaneous T cell lymphoma (CTCL).
With its anatomic location, bladder is an easily accessible structure and appears to be an ideal site for local administration of treatment agents by intravesical delivery. Direct intravesical treatment has low toxicity and thus can be readily combined with existing treatment. Local administration of cytotoxic agents is commonly used in urological practice for treatment of superficial bladder cancer. The main objective of the intravesical drug administration is optimisation of drug delivery in the tumour and its vicinity and reduction of systemic toxicity. Until now, there is no data about the effects of SAHA on bladder cancer in vivo. This study was designed to define the therapeutic effects of SAHA in treating bladder cancer in vitro and in vivo. We examined whether SAHA can mediate inhibition of cell growth and induce apoptosis in bladder cancer cells, the synergistic effects of SAHA when combined with mitomycin c (MMC), Cisplatin (DDP) and Adriamycin (ADM), furthermore we examined tumor growth inhibition of intravesical inject SAHA in MNU induced bladder cancer.
2. Materials and Methods
2.1. Cell Lines and Chemicals
T24, BIU87, 5637(bladder TCC) cells were maintained in RPMI 1640(Gibco, USA) supplemented with 10% fetal bovine serum (FBS). SAHA and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma Chemical Co. (USA).
2.2. Cell Viability Assay
To study the effects of SAHA on cell viability, all cell lines (3 × 103/well) were plated in 96-well plates and incubated in PRMI 1640 culture medium plus 10% FBS. After 24 hours, the cells were treated with medium alone or with medium containing different doses of SAHA (5, 7.5 or 10 µM). Cell viability was determined after 24, 48 or 72 h of treatment by incubation by MTT assay. Briefly, 10 μl of 12 mM MTT were added to each well; after a further 4 hours incubation, DMSO 150 μL/well were added and absorbance at 490 nm was measured using a plate reader (Bioelisa Reader, EL 800. Bio-Kit, USA). 6 replicates were done to determine each data point. The cells for synergy effect assay were treated with medium alone or with medium containing different doses of SAHA and/or chemical agents (5 mg/L DDP, 5 mg/L MMC or 2 mg/L ADM). MTT assay was performed after 24, 48 and 72 hours treatment. The coefficient of drug interaction (CDI) was used to analyze the synergistically inhibitory effect of drug combinations [6]. CDI is calculated as follows: CDI = AB/(A × B). According to the absorbance of each group, AB is the ratio of the combination groups to control group; A or B is the ratio of the single agent groups to control group. Thus CDI value less than, equal to or greater than 1 indicates that the drugs are synergistic, additive or antagonistic, respectively. CDI less than 0.7 indicate that the drugs are significantly synergistic.
2.3. Cell Morphology Observation by Hoechst 33258 Staining
Hoechst staining was used to view apoptotic cells in bladder cancer cell lines and cancerous tissues from MNU induced bladder cancer rats. The bladder cancer cells were separately incubated with 7.5 µM SAHA, 5 mM DDP or 7.5 µM SAHA combine 5 mM DDP for 72 h. The culture medium was discarded and washed three times in ice-cold PBS. Cells were fixed with 4% paraformaldehyde and stained with Hochest 33258 (1 µg/ml) for 30 min at room temperature. The rats with MNU induced bladder cancer were treated once a week with 100 mg/kg SAHA intravesical instillation and/or 2 mg/kg DDP by intraperitoneal injection for 15 weeks. Sections from the cancerous tissues were fixed with 4% paraformaldehyde and stained with Hochest 33258 (1 µg/ml) for 30 min at room temperature. The cells and tissues were observed under fluorescence microscope.
2.4. Detect the Survivin and Acetylated Histone H3 by Western Blotting
T24 cells were incubated with 7.5 µM or 10 µM SAHA for 72 h then homogenized and re-suspended in M-PER (Mammalian Protein Extraction Reagent) (Pierce, Rockford, IL). The BCA protein assay kit (Bio-Rad, Hercules, CA) was used to determine total protein concentration. Proteins were separated on a 12% Tris-HCL Polyacrylamide gel (Bio-Rad, Hercules, CA) and transferred to PVDF Membrane. The membrane was blocked for an hour in Blocking Buffer (100 mM Tris-HCL pH 7.5, 150 mM NaCl, 0.1% Tween 20) with 5% nonfat dry milk, then incubated overnight with a 1:1000 dilution of rabbit anti-survivin, rabbit anti-acetylated histone H3 and rabbit anti-beta-actin antibody (Cell Signaling Technology, Danvers, MA) respectively followed by anti-rabbit IgG Peroxidase Conjugate (1:20,000) (Beyotime, China) for 1.5 hours at room temperature. Immunoreactive bands were detected using the BeyoECL Plus Western Blotting detection System (Beyotime, China), according to the manufacturer’s instructions
2.5. Animal Study on Effect of SAHA in Combination with DDP on MNU Induced Bladder Cancer
A cohort of 60 six to eight week old female Wistar rats (Lanzhou University experiment animal center) was used for this study. The animals were anesthetized with intraperitoneal chloral hydrate and received 0.15 ml 10 mg/ml (1.5 mg.) N-methyl-N-nitrosourea (MNU) via a 22 gauge Teflon angiocath intravesically every other week (week 1, 3, 5, 7 and 9) for a total of five doses after the bladder was drained. The animals remained anesthetized for approximately 2 hours after catheterization. 4 rats were excluded from the experiment (1 died and 3 with urosepesis secondary to urethral stricture or urinary obstruction). Remain 56 rats were divided into 4 treatment groups: 1. control (n = 14); 2. SAHA (n = 14); 3. DDP (n = 14); 4. SAHA combine DDP (n = 14). From week 11, for 15 weeks the rats were treated intravesically with 0.4 ml of either saline (group1 and group 3); or 100 mg/kg SAHA (group2 and group 4) every first day of the weeks. 2 hours after intravesically injection, the rats were treated by intraperitoneal injection with either saline (group1 and group 3); or 2 mg/kg/dose DDP (group2 and group 4). The animals were sacrificed at 25 weeks and necropsy was performed. The urinary bladders were excised and the bladders were bivalved at the dome and fixed in 10% phosphate buffered formalin for 24 hours and embedded in paraffin for histopathology. Sections were stained with hematoxylin and eosin (H&E). The incidence of tumor growth was scored while blinded to the treatment protocol. All sections from each bladder specimen were reviewed under light microscopy. The section that appeared to have the greatest amount ofchange from normal rat bladder was selected for histological grading. The sections were assessed and categorized into 3 stages: a) hyperplasia, flat or papillary atypia, or mild and moderate dysplasia; b) superficial TCC, which includes Stages Pa (papillary exophytic tumors with fibrovascular cores and nuclear pleomorphism of the epithelial cells with no evidence of invasion), Pis [tumors confined to the mucosa with full mucosal thickness of marked atypia/dysplasia (i.e., carcinoma in situ)], and P1, (tumors that demonstrate evidence of lamina propria invasion); or c) bladder wall muscle-invasive transitional cell carcinomas.
2.6. Statistical Analysis
The data were expressed throughout as means ±SEM and analysised with SPSS 11.0. The data of animal study was analysis with the method of Kruskal-Wallis H test. (Wilcoxon W test as post hoc test).
3. Results
3.1. SAHA Inhibit Bladder Cancer Cell Proliferation
A panel of bladder cancer cells (T24, BIU87, 5637) was treated with different doses of SAHA for up to a maximum of 3 days. As shown in Figure 1, SAHA significantly reduced the number of viable cells of bladder cancer cell lines. The effect appeared on all 3 cell lines. Cell viability decreased in a does-dependent manner in all 3 cell lines. 7.5 µM SAHA can inhibit the growth of all 3 bladder cancer cell lines significantly.
3.2. SAHA and/or DDP Induce Apoptosis in Bladder Cancer Cell Lines and in Cells of MNU Induced Bladder Cancer
Hoechst 33258 staining was used to determine whether apoptosis was involved after SAHA treatment. The morphology of bladder cancer cells apoptosis in vitro and in vivo by Hoechst 33258 staining show that viable cells display diffuse fluorescence in cellular nuclei. Apoptotic
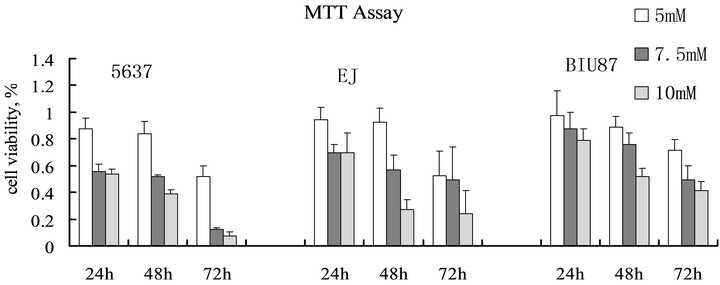
Figure 1. Effect of SAHA on bladder cancer cell survival. Cells were seeded at 3 × 103 cells/well in 96 multiwell plates and treated with medium alone or with medium containing different doses of SAHA (5, 7.5 and 10 µM) for up to 3 days. Viable cells were determined by MTT assay AT 24, 48 and 72 h. SAHA significantly reduced the number of survival bladder cancer cells. Cell viability decreased in a does-dependent manner in all 3 cell lines.
cells show the concentrated dense granular fluorescence. There are many apoptotic cells seen in the group treated with SAHA. But the apoptosis of control cells was not shown. As shown in Figure 2.
3.3. SAHA Decreased Survivin Expression and Increase Acetylated Histone H3 Expression in T24 Cells
The T24 cells were treated with medium alone or with medium contain SAHA for 72 hours, the expression of survivin and acetylated histone H3 proteins were detected by the method of western blotting. As can be seen in Figure 3, SAHA inhibit survivin expression in T24 cells. Acetylated histone H3 was increased significantly in T24 cells after treatment with SAHA at concentration 7.5 mM or 10 mM. The proteins expression in BIU87 and 5637 are similarly (data not show here).
3.4. The Synergy Effect of SAHA in Combination with DDP, MMC and ADM on Bladder Cancer Cell Survival
By MTT assay, the synergistic inhibition was observed in the inhibition of bladder cancer survival when combined SAHA with DDP, MMC and ADM (Figure 4), that is, the individual effect of 7.5 µM SAHA or DDP, MMC and ADM caused a decrease of the cancer cell survivals respectively. However, when SAHA was combines with DDP, MMC or ADM, the survival markedly decreased.

Figure 2. Cell morphology observation after Hoechst 33258 staining. The bladder cancer cells were separately incubated with 7.5 µM SAHA and/or DDP for 72 h. The rats with bladder cancer were treated with SAHA and/or DDP intraperitoneally. Cells and sections were fixed with 4% paraformaldehyde and stained with Hochest 33258. Apoptotic cells show the concentrated dense granular fluorescence. (A) BIU87, control. (B) BIU87, SAHA and DDP treated. (C) MNU induced bladder cancer without drug treatment. (D) MNU induced bladder cancer were treated with SAHA and DDP intraperitoneally. Scale bar = 10 µm.

Figure 3. Western Blot Analysis of acetylated Histone H3 and survivin expression in T24 cells. T24 cells were treated with 0, 7.5 µM and 10 µM SAHA then analyzed for acetylated histone H3 and survivin expression by western blot. Relative fold increase was determined by scanning densitometry of the Western blot normalized to β-actin. SAHA treatment results in an increase in acetylated H3 expression and decreased survivin expression in T24 cells.
3.5. SAHA and/or DDP Can Inhibit Tumor Progression in MNU Induced Bladder Cancer
All rats bladder treated with intravesical MNU develop progressive neoplastic changes, those lesions progress from hyperplasia; superficial transitional cell carcinoma; or bladder wall muscle invasive transitional cell carcinomas (Figure 5). In the animals that began treatment at 11 weeks after initiation of MNU. As show in Figure 6, intravesical SAHA can prevent progression of bladder cancer (P < 0.05). Better results achieved when combine SAHA and DDP in treating bladder cancer (P < 0.01).
4. Discussion
Treatment with HDACi results in induction of a large number of candidate genes and repression of anti-apoptosis genes [7]. SAHA acts in part by selectively altering the expression of genes and the activity of proteins that control cell growth and death [8]. Those proteins are abnormal in many different cancers. SAHA blocks the HDACs which remove the acetyl group from those proteins. The treatment of SAHA results in changes in their structure and thwarts the abnormal function [9]. This can cause the death of cancer cells. However, we still don’t know whether SAHA can be used in bladder cancer treatment, especially by Intravesical administration for the treatment. This study showed that SAHA could downregulate survivin expression and induce apoptosis in bladder cancer. Survivin is a member of the inhibitor of apoptosis protein family and is involved in both inhibition of apoptosis and regulation of cell division. Although rarely expressed in terminally differentiated normal adult tissues, Survivin is upregulated in most malignnancies. We show that treatment with SAHA dramatically and significantly increased the number of apoptotic
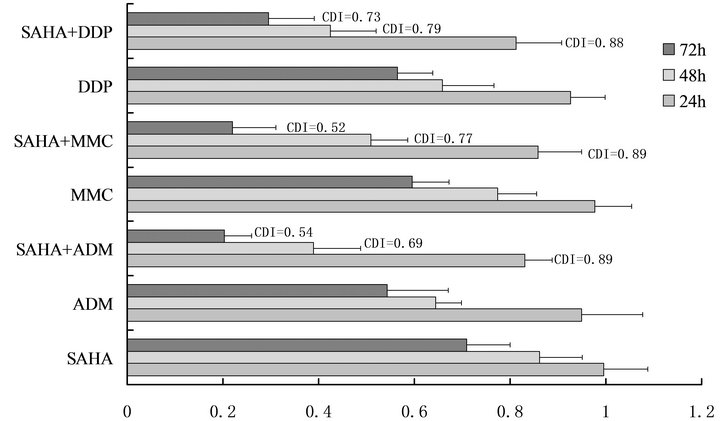
Figure 4. Effect of SAHA in combination with DDP, MMC and ADM on bladder cancer cell survival. By MTT assay, the synergistic effects was observed in the inhibition of bladder cancer survival when combined 7.5 µM SAHA with 5 mg/L DDP, 5 mg/L MMC and 2 mg/L ADM. The data show SAHA can act synergistically with MMC, DDP AND ADM.
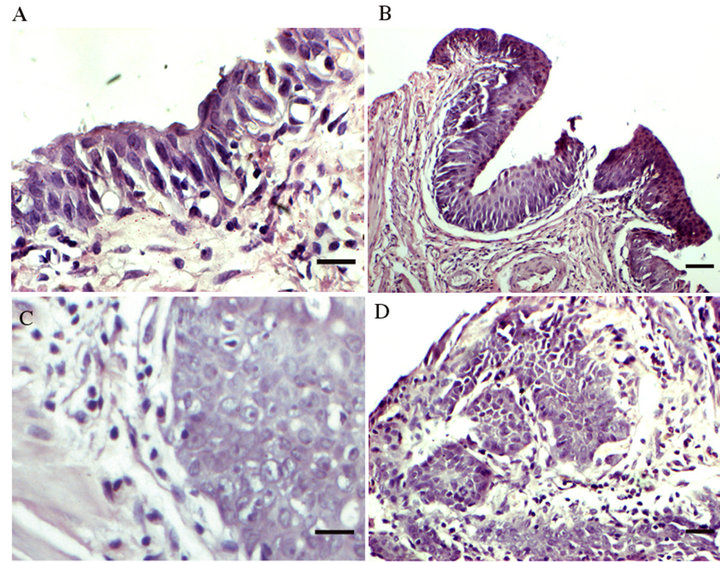
Figure 5. Histopathological findings in female Wistar rat bladders treated with 5 doses of intravesical MNU. Female Wistar rat bladder treated with 5 doses of MNU develop progressive neoplastic changes. These lesions progress from hyperplasia, superficial transitional cell carcinoma to large bulky muscle invasive transitional cell carcinomas. (A) Control. (B) Hyperplasia. (C) Superficial transitional cell carcinoma. (D) Muscle invasive transitional cell carcinomas. Scale bar = 10 µm.
cells in the bladder cancer cells. This effect may associate with a decrease in levels of the antiapoptotic protein survivin. It is likely that SAHA induced bladder cancer cell apoptosis by down regulating Survivin expression.
While SAHA show promise as single agent chemotherapeutic drugs, its use in combination with other anticancer agents may to be their most useful application [10]. The synergy effects of SAHA seemingly had no preconceived mechanistic basis. We show here that treatment with SAHA results in down regulation of antiapoptotic protein survivin. In those scenarios, the SAHA would act to sensitize the cancer cells to various apoptotic stimuli including chemotherapeutic drugs. Intravesical injection of SAHA can prevent tumor progression in MNU induced bladder cancer, better results obtained when combine SAHA intravesical administration with DDP intraperitoneal injection. The in vivo data also indicated that SAHA can inhibit tumor progress and also sensitize bladder cancer to chemotherapeutic drugs.
In summary, SAHA exhibit antiproliferative activity
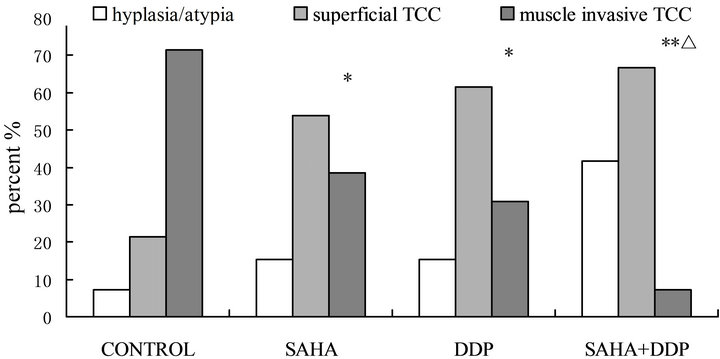
Figure 6. In vivo effects of SAHA and/or DDP on MNU induced bladder cancer. Histopathological findings in the female Wistar rat bladders treated with four doses of intravesical MNU, commenced therapy at 11 weeks and sacrificed at 25 weeks.( *P < 0.05, **P < 0.001, vs control; ΔP < 0.05, vs SAHA).
and potently induce apoptosis in human bladder cancer cells. Furthermore, SAHA can down regulate the survivin expression and increase the sensitivity of bladder cancer to chemotherapeutic dugs. Intravesical application with SAHA or SAHA combine DDP can prevent tumor progression in rats. The present findings raise the possibility that SAHA may prove particularly effective in treating bladder cancers and SAHA can act synergistically with chemotherapeutic drugs.
5. Acknowledgements
Projects 81171954 and 81160287 are supported by NSFC; Project 1107RJZA265 is supported by Natural Science Foundation of Gansu; Project 07-1-100 is supported by Natural Science Foundation of Lanzhou.
REFERENCES
- H. Wallerand, J. C. Bernhard, S. Culine, P. Ballanger, G. Robert, R. E. Reiter, et al., “Targeted Therapies in NonMuscle-Invasive Bladder Cancer According to the Signaling Pathways,” Urologic Oncology: Seminars and Original Investigations, Vol. 29, No. 1, 2011, pp. 4-11. doi:10.1016/j.urolonc.2009.07.025
- M. Kaiser, I. Zavrski, J. Sterz, C. Jakob, C. Fleissner, P. M. Kloetzel, et al., “The Effects of the Histone Deacetylase Inhibitor Valproic Acid on Cell Cycle, Growth Suppression and Apoptosis in Multiple Myeloma,” Haematologica, Vol. 91, No. 2, 2006, pp. 248-251.
- M. J. Lee, Y. S. Kim, S. Kummar, G. Giaccone and J. B. Trepel, “Histone Deacetylase Inhibitors in Cancer Therapy,” Current Opinion in Oncology, Vol. 20, No. 6, 2008, pp. 639-649. doi:10.1097/CCO.0b013e3283127095
- D. Mottet and V. Castronovo, “Histone Deacetylases: AntiAngiogenic Targets in Cancer Therapy,” Current Cancer Drug Targets, Vol. 10, No. 8, 2010, pp. 898-913. doi:10.2174/156800910793358014
- V. Santini, A. Gozzini and G. Ferrari, “Histone Deacetylase Inhibitors: Molecular and Biological Activity as a Premise to Clinical Application,” Current Drug Metabolism, Vol. 8, No. 4, 2007, pp. 383-393. doi:10.2174/138920007780655397
- W. S. Xu, R. B. Parmigiani and P. A. Marks, “Histone Deacetylase Inhibitors: Molecular Mechanisms of Action,” Oncogene, Vol. 26, 2007, pp. 5541-5552. doi:10.1038/sj.onc.1210620
- T. Ai, H. Cui and L. Chen, “Multi-Targeted Histone Deacetylase Inhibitors in Cancer Therapy,” Current Medicinal Chemistry, Vol. 19, No. 4, 2012, pp. 475-487. doi:10.2174/092986712798918842
- Q. T. Luong, J. O’Kelly, G. D. Braunstein, J. M. Hershman and H. P. Koeffler, “Antitumor Activity of Suberoylanilide Hydroxamic Acid against Thyroid Cancer Cell Lines in Vitro and in Vivo,” Clinical Cancer Research, Vol. 12, 2006, pp. 5570-5577. doi:10.1158/1078-0432.CCR-06-0367
- A. Tong, H. Zhang, Z. Li, L. Gou, Z. Wang, H. Wei, et al., “Proteomic Analysis of Liver Cancer Cells Treated with Suberonylanilide Hydroxamic Acid,” Cancer Chemotherapy and Pharmacology, Vol. 65, No. 5, 2008, pp. 791- 802.
- M. Ouaissi, U. Giger, I. Sielezneff, N. Pirro, B. Sastre and A. Ouaissi, “Rationale for Possible Targeting of Histone Deacetylase Signaling in Cancer Diseases with a Special Reference to Pancreatic Cancer,” Journal of Biomedicine and Biotechnology, Vol. 2011, 2011, Article ID: 315939.
NOTES
*Corresponding authors.

