Journal of Cancer Therapy
Vol.4 No.2(2013), Article ID:29678,7 pages DOI:10.4236/jct.2013.42060
Chemoembolization Combined with RFA for HCC: Survival Benefits and Tumor Treatment Response*
![]()
1Interventional Radiology and Image Guided Medicine, Emory University School of Medicine, Atlanta, USA; 2Surgery, Emory University School of Medicine, Atlanta, USA; 3Hematology and Medical Oncology, Emory University School of Medicine, Atlanta, USA; 4Biostatistics, Winship Cancer Institute, Emory University School of Medicine, Atlanta, USA.
Email: #kevin.kim@emory.edu
Copyright © 2013 Renumathy Dhanasekaran et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 11th, 2013; revised February 13th, 2013; accepted February 21st, 2013
Keywords: TACE; RFA; Survival; RECIST
ABSTRACT
Purpose: To investigate survival benefits and tumor treatment response among patients who received treatment with transarterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) and TACE alone. Materials and Methods: A total of 108 HCC patients were treated with TACE between the period of 1998 and 2008. 51 (47.2%) received TACE followed by planned RFA and 57 (52.8%) received TACE alone. 57 patients received Precision TACE with Doxorubicin drug eluting beads and 51 received conventional TACE. Survival analysis was performed using Kaplan Meier Estimator with a log rank test, Fischer exact test was performed for categorical variables and the t test for continuous variables. Results: Mean MELD (Model for End Stage Liver Disease) score among the TACE-RFA and TACE-only groups were 12.87 and 12.33 respectively (p = 0.64). The number of patients in Child’s Class A, B, C in the two groups were 28/15/8 and 23/23/11 (p = 0.30); in Okuda Class I, II and III in the two groups were 22/23/6 and 14/30/9 (p = 0.2). Median survival among patients who received TACE-RFA and TACE alone were 566 days and 209 days (p = 0.01). Median survival of patients treated with Precision-TACE + RFA was 566 days and that of patients treated with conventional TACE + RFA was 336 days (p = 0.510). Mean progression-free duration by RECIST criteria among the TACE + RFA group was 210 days vs. TACE only group 97 days (p = 0.04). Conclusion: Combination therapies of TACE and RFA were associated with improved overall survival than TACE alone. Patients with single tumors <5 cm appeared to have a survival advantage with combination therapy when compared to larger tumors. TACE-RFA was associated with improved tumor response and progression-free duration than TACE alone.
1. Introduction
Hepatocellular carcinoma (HCC) is a highly fatal malignancy and is in fact the third most common cause of cancer deaths in the world [1]. Though it is not a common cancer in the US, its incidence has been increasing and has almost doubled in the last two decades [2]. The rising incidence poses challenges to the clinician as a definitive cure is still not in sight. Though orthotopic liver transplantation and surgical resection are the two potentially curative modes of treatment for HCC only, 5% - 37% of HCC patients are actually eligible for surgical cure [3,4]. Recent advances in the treatment of HCC have led to the availability of various locoregional therapeutic options for those who are ineligible for surgery [5-7]. Image guided delivery of chemotherapeutic agents directly into the vessels supplying the tumor (TACE) and local radio ablative procedures (RFA) have revolutionized the treatment of unresectable HCC [8].
TACE is generally preferred for large and multifocal tumors while RFA is preferred small and oligonodular HCC. In a prospective randomized trial by Kenneth et al. it was demonstrated that TACE and RFA have comparable efficacy and survival benefit in patients with unresectable HCC [9]. But both modalities have their own inherent advantages and disadvantages. Combination therapy of TACE and RFA has been tried in an attempt to counterbalance their limitations and enhance their efficacy [10-13]. One of the limitations of RFA is that the heat generated from the probe is dispersed, via the rich vascular supply of the tumor, from the target area to the non-tumorous areas [14]. Performing TACE prior to RFA can lead to tumor devascularization which, it is hypothesized, can lead to all the heat generated to be confined to the tumor area. Thus the devascularization and thermal ablation can synergistically lead to increased tumor necrosis [15].
Studies have already proposed a synergistic action of combination TACE-RFA in HCC [10,13,16,17]. To test the above hypothesis we have undertaken a comparison of the survival benefits and tumor response among HCC patients treated with a combination of TACE-RFA against those treated with TACE alone.
2. Materials and Methods
2.1. Patient Selection
The study was approved by our institutional review board. Consecutive patients with HCC who underwent transcatheter chemoembolization (TACE) in our Institution over a period of ten years (June 1998 to June 2008) were reviewed. Inclusion criteria for the study were as follows: 1) TACE was performed with Doxorubicin eluting beads or conventional chemoembolization; 2) Planned RFA was performed within 1 week of TACE. Exclusion criteria were: 1) Patients who underwent resection or transplant; 2) Patients who received bland embolization, radioembolization with Y-90; 3) Patients who received therapy with more than one type of embolic agent.
2.2. Pre-Procedure Imaging
All patients underwent CT or MR imaging within 1 month before the procedure to assess tumor burden and presence of portal vein thrombus. Number, size and location of tumors were recorded. In this study, HCC with more than 5 discrete nodules is referred to as diffuse tumor. Mean tumor size was calculated by the sum of the longest diameter of all measurable tumors. Presence of portal vein thrombus, its location, nature and extent were also noted down.
2.3. Procedure
All TACE and RFA were performed by the same interventional radiologists. TACE was performed by introducing the catheter through femoral artery according to the Seldinger technique under local anesthesia. Celiac and Mesenteric arteriogram were obtained to assess arterial vascularization of the liver and to evaluate portal vein patency. The chemotherapeutic agents were infused into the hepatic artery which feeds the tumor. Conventional TACE was performed by introducing a mixture 100 mg Adriamycin, 100 mg of Cisplatin and Ethiodol. Those who underwent Precision TACE received LC beads impregnated with 50 mg of Doxorubicin. In both cases PVA particles (size 300 - 500, 500 - 700) were used to attain stasis in the tumor feeding artery. After embolization arteriogram was obtained to confirm vascular occlusion and to assess blood flow in other vessels. All patients were kept under observation for a period of 24 hours and analgesia administered as necessary.
In those who received combination therapy, RFA was planned and performed within a week after TACE. Appropriate anesthesia and sedation were used during the procedure. Radiofrequency ablation was performed using RITA prongs or Valley Lab RFA electrode under image guidance by the open or percutaneous route depending on the size and location of tumors. The ablation area included the tumor and is up to 1.0 cm of the surrounding tissue. The track was ablated during withdrawal.
2.4. Follow-Up
Four to six weeks after the transcatheter therapy/RFA patients underwent cross-sectional imaging (contrast material-enhanced computed tomography [CT] or magnetic resonance [MR] imaging). Comparison was made with the pre-procedure imaging and patients with residual or new tumor underwent repeat TACE. Patients who had no evidence of tumor enhancement underwent screening with repeat imaging performed every 3 months. If marginal recurrences or new tumors were identified, patients were treated again.
2.5. Study Endpoints
The primary end point of the study was survival and the secondary was tumor response. All patients were followed up unto death or to the date of last follow-up. Tumor response was assessed using RECIST criteria-CR (complete response) = disappearance of all target lesions; PR (partial response) = 30% decrease in the sum of the longest diameter of target lesions; PD (progressive disease) = 20% increase in the sum of the longest diameter of target lesions; SD (stable disease) = small changes that do not meet above criteria [18-20]. The duration of overall response was measured from the time that measurement criteria are met for complete response or partial response (whichever status is recorded first) until the first date that recurrent or progressive disease is objectively documented.
2.6. Statistics
Survival time is defined as the time from the date of first embolization to the date of death or last follow-up. Survival analysis was performed using Kaplan Meier Estimator and Cox proportional Hazard model. Death from any cause was considered as the censoring event. Survival time in the different groups was compared using the Log rank test. Pearson Chi square test and Fisher exact probability were used to compare the distribution of categorical variables. Independent t tests were used to compare means of continuous variables. The software SPSS Graduate version 16.0 was used for all computations.
3. Results
3.1. Patient Selection
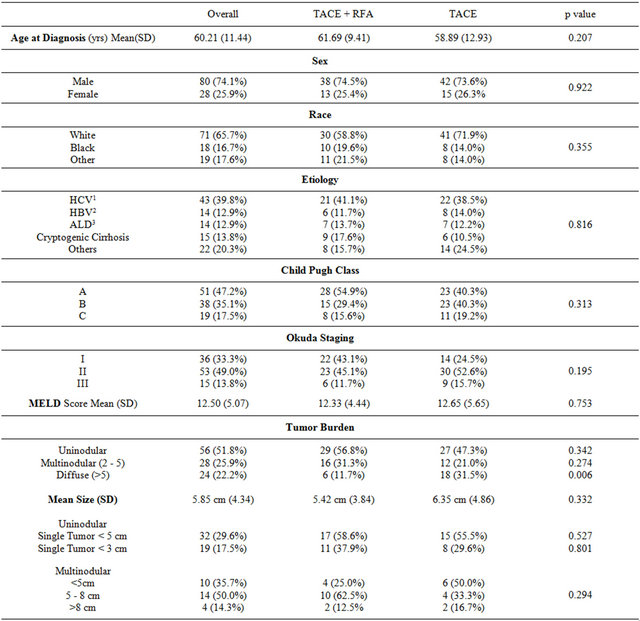
A total of 108 patients met the inclusion criteria and were included in the study, 51 (47.2%) patients received TACE followed by planned RFA and 57 (52.8%) patients received TACE alone. A total of 176 TACE procedures were performed in the 108 patients. Fifty-six patients received single session of transcatheter therapy and fifty one received multiple sessions. Demographic and clinical characteristics of the patients are described in Table 1. There were no significant differences in the variables considered including demographics, liver function and tumor burden.
3.2. Survival
At the end of the chosen study period 70.17% (40/57) of patients in the TACE alone group and 60.78% (31/51) of the patients in the TACE-RFA group suffered mortality.
Table 1. Demographic and clinical characteristics of study group.
Median survival among patients who received combination TACE + RFA was 566 (152 - 980) days and it was 209 (12 - 425) days in those treated with TACE alone (p = 0.01) (Figure 1). Survival rates at 1, 2 and 3 years among patients who received TACE + RFA and TACE alone were −53%, 44%, 30% and 35%, 15%, 7% respectively.
A sub-stratification analysis was performed comparing survival rates among patients who received Precision TACE and Conventional TACE. Median survival of patients treated with Precision-TACE +RFA was 566 (95% CI 109 - 1023) days and that of patients treated with conventional TACE + RFA was 336 days (95% CI 28 - 643) days (p = 0.510). Among the patients who received Precision TACE alone median survival was 295 (95% CI 12 - 590) days and among those who received conventional TACE alone it was 114 (95% CI 12 - 275) days (p = 0.458).
3.3. Tumor Treatment Response
In the group of patients who received only TACE 19.29% (10/57) and in the group that received TACERFA 3.88% (3/51) showed progressive disease by RECIST criteria in the initial follow up imaging at 4 - 6 weeks after therapy (p = 0.015). Mean progression-free duration by RECIST criteria among the TACE-RFA group was 210 (SD 312) days and the same among those who received TACE alone was 96 (SD 157) days (p = 0.001). In the TACE-RFA group, mean progression-free duration among patients with single tumor was 280 (SD 369) days and was 49 (SD 42) days among patients with multiple tumors. When progression free duration was compared amongst the TACE-RFA group stratified by
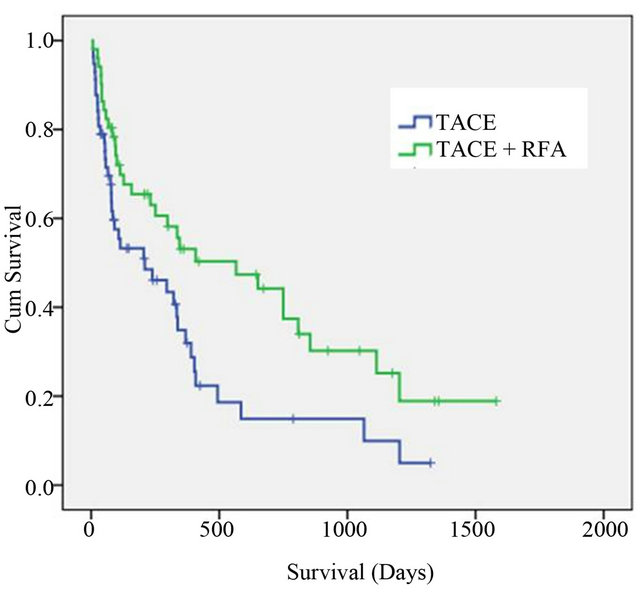
Figure 1. Kaplan Meier survival curves showing improved survival in patients treated with combination TACE-RFA (Transarterial chemoembolization-Radiofrequency ablation) versus TACE alone.
tumor size no significant difference was found (p = 0.427).
3.4. Number of Tumors
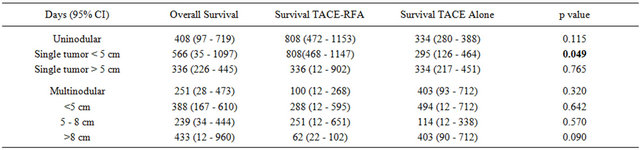
On subgroup analysis of survival among patients with a single tumor, those who received combination therapy with TACE and RFA had a median survival of 808 (95% CI 472 - 1153) days and those who received TACE alone had a median survival of 334 (95% CI 280 - 388) days (p = 0.115). Among patients with multinodular HCC, median survival in the combination TACE-RFA group was 100 (95% CI 12 - 268) days and among the TACE alone group it was 403 (95% CI 93 - 712) days (p = 0.321). Median survival in patients with diffuse tumor (>5 nodules) treated with combination TACE-RFA was 345 (95% CI 12 - 1000) days and among those treated with TACE alone it was 57 (95% CI 11 - 102) days (p = 0.019).
3.5. Size of Tumors
The patients with single tumor were sub-stratified into those with tumor size <5 cm and >5 cm. Median survival among patients with tumor size <5 cm among the patients treated with combination TACE-RFA was 808 (95% CI 468 - 1147) days and among those treated with TACE alone was 295 (95% CI 126 - 464) days (p = 0.049) (Figure 2). Median survival was not significantly different between patients treated with combination TACERFA and TACE alone when stratified by mean tumor size as, 5 - 8 cm or >8 cm (p = 0.581) (Table 2).
3.6. Complications
All patients experienced one or more minor complications like pain, nausea and loss of appetite which were
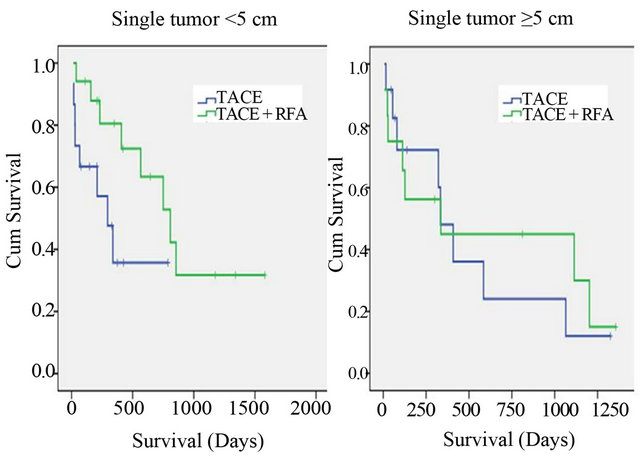
Figure 2. Kaplan Meier survival curves comparing survival in patients with single tumor treated with combination TACERFA (Transarterial chemoembolization-Radiofrequency ablation) versus TACE alone with patients stratified by tumor size.
Table 2. Survival analysis stratified by tumor number and cumulative size.
self-limited and managed with supportive care. Five patients suffered procedure related mortality, due to liver failure. Four of them belonged to Child Class C. No significant differences in incidence of adverse effects were noted between the TACE-RFA and TACE alone group as illustrated in Table 3.
4. Discussion
In our study, combination therapy with TACE and RFA shows both increased survival and better treatment response than treatment with TACE alone. Prognostic factors affecting survival in patients with unresectable HCC have been published before including one from this study group [21]. Tumor stage and liver dysfunction are the usual factors which influence outcome. In the current study the patients treated with TACE-RFA or TACE alone were found to have comparable liver reserve (as assessed by Child Pugh score and MELD score), comparable tumor staging (as assessed by Okuda staging) and comparable tumor burden (assessed by tumor size and multiplicity).
In this study, when survival curves between the TACERFA group and TACE alone group were compared it was seen that the combination therapy significantly prolonged overall survival. A prospective randomized controlled trial by Cheng et al. demonstrated a similar survival benefit of combination TACE-RFA therapy over TACE alone (HR = 1.87, 95% CI, 1.33 - 2.63), in patients with unresectable HCC larger than 3 cm in size [10]. Another study Liao et al. in 36 patients with unresectable HCC also showed survival benefit of combination TACE-RFA over untreated historical control [12]. A recent metaanalysis also concluded that combination therapy improved survival over TACE alone [22]. While most of these studies included patients with unresectable HCC another study by Kagawa et al. included patients within Milan criteria who were treated with combination TACERFA. They showed improved survival with the combination therapy when compared to surgical resection [23].
We have used the term TACE as an umbrella term to describe both Drug eluting beads and conventional TACE. When we compared the groups treated with Precision TACE-RFA and Conventional TACE-RFA in terms of survival and tumor response we did not find any significant differences between the two techniques. Most of the studies in literature have studied Conventional TACE in combination with RFA. But a recent Italian study in a small group of patients has shown that drug eluting beads enhance the effects of RFA leading to better tumor response [14]. But we did not find any study which compared the two techniques. Though statistical significance has not been achieved we observed a trend that Precision TACE was better than Conventional TACE when combined with RFA. The relatively small size of the study group is probably decreasing the predictive power. Since, in a study comparing DEB-TACE versus conventional TACE alone from the study group, we have shown survival benefit with DEB-TACE [24].
The tumor response to treatment has been assessed, in our study, using RECIST criteria. The duration for which the intrahepatic disease remained stable is significantly longer in the TACE-RFA group. This probably reflects the increased tumor necrosis obtained by the synergistic action of the combination therapy. In a study by Cheng et al., TACE-RFA was shown to demonstrate better tumor response than TACE alone when tumors were stratified by both multiplicity and size [10]. Gaspirni et al. observed that when TACE was sequentially followed by RFA, 100% tumor necrosis was attained in single tumors <5 cm, 95% in multifocal tumors with tumor mass <40% of liver volume and 90% necrosis in multifocal tumors with tumor mass >40% of liver volume [11].
Animal studies have also shown increased tumor necrosis with combination TACE-RFA [25-27]. Ahmed et al. [26] studied this combination therapy in by implanting mammary adenocarcinoma cells in Fischer rats. They showed that combination RFA and embolotherapy increased tumor necrosis when compared to either treatment alone. The intratumoral dose of Doxorubicin was also found to be higher in combination therapy. Monsky et al. [28] demonstrated that this increase in necrosis occurs in peripheral zones which are exposed to sublethal hyperthermia. It has been proposed that the chemotherapeutic agent synergistically increases the hyper-
Table 3. Complications after TACE-RFA and TACE.
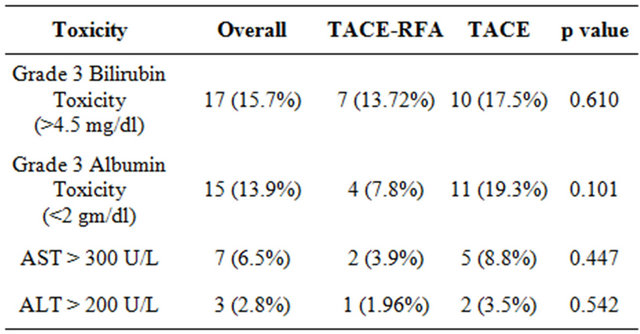
thermia induced lipid peroxidation and free radical generation, which translate into increased necrosis. Heat induced increased vascular permeability is said to play a role in augmenting intratumoral drug concentration.
When the study group was stratified by number and size of tumors it was observed in our study, that survival benefit of combination TACE-RFA was significantly better in those with single tumor <5 cm in size. Similar results have been reported in another study where, complete necrosis was attained in a significantly higher proportion of patients with tumor size <5 cm than in those with tumors larger than 5 cm when treated with TACERFA [13]. The combination therapy is also seen to result in longer survival in patients with diffuse (>5 nodules) tumor. As mentioned in Table 1, the distribution of diffuse tumors between the two arms of treatment is significantly different with more patients receiving TACE alone. So the results are confounded by patients with lesser tumor burden receiving the combination therapy more often.
One of the chief concerns of combination therapy is that it might lead to further decline in liver function. We compared the rate of complications between the two arms of treatment and did not find any significant differences. In Child Class A no major complications, like death or hepatic failure, were recorded in TACE-RFA or TACE only groups. Minor complications like fever and nausea were common but self-limited. Most of the patients who developed liver failure belonged to Child class C, so the failure can itself be attributed to the natural course of the disease. No significant differences were found in the complication rate between TACE-RFA and TACE alone in the study by Cheng et al. either [10].
The chief limitations of this study are its retrospective design and relatively small size of the study group. Though the two groups were not prospectively matched, they are statistically similar for tumor and liver factors which add strength to our results. We have used the RECIST criteria to assess tumor response, which is a standard and widely used classification. These criteria allow objective assessment of the change in tumour burden which is a crucial component in the evaluation of cancer therapeutics. And using such standard criteria also facilitates comparison between clinical trials. However, it does not take into account tumor viability factors, which are crucial for response assessment. Finally, this is a single-center study from a referral unit, which leads to limited diversity in the demographics. But overall the study adds to the literature on this important aspect of HCC treatment. And we bring up some interesting observations regarding tumor size and safety in advanced tumors. Our study is also unique in including patients treated with DEB TACE and RFA, most other studies have only reported on conventional TACE.
5. Conclusion
In patients with HCC, combination therapy with TACE and RFA leads to longer survival and progression free duration than treatment with TACE alone. Patients with single tumors <5 cm appeared to have a survival advantage with combination therapy when compared to larger tumors. The safety profile of the combination therapy is similar to treatment with TACE alone. Multimodality therapy appears to be a safe and effective option that can be included in the armamentarium of palliative treatments for HCC.
REFERENCES
- R. Dhanasekaran, A. Limaye and R. Cabrera, “Hepatocellular Carcinoma: Current Trends in Worldwide Epidemiology, Risk Factors, Diagnosis, and Therapeutics,” Hepatic Medicine: Evidence and Research, Vol. 4, 2012, pp. 19-37.
- Surveillance Research Program, “National Cancer Institute. Fast Stats: An Interactive Tool for Access to SEER Cancer Statistics,” 2012. http://seer.cancer.gov/faststats
- W. T. Kassahun, J. Fangmann, J. Harms, J. Hauss and M. Bartels, “Liver Resection and Transplantation in the Management of Hepatocellular Carcinoma: A Review,” Experimental and Clinical Transplantation, Vol. 4, No. 2, 2006, pp. 549-558.
- Y. S. Guan and Y. Liu, “Interventional Treatments for Hepatocellular Carcinoma,” Hepatobiliary & Pancreatic Diseases International, Vol. 5, No. 4, 2006, pp. 495-500.
- M. Schwartz, S. Roayaie and M. Konstadoulakis, “Strategies for the Management of Hepatocellular Carcinoma,” Nature Clinical Practice Oncology, Vol. 4, No. 7, 2007, pp. 424-432.
- H. B. El-Serag, J. A. Marrero, L. Rudolph and K. R. Reddy, “Diagnosis and Treatment of Hepatocellular Carcinoma,” Gastroenterology, Vol. 134, No. 6, 2008, pp. 1752-1763.
- S. G. Christos, E. R. Douglas, S. Stephen and H. G. JeanFrancois, “New Nonsurgical Therapies in the Treatment of Hepatocellular Carcinoma,” Techniques in Vascular and Interventional Radiology, Vol. 4, No. 3, 2001, pp. 193- 199. doi:10.1016/S1089-2516(01)90025-3
- A. Lubienski, M. Simon, K. Lubienski, J. Gellissen, R. T. Hoffmann, T. F. Jakobs, et al., “Update on Chemoinfusion and Chemoembolization Treatments,” Radiologe, Vol. 47, No. 12, 2007, pp. 1097-1106, 1108.
- K. S. Chok, K. K. Ng, R. T. P. Poon, C. M. Lam, J. Yuen, W. K. Tso, et al., “Comparable Survival in Patients with Unresectable Hepatocellular Carcinoma Treated by Radiofrequency Ablation or Transarterial Chemoembolization,” Archives of Surgery, Vol. 141, No. 12, 2006, pp. 1231-1236. doi:10.1001/archsurg.141.12.1231
- B. Q. Cheng, C. Q. Jia, C. T. Liu, W. Fan, Q. L. Wang, Z. L. Zhang, et al., “Chemoembolization Combined with Radiofrequency Ablation for Patients with Hepatocellular Carcinoma Larger than 3 cm: A Randomized Controlled Trial,” JAMA, Vol. 299, No. 14, 2008, pp. 1669-1677.
- D. Gasparini, M. Sponza, A. Marzio, R. Zanardi, M. Bazzocchi and Y. Cemal, “Combined Treatment, TACE and RF Ablation, in HCC: Preliminary Results,” La Radiologia Medica (Torino), Vol. 104, No. 5-6, 2002, pp. 412- 420.
- G. S. Liao, C. Y. Yu, M. L. Shih, D. C. Chan, Y. C. Liu, J. C. Yu, et al., “Radiofrequency Ablation after Transarterial Embolization as Therapy for Patients with Unresectable Hepatocellular Carcinoma,” European Journal of Surgical Oncology, Vol. 34, No. 1, 2008, pp. 61-66. doi:10.1016/j.ejso.2007.02.006
- A. Veltri, P. Moretto, A. Doriguzzi, E. Pagano, G. Carrara and G. Gandini, “Radiofrequency Thermal Ablation (RFA) after Transarterial Chemoembolization (TACE) as a Combined Therapy for Unresectable Non-Early Hepatocellular Carcinoma (HCC),” European Radiology, Vol. 16, No. 3, 2006, pp. 661-669. doi:10.1007/s00330-005-0029-9
- R. Lencioni and L. Crocetti, “Image-Guided Thermal Abla- tion of Hepatocellular Carcinoma,” Critical Reviews in Oncology/Hematology, Vol. 66, No. 3, 2008, pp. 200-207. doi:10.1016/j.critrevonc.2008.01.003
- S. Rossi, F. Garbagnati, R. Lencioni, H. P. Allgaier, A. Marchiano, F. Fornari, et al., “Percutaneous Radio-Frequency Thermal Ablation of Nonresectable Hepatocellular Carcinoma after Occlusion of Tumor Blood Supply,” Radiology, Vol. 217, No. 1, 2000, pp. 119-126.
- M. Bloomston, O. Binitie, E. Fraiji, M. Murr, E. Zervos, S. Goldin, et al., “Transcatheter Arterial Chemoembolization with or without Radiofrequency Ablation in the Management of Patients with Advanced Hepatic Malignancy,” The American Journal of Surgery, Vol. 68, No. 9, 2002, pp. 827-831.
- K. Yamakado, A. Nakatsuka, M. Akeboshi, K. Shiraki, T. Nakano and K. Takeda, “Combination Therapy with Radiofrequency Ablation and Transcatheter Chemoembolization for the Treatment of Hepatocellular Carcinoma: Short-Term Recurrences and Survival,” Oncology Reports, Vol. 11, No. 1, 2004, pp. 105-109.
- A. R. Padhani and L. Ollivier, “The RECIST (Response Evaluation Criteria in Solid Tumors) Criteria: Implications for Diagnostic Radiologists,” British Journal of Radiology, Vol. 74, No. 887, 2001, pp. 983-986.
- C. C. Jaffe, “Measures of Response: RECIST, WHO, and New Alternatives,” Journal of Clinical Oncology, Vol. 24, No. 20, 2006, pp. 3245-3251. doi:10.1200/JCO.2006.06.5599
- P. K. Julka, D. C. Doval, S. Gupta and G. K. Rath, “Response Assessment in Solid Tumours: A Comparison of WHO, SWOG and RECIST Guidelines,” British Journal of Radiology, Vol. 81, No. 966, 2008, pp. 444-449. doi:10.1259/bjr/32785946
- R. Dhanasekaran, D. A. Kooby, C. A. Staley, J. S. Kauh, V. Khanna and H. S. Kim, “Prognostic Factors for Survival in Patients with Unresectable Hepatocellular Carcinoma Undergoing Chemoembolization with Doxorubicin Drug-Eluting Beads: A Preliminary Study,” HPB, Vol. 12, No. 3, 2010, pp. 174-180. doi:10.1111/j.1477-2574.2009.00138.x
- W. Wang, J. Shi and W. F. Xie, “Transarterial Chemoembolization in Combination with Percutaneous Ablation Therapy in Unresectable Hepatocellular Carcinoma: A MetaAnalysis,” Liver International: Official Journal of the International Association for the Study of the Liver, Vol. 30, No. 5, 2010, pp. 741-749.
- T. Kagawa, J. Koizumi, S. Kojima, N. Nagata, M. Numata, N. Watanabe, et al., “Transcatheter Arterial Chemoembolization plus Radiofrequency Ablation Therapy for Early Stage Hepatocellular Carcinoma: Comparison with Surgical Resection,” Cancer, Vol. 116, No. 15, 2010, pp. 3638-3644.
- R. Dhanasekaran, D. A. Kooby, C. A. Staley, J. S. Kauh, V. Khanna and H. S. Kim, “Comparison of Conventional Transarterial Chemoembolization (TACE) and Chemoembolization with Doxorubicin Drug Eluting Beads (DEB) for Unresectable Hepatocelluar Carcinoma (HCC) ,” Journal of Surgical Oncology, Vol. 101, No. 6, 2010, pp. 476- 480.
- D. I. Giuseppe, A. Muneeb, D. G. Geoffrey, E. S. Keith, B. K. Jonathan, F. H. Elkan, et al., “Percutaneous Tumor Ablation: Reduced Tumor Growth with Combined Radio-Frequency Ablation and Liposomal Doxorubicin in a Rat Breast Tumor Model,” Radiology, Vol. 228, No. 1, 2003, pp. 112-118. doi:10.1148/radiol.2281020358
- A. Muneeb, E. M. Wayne, G. Geoffrey, L. Anatoly, D. I. Giuseppe, B. K. Jonathan, et al., “Radiofrequency Thermal Ablation Sharply Increases Intratumoral Liposomal Doxorubicin Accumulation and Tumor Coagulation,” Cancer Research, Vol. 63, No. 19, 2003, pp. 6327-6333.
- A. Muneeb, N. L. Anatoly, T. Vladimir, T. Herve, N. S. Anatoly and N. S. Goldberg, “Combined Radiofrequency Ablation and Adjuvant Liposomal Chemotherapy: Effect of Chemotherapeutic Agent, Nanoparticle Size, and Circulation Time,” Journal of Vascular and Interventional Radiology: JVIR, Vol. 16, No. 10, 2005, pp. 1365-1371. doi:10.1097/01.RVI.0000175324.63304.25
- L. M. Wayne, B. K. Jonathan, N. L. Anatoly, D. G. Geoffrey, A. Muneeb, G. S. Gazelle, et al., “Radio-Frequency Ablation Increases Intratumoral Liposomal Doxorubicin Accumulation in a Rat Breast Tumor Model,” Radiology, Vol. 224, No. 3, 2002, pp. 823-829. doi:10.1148/radiol.2243011421
NOTES
*There is no conflict of interest of the authors.
#Corresponding author.

