Journal of Cancer Therapy
Vol.3 No.5(2012), Article ID:23438,5 pages DOI:10.4236/jct.2012.35061
Evaluation of Surgical Outcome after Resection of Pancreatic Tumors, Our Experience in Mansoura Oncology Center, a Middle-Volume Center in Egypt
![]()
Oncology Center, Mansoura University, Mansoura, Egypt.
Email: *mmosbah74@yahoo.com
Received June 20th, 2012; revised July 25th, 2012; accepted August 6th, 2012
Keywords: Pancreatic Cancer; Whipple; Complication; Survival
ABSTRACT
Background: Pancreatic cancer is the fourth most common cause of cancer related deaths in the world. Surgical resection remains the only potentially curative treatment for adenocarcinoma of the pancreas; only 10% - 20% of patients are candidate for standard pancreatic resection. Objective: To evaluate perioperative mortality, morbidity and survival for patients underwent PD in middle-volume center. Patients and Methods: Sixty patients with pancreatic tumors were enrolled in this study for different surgical procedure according to type of tumors. Results: No early postoperative complications were observed in 60% of patients, whereas 40% of patients developed one or more complication, the median survival for this group was 12.3 months with standard deviation 3.8 months.
1. Introduction
Pancreatic cancer is a deadly disease. In 2007, death due to pancreatic cancer is projected to approximate the incidence of the disease, with about 34,000 patients being diagnosed with pancreatic cancer in the United States [1].
The prognosis of patients with pancreatic cancer remains poor. Patients frequently present with distant metastases, which are often occult at the time of diagnosis and the 5-year survival rate is 5.6% for all patients diagnosed with pancreatic adenocarcinoma. Treatment for patients with potentially curable disease remains challenging, and the 5-year survival for patients with early stage disease is estimated at 15%, with median survival for patients with locally advanced disease remains limited at 6 to 11 months [2].
Advances in operative technique and patient care have limited perioperative morbidity and mortality for those fortunate enough to undergo resection. Nonetheless, surgical therapy (i.e. pancreatectomy) despite curative intent results in high rates of recurrence and disappointing median survivals of about 12 months [3].
The morbidity related to PD is still high, even in high-volume centers, although there has been improvement in the management of complications [4].
So in this study we evaluate perioperative mortality, morbidity and survival for patients underwent PD in middle-volume center.
2. Patient and Methods
This study was conducted at Surgical Oncology Unit, Oncology Centre, Mansoura University (OCMU) during the period between January 2005 & January 2012. Sixty patients with pancreatic tumors were enrolled in this study for different surgical procedure according to type of tumors.
Our inclusion criteria were operable pancreatic tumors candidates for surgery and exclusion criteria were metastatic cancer pancreas and celiac trunk encasement.
The following factors were analyzed: Patient demographics, intraoperative factors, as type of resection, blood loss, blood transfusion, and operative time, Tumor characteristics, including diameter, histologic grade, lymph node status and finally the primary outcome variable analyzed was survival. Follow-up was performed by office records, telephone contact; Survival was analyzed by the method of Kaplan and Meier test.
3. Results
Table 1 summarizes the patients characteristics and operative details.

Table 1. Operative findings in 60 patients undergoing pancreatic resection.
The mean age of the patients was 57 ± 11.7 years and ranged from 20 - 74 years. There were 37 male (61.7%) and 23 female (38.3%).
Jaundice was the commonest presentation as it was present in 78.3% patients followed by abdominal pain in 21.7% patients.
At time of diagnosis 55% of the patients were diabetic and the duration of symptoms was more than four weeks in 86.7% patients and less than four weeks in 13.3% patients.
Regarding site of tumor 44 patients (73.3%) were in head of pancreas, 7 patients (11.7%) in body, 7 patients (11.7%) in duodenum and periampulary and 2 patients (3.3%) in tail.
As regard to mass diameter determined by assessment of the pathology specimen, were less than 3 cm in 45% Patients and more than 3 cm in 55% patients.
Classical Whipple’s pancreaticoduodenectomy was done 36 patients (60%) while extended radical resection was performed for 13 (21.7%) patients, distal pancreatectomy for 9 patients (15%) and ennuclation of cyst for 2 patients (3.3%).
Pancreaticogasrostomy (PG) for reconstruction after resection was performed for 50 patients (88.3%) whereas pancreaticojejunostomy (PJ) was performed in 8 patients (13.3%) patients. Two patient performed ennuclation of cyst with no need for reconstruction.
LN was infiltrated in 38 patients (63.3%) and not infiltrated in 22 patients (36.7 %).
In the current study, the mean operative time was 7.17 ± 0.50 hours. Mean blood unit transfused was 1.6 ± 0.9 unit.
The final pathological diagnoses of the resected specimens was pancreatic adenocarcinoma in 45 (75%) of patients, duodenal tumor in 6 (10%), periampullary adenocarcinoma in 3 (5%), 3 patients with cystic neoplasm (5%) one of them proved to be carcinoid tumor, Chronic pancreatitis in 2 (3.3%), and 1 patient with sarcomatoid carcinoma (1.7%).
Well-differentiated tumors were 59.3% of all tumors moderate differentiated tumors in 15.3% and poorly differentiated in 25.4%.
No early postoperative complications were observed in 60% of patients, whereas 40% of patients developed one or more complication.
The commonest complications were wound infection 8 patients (13.3%), delayed gastric emptying; DGE 7 patients (11.7%), pancreatitis 4 patients (6.7%), pancreatic leak 3 patients (5%), abdominal collection 2 patients (3.3%).
Three deaths occurred in-hospital or within thirty days of operation for an operative mortality rate of 5%. The precipitating cause was pancreatic fistula (one patient) and sudden cardiac arrest (two patients).
Pancreatic leak is considered the most serious early postoperative complication after pancreaticoduodenectomy, the type of reconstruction was found to have a statistically significant association with the development of pancreatic leak. Pancreaticojejunostomy (p = 0.047) were statistically significant risk factors for the occurrence of pancreatic leak.
Postoperative hospital stay (mean = 10.8 ± 5, median = 8 days).
The median survival for this group was 12.3 months with standard deviation 3.8 months. The median free survival for this group was 9.1 months with standard deviation 4 months.
Factors which did not influence survival included age, gender, type of resection whether Whipple’s PD, extended resection or distal pancreatectomy. Factors that did influence survival included tumor diameter, lymph node status and tumor differentiation (Table 2).

Table 2. Factors influencing survival after pancreaticoduodenectomy.
There is no significant change between Type of resection and complication (p = 0.2).
4. Discussion
The incidence rate for PC is approximately nine new cases per 100,000 people, with the peak incidence in the seventh and eighth decades of life and an average age of 60 to 65 years at diagnosis. The incidence rate is slightly higher in men than in women [5].
Pancreatic cancer remains a lethal disease with overall poor outcome following “curative” surgery. Despite this, surgical resection offers the only possibility of long-term cure. The morbidity and mortality associated with pancreatic surgery has declined significantly in the last two decades. Advances in diagnostic imaging and laparoscopy have contributed to limiting the number of pancreatic cancer patients subjected to nontherapeutic laparotomy. Advances in surgical technique and aftercare have made the design and completion of large randomized trials of adjuvant therapy possible in recent years. This is a critical development as it is clear that significant improvements in survival for pancreatic cancer patients await the development and testing of more effective multimodality therapies [3].
The northeast Nile Delta region exhibits a high incidence of early-onset pancreatic cancer. It is well documented that this region has one of the highest levels of pollution in Egypt. Epidemiologic studies have suggested that cadmium, a prevalent pollutant in the northeast Nile Delta region, play a role in the development of pancreatic cancer and the results of study on level of serum cadmium suggest that pancreatic cancer in the East Nile Delta region is significantly associated with high levels of serum cadmium and farming [6].
In our study resection for pancreatic tumors included 60 patients with mean age 57 ± 11.7 years, sex ratio (M: F) 61.7%:38.3%, the mean operative time was 7.17 ± 0.50 hours, mean blood loss 660 ± 180 which is comparable to Nagai et al. 2011 who reported in his study mean age was 63.3 ± 9.1 years, the mean operation time and OBL were 7.7 ± 2.1 hours and 1693 ± 1734 mL, respecttively. The MST was 14.6 months (range, 12.8 - 16.5 months) [7].
The hospital mortality was 5% and postoperative complications developed in 40% of patients in our study while mortality rate has decreased dramatically to less than 2% in recent series in high-volume centers, but morbidity was still around 40% despite great efforts to reduce the incidence of DGE, fistula formation, wound infection, and hemorrhage, which are the most common complications after PD [8].
In Capussotti et al. hospital and 60-day operative mortality was 5.4%. Morbidity was 37.5% [9].
In experienced centers a postoperative morbidity of 30% - 50% and a mortality around or underneath 5% is reported. As long term-survival is rare and complications are frequent the quality of life for the remaining months or years is of paramount importance [10].
In our study pancreatic leak according to the ISGPF criteria occurred in 3 patients (5%) out of 60 patients underwent resection whereas in Dong et al. the overall incidence of PF was 19.4%, which seemed lower than that in other reports by ISGPF definition, the morbidity rate was still as high as 52.4%. This may be because that the infection rate was too high (35.7%, 105/294), especially pulmonary infection that occurred in 19.7% (58/ 294) patients, which was higher than that reported in previous studies. However, the total mortality was only 4.8%, which was similar to that in high volume centers [11].
Reports from The Johns Hopkins Hospital have analyzed the outcomes of patients with pancreatic adenocarcinoma undergoing successful pancreaticoduodenectomy. In the first report, 201 patients undergoing resection between 1970 and 1994 were analyzed. This group of patients had an actuarial 5-year survival of 21% and a median Survival of 15.5 months. Survival was noted to improve significantly over the study period, with a 3-year actuarial survival of 14% in the 1970s, 21% in the 1980s, and 36% in the 1990s. Multivariate analysis in this study indicated the strongest predictors of long-term survival were tumor diameter less than 3 cm, negative resected lymph node status, negative resection margin status, diploid tumor DNA content, and decade of resection [12].
In our study median survival was 12.3 months with standard deviation 3.8 month and factors influence survival included tumor diameter, lymph node status and tumor differentiation (Figures 1-3).
Three previous studies have investigated the role of LN count and survival after pancreaticoduodenectomy. Sierzega et al. [13] concluded that the number of resected LN was not a prognostic factor for survival in N1 or N0 patients. Similar results were noted by Berger et al. [14]. Recently, an analysis of a smaller cohort of 770 N0 SEER registry patients from 1973 to 2000 was conducted and found significant stage-specific survival according to the number of negative and total LN.46 [15].
Independent factors influencing survival are reported
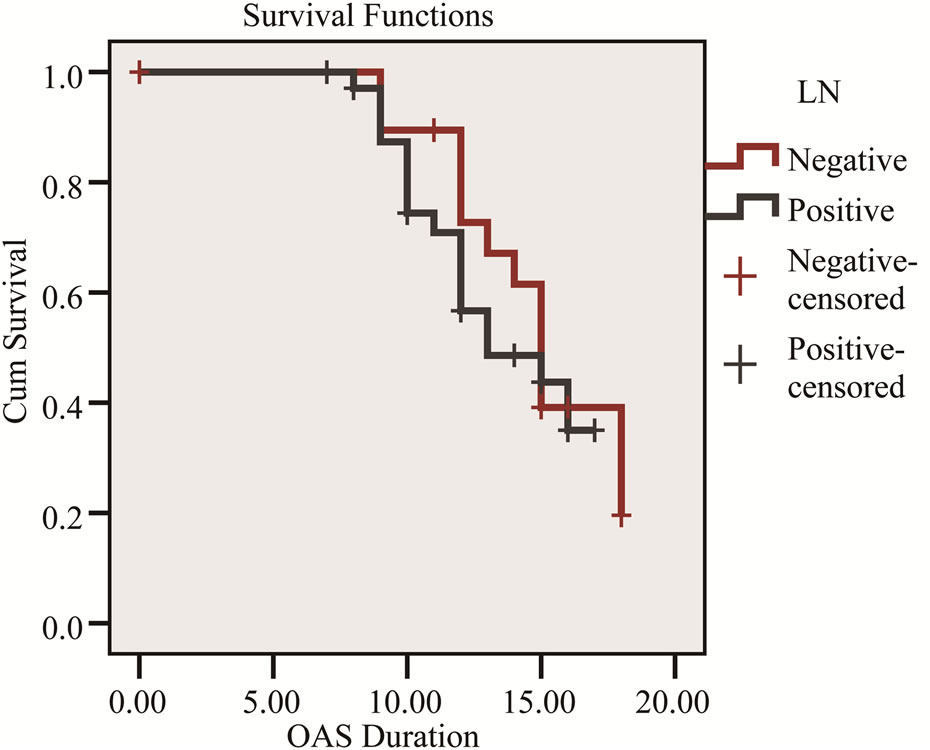
Figure 1. Kaplan-Meier survival curve shows the relation of overall survival and LN status.
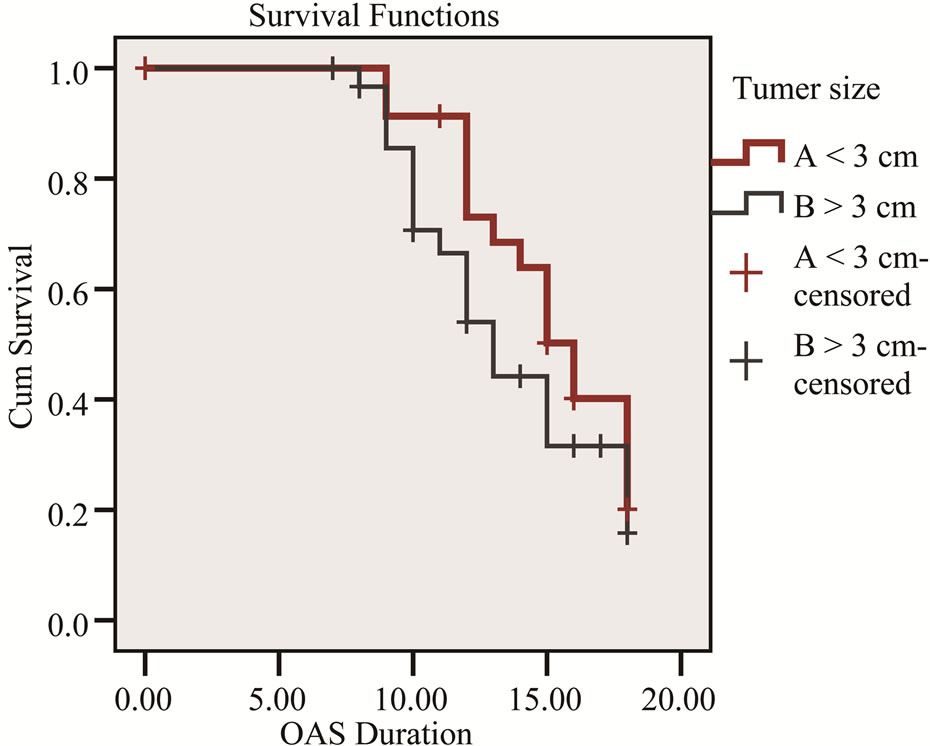
Figure 2. Kaplan-Meier survival curve shows the relation of overall survival and tumor size.
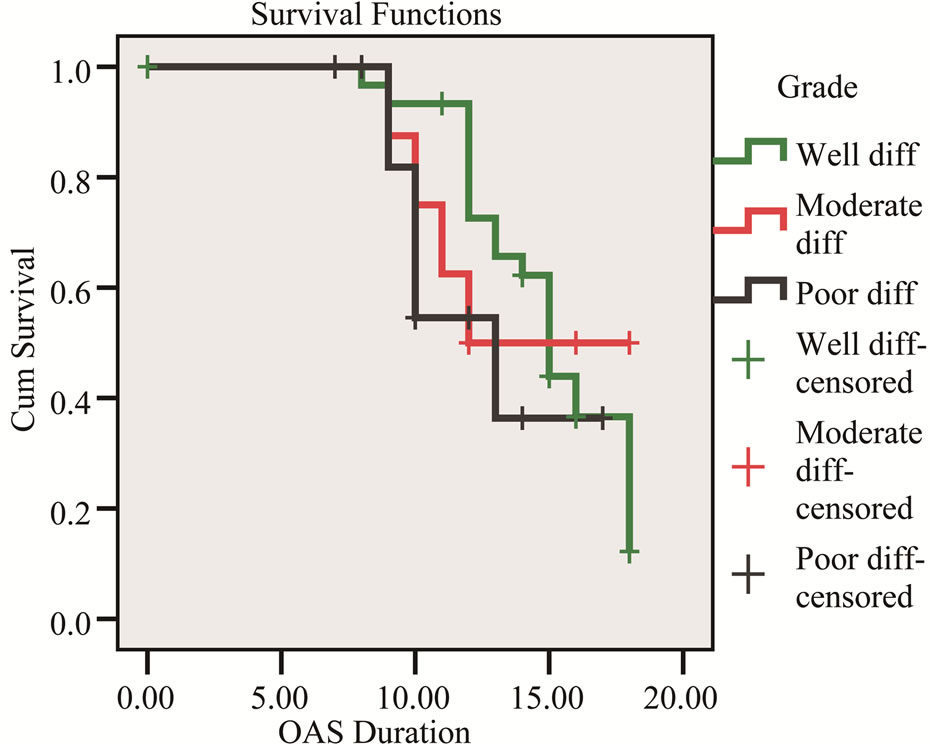
Figure 3. Kaplan-Meier survival curve shows the relation of overall survival and tumor differentiation.
in the literature as tumors less than 30-mm and pTNM classification and tumor differentiation .Recent studies showed that among pTNM, the number of resected lymph node and their involvement are powerful predictor of survival [16-19].
Many authors have emphasized that a minimal number of major pancreatectomies per year, ranging from 6 to 30, are required to obtain and conserve the expertise that is necessary to perform a complex procedure, such as PD. Although no one could ignore the significance of experience in medicine and in particular surgery, recently there are some reports indicating that good results could be achieved even in middle-volume centers [20].
In study in middle volume center although mortality following PD in was zero, the morbidity rate was higher than in a high-volume center. Not only the operative techniques, but also the intensive perioperative management of the patient, uniform definition of complications, use of a multidisciplinary approach, and identification of the risk factors are essential elements of decreasing morbidity. Better outcomes can be achieved even in lowto medium-volume centers in developing countries where a dedicated team with special interest in pancreatic surgery is treating all affected patients [21].
In the contrary other studies show that centralization has resulted in improved clinical outcomes of patients who underwent pancreatic surgery for a malignancy [22] and pancreaticoduodenectomy in high-volume centers can be performed with very low operative mortality [23].
5. Conclusion
Pancreatic resection either classic Whipple or extended radical resection can be safely performed in middle volume center with accepted morbidity, mortality and survival.
REFERENCES
- J. M. Hernandez, S. M. Cowgill, S. Al-Saadi, A. Collins, S. B. Ross, J. Cooper, et al., “CA 19-9 Velocity Predicts Disease-Free Survival and Overall Survival after Pancreatectomy of Curative Intent,” Journal of Gastrointestinal Surgery, Vol. 13, No. 2, 2009, pp. 349-353. doi:10.1007/s11605-008-0696-3
- S. M. Russo, R. Ove and M. W. Saif, “Identification of Prognostic and Predictive Markers in Pancreatic Adenocarcinoma. Highlights from the 2011 ASCO Gastrointestinal Cancers Symposium,” Journal of the Pancreas, Vol. 12, No. 2, 2011, pp. 92-95.
- C. J. Wray, S. A. Ahmad, J. B. Matthews and A. M. Lowy, “Surgery for Pancreatic Cancer: Recent Controversies and Current Practice,” Gastroenterology, Vol. 128, No. 6, 2005, pp. 1626-1641. doi:10.1053/j.gastro.2005.03.035
- C. Bassi, M. Falconi, R. Salvia, G. Mascetta, E. Molinari and P. Pederzoli, “Management of Complications after Pancreaticoduodenectomy in a High Volume Centre: Results on 150 Consecutive Patients,” Digestive Surgery, Vol. 18, No. 6, 2001, pp. 453-457. doi:10.1159/000050193
- American Cancer Society, “Global Cancer Facts and Figures [Database on the Internet],” American Cancer Society, Atlanta, 2002.
- A. M. Kriegel, A. S. Soliman, Q. Zhang, N. El-Ghawalby, F. Ezzat, A. Soultan, et al., “Serum Cadmium Levels in Pancreatic Cancer Patients from the East Nile Delta Region of Egypt,” Environmental Health Perspectives, Vol. 114, No. 1, 2006, pp. 113-119.
- S. Nagai, T. Fujii, Y. Kodera, M. Kanda, T. T. Sahin, A. Kanzaki, et al., “Impact of Operative Blood Loss on Survival in Invasive Ductal Adenocarcinoma of the Pancreas,” Pancreas, Vol. 40, No. 1, 2011, pp. 3-9. doi:10.1097/MPA.0b013e3181f7147a
- C. J. Yeo, “Management of Complications Following Pancreaticoduodenectomy,” Surgical Clinics of North America, Vol. 75, No. 5, 1995, pp. 913-924.
- L. Capussotti, P. Massucco, D. Ribero, L. Vigano, A. Muratore and M. Calgaro, “Extended Lymphadenectomy and Vein Resection for Pancreatic Head Cancer: Outcomes and Implications for Therapy,” Archives of Surgery, Vol. 138, No. 12, 2003, pp. 1316-1322. doi:10.1001/archsurg.138.12.1316
- M. Niedergethmann, M. Farag Soliman and S. Post, “Postoperative Complications of Pancreatic Cancer Surgery,” Minerva Chirurgica, Vol. 59, No. 2, 2004, pp. 175-183.
- X. Dong, B. Zhang, M. X.Kang, Y. Chen, Q. Q. Guo and Y. L. Wu, “Analysis of Pancreatic Fistula According to the International Study Group on Pancreatic Fistula Classification Scheme for 294 Patients Who Underwent Pancreaticoduodenectomy in a Single Center,” Pancreas, Vol. 40, No. 2, 2011, pp. 222-228. doi:10.1097/MPA.0b013e3181f82f3c
- C. G. Yeo, “Whipple Procedure: 1935 to Present,” In: Douglas Evans PPaJA, Ed., Pancreatic Cancer, 2002, pp. 125-137.
- J. D. Birkmeyer, A. L Warshaw, S. R Finlayson, M. R. Grove and A. N. Tosteson, “Relationship between HospiTal Volume and Late Survival after PancreaticoduodenecTomy,” Surgery, Vol. 126, No. 2, 1999, pp. 178-183. doi:10.1016/S0039-6060(99)70152-2
- A. C. Berger, J. C. Watson, E. A. Ross and J. P. Hoffman, “The Metastatic/Examined Lymph Node Ratio is an Important Prognostic Factor after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma,” The American surgeon, Vol. 70, No. 3, 2004, pp. 235-240.
- M. Hellan, C. L. Sun, A. Artinyan, P. Mojica-Manosa, S. Bhatia, J. D. Ellenhorn, et al., “The Impact of Lymph Node Number on Survival in Patients with Lymph NodeNegative Pancreatic Cancer,” Pancreas, Vol. 37, No. 1, 2008, pp. 19-24. doi:10.1097/MPA.0b013e31816074c9
- T. M. Pawlik, A. L. Gleisner, J. L. Cameron, J. M. Winter, L. Assumpcao, K. D. Lillemoe, et al., “Prognostic Relevance of Lymph Node Ratio Following Pancreaticoduodenectomy for Pancreatic Cancer,” Surgery, Vol. 141, No. 5, 2007, pp. 610-618. doi:10.1016/j.surg.2006.12.013
- T. Zacharias, D. Jaeck, E. Oussoultzoglou, A. Neuville and P. Bachellier, “Impact of Lymph Node Involvement on Long-Term Survival after R0 Pancreaticoduodenectomy for Ductal Adenocarcinoma of the Pancreas,” Journal of Gastrointestinal Surgery: Official Journal of the Society for Surgery of the Alimentary Tract, Vol. 11, No. 3, 2007, pp. 350-356. doi:10.1007/s11605-007-0113-3
- M. Adham, D. Jaeck, J. Le Borgne, E. Oussoultzouglou, M. P. Chenard-Neu, J. F. Mosnier, et al., “Long-Term Survival (5 - 20 Years) after Pancreatectomy for Pancreatic Ductal Adenocarcinoma: A Series of 30 Patients Collected from 3 Institutions,” Pancreas, Vol. 37, No. 4, 2008, pp. 352-357. doi:10.1097/MPA.0b013e31818166d2
- K. Tobita, H. Kijima, S. Dowaki, Y. Oida, H. Kashiwagi, M. Ishii, et al., “Thrombospondin-1 Expression as a Prognostic Predictor of Pancreatic Ductal Carcinoma,” International Journal of Oncology, Vol. 21, No. 6, 2002, pp. 1189-1195.
- G. Peros, G. A. Giannopoulos, S. Christodoulou, G. Konstantoudakis, K. Petropoulou and G. H. Sakorafas, “Good Results after Major Pancreatic Resections in a Middle-Volume Center,” Pancreas, Vol. 39, No. 3, 2010, pp. 411-414. doi:10.1097/MPA.0b013e3181bd94ce
- P. J. Lakhey, R. S. Bhandari, B. Ghimire and M. Khakurel, “Perioperative Outcomes of Pancreaticoduodenectomy: Nepalese Experience,” World Journal of Surgery, Vol. 34, No. 8, 2010, pp. 1916-1921. doi:10.1007/s00268-010-0589-y
- G. A. Gooiker, L. G. van der Geest, M. W. Wouters, M. Vonk, T. M. Karsten, R. A. Tollenaar, et al., “Quality Improvement of Pancreatic Surgery by Centralization in the Western Part of the Netherlands,” Annals of Surgical Oncology, Vol. 18, No. 7, 2011, pp. 1821-1829. doi:10.1245/s10434-010-1511-4
- R. Pezzilli, M. Falconi, A. Zerbi, R. Casadei, L. Valli, R. Varale, et al., “Clinical and Patient-Reported Outcomes after Pancreatoduodenectomy for Different Diseases: A Follow-Up Study,” Pancreas, Vol. 40, No. 6, 2011, pp. 938-945. doi:10.1097/MPA.0b013e318216f693
NOTES
*Corresponding author.

