Health
Vol.08 No.15(2016), Article ID:72552,9 pages
10.4236/health.2016.815160
A Simple Method for Detection of Multiple Chemical-Specific IgGs in Serum Based on Dot Blotting
Mayumi Tsuji1*, Hsu-Sheng Yu1,2, Yasuhiro Ishihara3, Toyohi Isse1, Nami Ikeda-Ishihara4, Takuto Tuchiya1, Toshihiro Kawamoto1
1Department of Environment Health, University of Occupational and Environmental Health, Kitakyusyu, Japan
2Department of Food Science, College of Agriculture, National Pingtung University of Science and Technology, Taiwan
3Laboratory of Molecular Brain Science, Graduate School of Integrated Arts and Sciences, Hiroshima University, Higashi-Hiroshima, Japan
4Division of Gene Research, Natural Science Center for Basic Research and Development, Hiroshima University, Higashi-Hiroshima, Japan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: July 29, 2016; Accepted: December 3, 2016; Published: December 6, 2016
ABSTRACT
Plastic resins are known to cause occupational allergies. Therefore, serum-specific antibodies against plastic resins have been widely investigated as diagnostic markers for occupational allergies. In this study, we aimed to establish a convenient method for detection of multiple chemical-specific IgG antibodies in human serum based on dot blot analysis. Toluene diisocyanate (TDI), phthalic anhydride (PA), and formaldehyde (FA), which are frequently used to synthesize various resins, reacted well with lysine residues of human serum albumin (HSA) under alkaline conditions. Native polyacrylamide gel electrophoresis (PAGE) showed that the structures of chemical adducts of HSA were different from those of native HSA. Therefore, we performed dot blot assays using these adducts as artificial antigens. Serum samples from workers at plants utilizing plastic resins strongly reacted with TDI, PA, and FA adducts in HSA, while reduced signals were detecting using the serum from unexposed workers. These results suggested that dot blot assays using chemical-HSA adducts as antigens could be beneficial for simultaneously measuring multiple chemical-specific IgGs.
Keywords:
IgG, Dot Blot Assay, Plastic Resin

1. Introduction
The production and consumption of plastics have increased worldwide. In 2010, plastic production reached 265 million tonnes worldwide, and approximately 5% of world plastic production is carried out in Japan [1] . Plastics are present ubiquitously throughout society and the environment. Most chemicals used for producing plastic polymers are derived from various types of nonrenewable crude oils, and some of these polymers are hazardous. The polymers ranked as most hazardous include polyurethanes (PURs), polyacrylonitriles (PANs), polyvinyl chloride (PVC), epoxy resins, and styrenic copolymers. Moreover, several additional types of polymers are considered hazardous, including phenol formaldehyde resins (PFs), unsaturated polyesters (UPs), polycarbonate (PC), polymethyl methacrylate (PMMA), and urea-formaldehyde resins (UFs) [2] . Toluene diisocyanate (TDI), which causes serious upper respiratory health problems [3] , is the monomer used for PUR production, whereas phthalic anhydride (PA) is used for UP production, and formaldehyde (FA) is used for PF and UF production. FA can cause skin sensitivity, and PA can cause respiratory and skin sensitivity [2] [4] . Therefore, TDI, PA, and FA can induce occupational allergies in workers who come in contact with these chemicals [3] [5] .
Chemical-specific IgE and IgG antibodies in serum, particularly those targeting TDI, have been widely investigated as diagnostic markers of occupational allergies. Recently, enzyme immunoassay methods, e.g., enzyme-linked immunosorbent assays (ELISAs), have been used worldwide for detection of these IgE and IgG proteins. Using ELISA, specific IgE has been shown to identify inhalation challenge-positive workers with 14% - 31% sensitivity and 89% - 97% specificity. The corresponding sensitivity and specificity estimates for specific IgG were reported to be 46% - 72% and 74% - 92%, respectively [6] . Based on measurements of sensitivity, specific IgG can be a useful diagnostic marker of occupational allergy. However, although several chemical-specific IgEs can be measured commercially, chemical-specific IgG cannot be measured commercially in Japan.
In general, plants handling plastic resins do not only use one type of chemical substance. Furthermore, workers may be exposed different combinations of chemical substances, particularly in small plants. Therefore, appropriate methods for measurement of chemical exposure should simultaneously utilize many inexpensive antibodies and have the capacity for analysis of small serum samples.
Accordingly, in this report, we established a method for simultaneous detection of multiple chemical-specific IgG antibodies in human serum with high sensitivity using dot blot assays. We used three major chemical substances used for the production of plastic resins, i.e., the isocyanate TDI and the carbonyl compounds PA and FA. Our findings provide important advancements in the detection of chemicals in serum samples.
2. Materials and Methods
2.1. Measurement of the Amino Groups of HSA Conjugates
The reaction of 2,4,6-trinitrobenzenesulphonic acid (TNBS) with primary amino groups within proteins was performed as previously described [7] . HSA conjugate (20 μg protein) was mixed with 100 μL of 4% NaHCO3, followed by the addition of 100 μL of 0.01% TNBS. The reaction was carried out at 42˚C for 2 h and then was stopped by the addition of 50 μL HCl. The absorbance at 340 nm was measured. Known concentrations of L-lysine were used as standards.
2.2. Native Polyacrylamide Gel Electrophoresis (PAGE)
Qualitative assessment of structural changes in HSA conjugates was achieved by native PAGE performed on 8% (w/v) acrylamide resolving gels. Ten micrograms of HSA or HSA conjugate was applied to the gel, and electrophoresis was performed. Protein bands were visualized by staining with Coomassie brilliant blue.
2.3. Serum Samples
Sera from workers handling plastic resin (e.g., TDI, FA, and PA) were collected in Kyusyu, Japan from 2013 to 2015. Sera from unexposed workers who live in Kyusyu were collected in 2014. After collection of blood samples, the blood was allowed to clot for approximately 3 h and then centrifuged at 2568 ×g 30 min to separate the serum portion of the blood. The sera were transferred to clean tubes and stored at −80˚C until use. The study was approved by the UOEH Ethics Committee in 2013 (H25-008).
2.4. Preparation of Chemical-Albumin Complexes
Chemical-albumin complexes were prepared as described previously with slight modifications [8] . Briefly, TDI, FA, and PA (Wako Pure Chemical Industries) were mixed with HSA (final concentration of HSA: 1 mM) with four ratios of the HSA to chemical concentration (i.e., 1:0, 1:1, 1:10, and 1:100) at pH 6.0, 8.0, and 10.8. Samples were then incubated at 37˚C for 1 h. The reactants were centrifuged, and the supernatants containing the chemical-HSA adducts served as diagnostic antigens.
2.5. Detection of Chemical-Specific IgG
Dot blots were performed as previously reported [9] . The antigens were blotted to nitrocellulose membranes (Amersham, GE Healthcare), which were then blocked with blocking buffer (Nacalai Tesque). Membranes were also blotted with human IgG (Zymed Laboratories) as positive control. Next, the membranes were incubated with serum (1:200 dilution) at room temperature for 1 h and then with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (1:4000 dilution; Millipore) at room temperature for 1 h. Luminescence derived from HRP was detected by ECL detection reagents (Amersham, GE Healthcare) using LuminoGraph (ATTO: WSE-6100H). The signal intensity was quantified with CS Analyzer ver 3.0 (ATTO Densitograph Software Library).
3. Results and Discussion
First, we examined the reactivity of HSA with TDI, PA, and FA. The primary target of TDI, PA, and FA is the lysine residue of HSA because isocyanates and carbonyl compounds have electron poor carbon and are therefore highly reactive with nucleophiles. Therefore, protein lysine residues within native HSA or HSA reacted with chemicals were quantified using TNBS. Under acidic or weakly alkaline conditions, lysine residues of HSA did not react with TDI, PA, or FA because no loss was not detected on lysine residues of HSA (Figure 1). When TDI, PA, and FA were reacted with HSA under basic conditions, lysine residues of HSA were lost in a chemical concentration-dependent manner (Figure 1), indicating that alkaline conditions promoted the reaction of HSA lysine residues with TDI, PA, and FA.
Next, we investigated structural changes in HSA-chemical adducts by native PAGE. Compared with native HSA, PA- and FA-treated HSA proteins showed major changes in electrophoretic mobility (Figure 2). In contrast, TDI only caused slight changes in the electrophoretic mobility of HSA (Figure 2). These results indicated that chemical-HSA adducts underwent structural changes triggered by loss of lysine residues. Under alkaline conditions, most lysine residues are present in the nonionized form. Therefore, diagnostic antigens can be effectively prepared at alkaline pH when using isocyanates and carbonyls as target chemicals.
We have previously reported that the antibody for Product-X, a mixture of epoxy resin compounds, in human serum can be detected by dot blot assay using X-HSA adducts as the antigen. Moreover, we also showed that the levels of Product-X-specific IgG in human serum are strongly correlated with the onset of dermatitis [9] . In this study, we attempted to simultaneously detect several specific antibodies against chemicals, such as TDI, PA, and FA in human serum from four workers handling plastic resin who had a history of allergy and from five unexposed workers. The participants were six men and three women with a median age (95% confidence interval [CI]) of 42 (28 - 58) years. Serum from unexposed workers showed little or no signal on membranes blotted with chemical-HSA adducts (Figure 3). When serum from exposed workers was added to the membrane, the signal was detected at the spot blotted with chemicals that had been reacted with HSA under alkaline condition, with higher signals observed as the molar ratio of chemicals increased (Figure 3). The signal intensities for all chemical substances that had been reacted with HSA at an HSA to chemical concentration of 1:100 at pH 10.8 were significantly higher in sera from exposed workers than in that from unexposed workers (TDI: P = 0.014, PA: P = 0.028, FA: P = 0.007; Figure 4). Therefore, these data supported that chemical-specific IgG in human serum could be detected by dot blot assays and that chemical-HSA adducts generated under alkaline conditions could be used as antigens.
When using this method for epidemiological studies, it will be necessary to consider establishment of the optimal cut-off point and evaluation of signals derived from HSA alone. At least 50 samples from normal unexposed participants should be used to set the cut-off point in order to determine whether a participant has antibodies targeting certain chemicals [6] . HSA showed nonspecific signals in our detection process (data not shown). Thus, subtracting the signal of HSA alone from the conjugate signal before quantification of the data could improve performance [10] .

Figure 1.Decreases in amino groups of HSA by reaction with TDI, PA and FA at alkaline pH.

Figure 2.Detection of structural changes in chemical-HSA adducts.

Figure 3. Results of dot blot assay.
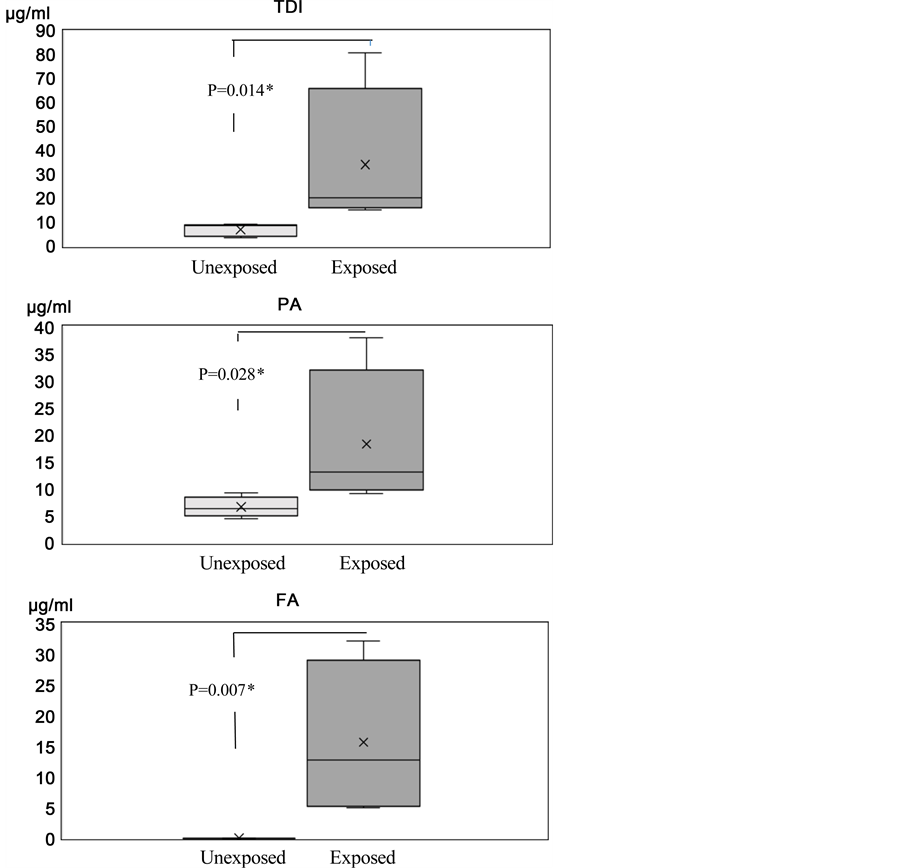
Figure 4. Comparison of chemical-specific IgG data between unexposed and exposed workers.
TDI-specific IgG and PA-specific IgG in serum have been previously detected by ELISA [11] [12] . Both ELISA and dot blotting can be used to measure chemical-specific antibodies in serum. However, in the ELISA system, it is difficult to confirm whether all chemical-HSA adducts bind to the wells of ELISA plates. In addition, although ELISA can be used to process many samples at the same time, this method is time consuming when numerous chemicals are tested. In contrast, dot blotting is advantageous because it can be used for simultaneous detection of multiple antibodies against chemicals. Moreover, because plants handling plastic resins typically use more than one type of chemical substance, exposing workers to various combinations of chemicals, simple methods are needed for analysis of multiple chemical-specific IgGs in serum. Thus, dot blotting is more suitable than ELISA in this regard.
Isocyanate and carbonyl groups can react with protein lysine residues under alkaline conditions, leading to structural changes in HSA. A high signal was detected when workers’ serum was applied to the spot with chemical-HSA adducts, which were generated under alkaline pH and with a high molar ratio. Namely, chemical-modified HSA, exhibiting variations in structure, had high reactivity to workers’ serum. Therefore, chemical-specific IgG was clearly detected by our dot blotting method.
The workers used TDI, PA, and FA in the workplace. However, both exposed and unexposed workers may have come in contact with these chemical substances in daily life, e.g., from smoking or during hobbies, etc. In such cases, the extent and frequency of exposure would be expected to be lower than that in the workplace. However, it is necessary to consider individual exposure levels when diagnosing occupational diseases.
In this study, we succeeded in the detection of chemical-specific IgG in human serum using conjugates of carbonyl and isocyanate compounds with HSA. Isocyanate and carbonyl compounds can react with lysine residues in HSA, leading to structural changes HSA, which can cause antigenicity. Moreover, our method could apply to the detection of chemical-specific antibodies other than TDI, PA, and FA if HSA can react with other chemicals. For example, methyl methacrylate and methyl acrylate, which are raw materials used in the production of acrylic acid resin and have sensitizing potential in humans, react with cysteine residues in proteins [13] . Therefore, our dot blotting method could be expanded for simultaneous detection of additional chemical-specific antibodies in the future.
4. Conclusion
TDI, PA, and FA induced structural changes in HSA by reacting with lysine residues of HSA under alkaline conditions. The dot blot assay developed in this study used chemical-HSA adducts as antigens and could simultaneously detect multiple chemical-spe- cific antibodies in human serum with high sensitivity. Our simple method is expected to contribute to the diagnosis of allergies in workers and the prevention of health hazards by harmful chemicals through determination of various chemical-specific antibodies in human serum.
Acknowledgements
This study was funded by an Industrial Disease Clinical Research Grant (to M.T.), UOEH Research Grant for Promotion of Occupational Health (to M.T.), and the JSPS KAKENHI (grant numbers 22790546 and 25860472 to M.T.).
Cite this paper
Tsuji, M., Yu, H.-S., Ishihara, Y., Isse, T., Ikeda-Ishihara, N., Tuchiya, T. and Kawamoto, T. (2016) A Simple Method for Detection of Multiple Chemical-Specific IgGs in Serum Based on Dot Blotting. Health, 8, 1645-1653. http://dx.doi.org/10.4236/health.2016.815160
References
- 1. Plastics Europe (2011) Plastics—The Facts 2011. An Analysis of European Plastics Production, Demand and Recovery for 2010.
- 2. Lithner, D., Larsson, A. and Dave, G. (2011) Environmental and Health Hazard Ranking and Assessment of Plastic Polymers Based on Chemical Composition. The Science of the Total Environment, 409, 3309-3324.
https://doi.org/10.1016/j.scitotenv.2011.04.038 - 3. Palikhe, N.S., Kim, J.H. and Park, H.S. (2011) Biomarkers Predicting Isocyanate-Induced Asthma. Allergy, Asthma & Immunology Research, 3, 21-26.
https://doi.org/10.4168/aair.2011.3.1.21 - 4. de Groot, A.C., White, I.R., Flyvholm, M.A., Lensen, G. and Coenraads, P.J. (2010) Formaldehyde-Releasers in Cosmetics: Relationship to Formaldehyde Contact Allergy. Part 1. Characterization, Frequency and Relevance of Sensitization, and Frequency of Use in Cosmetics. Contact Dermatitis, 62, 2-17.
https://doi.org/10.1111/j.1600-0536.2009.01615.x - 5. Kadivar, S. and Belsito, D.V. (2015) Occupational Dermatitis in Health Care Workers Evaluated for Suspected Allergic Contact Dermatitis. Dermatitis: Contact, Atopic, Occupational. Drug, 26, 177-183.
- 6. Bernstein, D.I., Ott, M.G., Woolhiser, M., Lummus, Z. and Graham, C. (2006) Evaluation of antibody Binding to Diisocyanate Protein Conjugates in a General Population. Annals of Allergy, Asthma & Immunology: Official Publication of the American College of Allergy, Asthma, & Immunology, 97, 357-364.
https://doi.org/10.1016/S1081-1206(10)60801-0 - 7. Sashidhar, R.B., Capoor, A.K. and Ramana, D. (1994) Quantitation of Epsilon-Amino Group Using Amino Acids as Reference Standards by Trinitrobenzene Sulfonic Acid. A Simple Spectrophotometric Method for the Estimation of Hapten to Carrier Protein Ratio. Journal of Immunological Methods, 167, 121-127.
https://doi.org/10.1016/0022-1759(94)90081-7 - 8. Pham, T.T., Oyama, T., Isse, T. and Kawamoto, T. (2009) Application of Tryptophan Fluorescence to Assess Sensitizing Potentials of Chemicals. Archives of Environmental Contamination and Toxicology, 57, 427-436.
https://doi.org/10.1007/s00244-009-9297-8 - 9. Kawamoto, T., Tsuji, M. and Isse, T. (2015) Comparison of IgG against Plastic Resin in Workers with and without Chemical Dermatitis. BMC Public Health, 15, 930.
https://doi.org/10.1186/s12889-015-2302-4 - 10. Ott, M.G., Jolly, A.T., Burkert, A.L. and Brown, W.E. (2007) Issues in Diisocyanate Antibody Testing. Critical Reviews in Toxicology, 37, 567-585.
https://doi.org/10.1080/10408440701419553 - 11. Ye, Y.M., Kim, C.W., Kim, H.R., Kim, H.M., Suh, C.H., Nahm, D.H., et al. (2006) Biophysical Determinants of Toluene Diisocyanate Antigenicity Associated with Exposure and Asthma. The Journal of Allergy and Clinical Immunology, 118, 885-891.
https://doi.org/10.1016/j.jaci.2006.06.026 - 12. Rosqvist, S., Nielsen, J., Welinder, H., Rylander, L., Lindh, C.H. and Jonsson, B.A. (2003) Exposure-Response Relationships for Hexahydrophthalic and Methylhexahydrophthalic Anhydrides with Total Plasma Protein Adducts as Biomarkers. Scandinavian Journal of Work, Environment & Health, 29, 297-303.
https://doi.org/10.5271/sjweh.734 - 13. Yarbrough, J.W. and Schultz, T.W. (2007) Abiotic Sulfhydryl Reactivity: A Predictor of Aquatic Toxicity for Carbonyl-Containing Alpha, Beta-Unsaturated Compounds. Chemical Research in Toxicology, 20, 558-562.
https://doi.org/10.1021/tx600344a

