Materials Sciences and Applications
Vol.09 No.09(2018), Article ID:86492,9 pages
10.4236/msa.2018.99052
Tribological Behavior of Binary B-C Films Deposited by Sputtering-PBII Hybrid System
Honei Chin1,2, Satoshi Yoshida1, Shuichi Watanabe3*
1Mechanical Systems Engineering Major, Graduate School of Engineering, Nippon Institute of Technology, Saitama, Japan
2USTRON Co. Ltd., Tokyo, Japan
3Department of Applied Chemistry, Faculty of Fundamental Engineering, Nippon Institute of Technology, Saitama, Japan

Copyright © 2018 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: June 29, 2018; Accepted: August 4, 2018; Published: August 7, 2018
ABSTRACT
In this article, the authors report on the use of Radio Frequency (RF) Magnetron Sputtering combined with Plasma-Based Ion Implantation (PBII) technique to synthesize the Boron-Carbon (B-C) films. High purity of boron carbide (99.5%) disk was used as a target with an RF power of 300 W. The mixtures of Argon (Ar)-Methane (CH4) ware used as reactive gas under varying CH4 partial flow pressure at the specified range of 0 - 0.15 Pa and fixed total gas pressure and total gas flow at 0.30 Pa and 30 sccm, respectively. The effect of CH4 flow ratio on the friction coefficient of B-C films was studied. The friction coefficient of the film depended on the concentration of B. When it was 10% or lower, the coefficient decreased to 0.2 or lower. In this concentration range of B, the specific wear rate also decreased to the order of 10−7 mm3/Nm, and excellent wear resistance was displayed.
Keywords:
Binary Boron-Carbon (B-C) Film, Sputtering, Plasma-Based Ion Implantation (PBII), Hybrid System, Bonding Structure, Friction Performance

1. Introduction
In the last decades, boron-carbon (B-C) binary system materials have attracted much attention due to their prospective properties. Among these, B4C is well known to be the crystal composition of the boron carbon (B-C) system; it is a very hard material, second to super-hard materials such as diamond and cubic boron nitride (c-BN). Therefore, boron carbide has excellent mechanical strength, and it is also an attractive material chemically and as a material for use in electronics; thus, synthetic studies on this system have been carried out in the past few decades [1] - [9] . However, there are few studies regarding the stable synthesis of this B-C material for coatings, as far as the authors know [10] - [15] , since the effect of deposition conditions and other conditions on the film structure and various characteristics, in thin film applications, is not well understood. The development of boron carbide films, which are highly adhesive and have stable tribological properties, is anticipated for wider applications including mechanical fields.
The objective of this study is to develop such B-C films with excellent tribological properties. For the deposition of films, a new unique composite system in which sputtering and PBII are combined was used (so-called Hybrid System). In the present system, sputtering deposition and PBII deposition, in which a radio frequency (RF)-superimposed high-voltage pulse bias is applied on the substrate, were carried out simultaneously. When the deposition of B-C films is conducted in a normal chemical vapor deposition process, a B-containing toxic gas compound must be used to add B into the film. In the present process, a relatively harmless B-containing solid (B4C) is used as the target; thus, the test samples can be safely prepared. Then, the mixing effect of argon (Ar) sputtering gas and PBII-treatment methane (CH4) gas was investigated. In particular, this study especially is focused on the tribological properties of films, and the results are described in this report.
2. Experimental
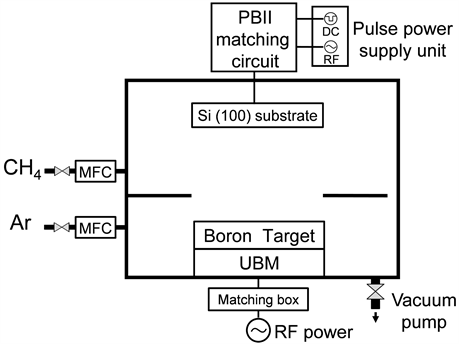
Figure 1 shows a schematic diagram of the sputtering-PBII combined system used for the deposition of B-C films, and the deposition conditions are listed in Table 1. As the sputtering target, B4C (purity: 99.5% or higher) was used, and an unbalanced magnetron was installed at the back. As the substrate, a silicon (Si) (100) wafer was used and this was fixed on a stainless steel substrate holder. The substrate holder was connected to the inlet terminal of the RF-superimposed pulse bias of PBII and installed so that it faces the above-described target. As the pre-deposition treatment, Ar-ion cleaning of the substrate and target was carried out for 10 min. Subsequently, CH4 gas was introduced so that the chamber pressure was 1.0 Pa. The substrate ion implantation treatment was carried out as preprocessing, under the conditions of pulse RF power = 300 W and direct current (DC) pulse bias = −20 kV, with CH4 plasma for 30 min. Thus, an intermediate layer consisting of the mixed layer of C and Si substrate was formed. After this ion-implantation pre-treatment, Ar and CH4 were introduced into the chamber so that the deposition pressure was 0.30 Pa. The deposition was carried out by controlling CH4 gas so that the CH4/Ar gas flow ratio was in the range of 0% - 50% (the CH4 partial pressure: 0 - 0.15 Pa). In the present process, sputtering and PBII were simultaneously carried out; therefore, a stainless steel partition plate was installed so that the gases, from the gas
Figure 1. Schematic diagram of Sputtering-PBII combined system used for this experiment.
Table 1. Deposition conditions.
inlets, were separated in the target side and in the substrate side. Thus, a system in which the proportion of Ar is larger in the target side and the proportion of CH4 is larger in the substrate side was adopted.
The composition of the deposited boron carbide film was investigated using Auger electron spectroscopy (AES) (JAMP-7800F, JEOL Ltd.). For the analysis of the film structure, a Raman spectrometer (MRS-1000DT, JASCO Co.), in which 532 nm green laser light was used as the excitation light, and an infrared (IR) spectrometer (FT/IR-4200ST, JASCO Co.) was used. The hardness of the film was determined with a nano-indentation tester (Hysitron, Inc.). A diamondindenter (Berkovich-type) was used, and the load of indentation was 1000 μN. In addition, the friction properties of B-C films were evaluated with a ball-on-disk friction tester (FPR-2100, Rhesca Co., Ltd.). On this occasion, AISI440C (SUS440C) balls and diamond-like-carbon (DLC)-coated aluminum oxide (Al2O3) balls (denoted as DLC/Al2O3) with a diameter of 6 mm were used as the indenter. Friction conditions were as follows: load = 3 N, friction radius = 3 mm, friction velocity = 31.4 mm/s, sliding distance = 100 m, and the test temperature was room temperature. The value of applied load was selected considering practical use. The relative humidity during the testing was 40% - 50%. The depth profile of wear marks was observed using a 3D-laser microscope (VK-9700, KEYENCE Corp.)
3. Results and Discussion
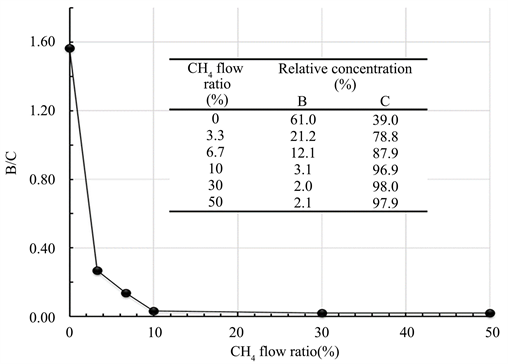
Figure 2 shows the results of AES composition analysis (B/C atomic composition ratio) for the films deposited at the respective CH4 flow ratios. In the figure, the values of the specific composition analysis results of the films are also shown. In addition to B and C, a very small amount of O (2at% or less) was observed in the film. Since the content of O is very small, in the experimental results described below, the influence of O is not discussed. The hydrogen content in the film could not be determined from these analysis results. As is clear from the figure, the amount of B in the film rapidly decreased when CH4 gas was mixed. When the flow ratio is 10% or higher, though not shown in the figure, the rate of deposition increased approximately 2 - 3 times compared with the case when there was no flow of CH4, and it may be assumed that the film deposition behavior changes at the flow ratio of 10%.
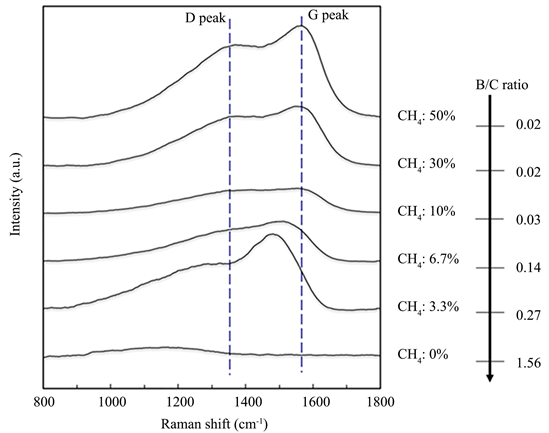
Figure 3 shows the variation in the Raman spectra for the films similarly deposited at the respective CH4 flow ratios. It is known that boron carbide exhibits a peak due to the B12 cluster at 600 - 1200 cm−1 [13] , and a small broad peak could be observed at 1000 - 1200 cm−1 for the film deposited with Ar only (CH4: 0%). On the other hand, the peaks of D band (1350 cm−1) and G band (1550 cm−1), which are specific to DLC film that is well known for its solid lubricity, appeared by introducing CH4 at a flow ratio of 6.7% or higher. Thus, a DLC film was found to become dominant with the increase of carbon due to the introduction of CH4. When the CH4 flow ratio was 3.3%, both the G peak/D peak shifted to lower wavenumber side, and it may be assumed that a different film structure is taken from that of the DLC-based film.
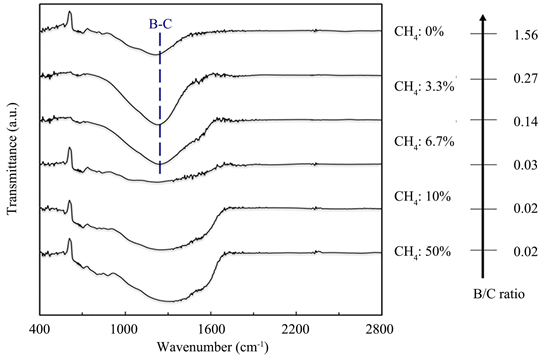
Figure 4 shows the variation in the Fourier transform-IR (FT-IR) spectra for the films similarly deposited at the respective CH4 flow ratios to compare with the results of Raman spectroscopic analysis. The IR absorption peak due to the B-C bond is supposedly in the vicinity of 1200 cm−1. This peak was present in the three kinds of films, which were deposited with a CH4 flow ratio of 6.7% or lower and distinctly contain B. When the CH4 flow ratio was higher than that, the clear-cut absorption peak was not observed.
Figure 5 shows the measurement results of nano-indentation hardness for the deposited films. Even when the CH4 flow ratio was varied, the hardness was within the range of 15 - 20 GPa, and a large variation was not observed. Thus, the variation in the B content of the film does not greatly affect the hardness. However, the hardness of the film deposited with the CH4 flow ratio of 10% displayed a relatively high value around 23 GPa.
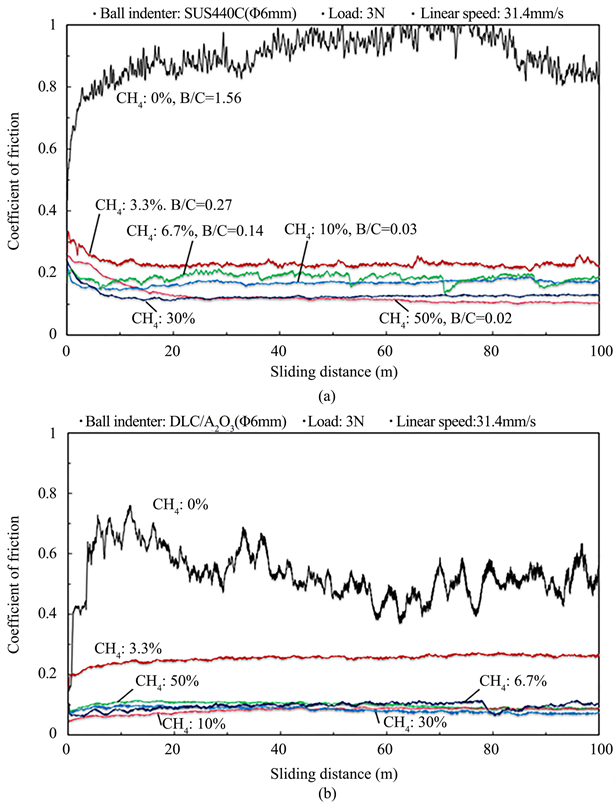
Figure 6 shows the results of the ball-on-diskfriction test for the deposited films. Here, the relationship between the sliding distance and the coefficient of friction is shown. Figure 6(a) shows the results when the indenter was AISI440C (SUS440C) balls, and Figure 6(b) shows the results when the indenter was DLC-coated Al2O3 balls (denoted asDLC/Al2O3). As seen from Figure 6(a), the film deposited with Ar only (CH4 flow ratio: 0%) peeled immediately after the start of friction, and the coefficient of friction was high. It can be seen that the frictional performance of the film having a high B content is not good. On the other hand, the friction coefficient of the film deposited by introducing CH4 was 0.1 - 0.2 and displayed steady low friction. The increase of carbon due to the introduction of CH4 largely contributed to the improvement of friction properties. However, the coefficient of friction exceeded 0.2 in the case of the film with the CH4 flow ratio of 3.3%. A similar trend was observed in Figure 6(b); however, except when the CH4 flow ratio was 3.3%, the coefficient of friction was small and steady because the counter material was DLC in this case. As can be seen from the Raman spectra shown in Figure 3, a DLC structure having excellent lubricity was predominant in the case of the film with a CH4 flow ratio of 6.7% or higher. Thus, it is presumed that this led to a drastic decrease in the friction coefficient.
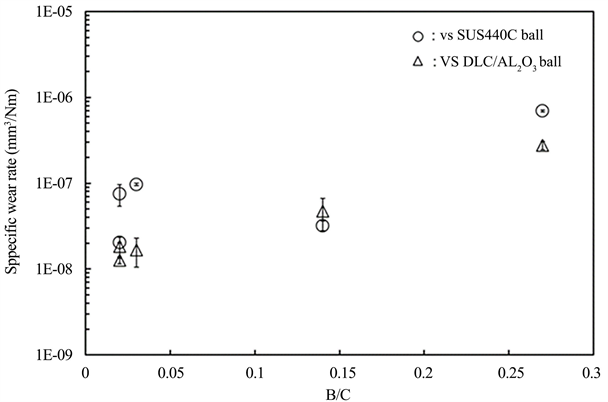
Figure 7 shows the specific wear rate (mm3/Nm) determined from the wear measured from the depth profile of film-side friction marks after the friction test (Figure 6). The B/C value 0.27 is that for when the CH4 flow ratio was 3.3%. In this case, the specific wear rate was on the order of 10−6, which was relatively high. However, the specific wear rate decreased to the order of 10−7 with a decrease in the B/C value (with an increase in the CH4 flow ratio), and excellent wear resistance was displayed. Though it is not shown in the figure, the specific wear rate was on the order of 10−4, and the wear was large in the case of the CH4 flow ratio of 0% (B/C: 1.56), which is consistent with the high friction coefficients (Figure 6) compared with those of other samples. I would like to clarify the influence of B content exerted by tribological properties in more detail in the near future.
Figure 2. Effect of CH4 gas flow ratio on the chemical compositions (B/C ratio) of the films by means of AES analysis.
Figure 3. Variation in Raman spectra and B/C ratio of the films.
Figure 4. Variation in FT-IR transmission spectra and B/C ratio of the films.
Figure 5. Relations between nano-indentation hardness of the film and CH4 gas flow ratio.
Figure 6. Coefficient of friction vs sliding distance of the films deposited various CH4 gas flow ratios. (Ball indenter: (a) SUS440C; (b) DLC/Al2O3).
Figure 7. Changes in specific wear rate values of the B-C films with various B/C ratios obtained after friction test.
4. Conclusions
The effect of deposition conditions on the film structure and tribological properties was evaluated for the B-C films deposited, by varying Ar:CH4 partial pressures, in a combined process of sputtering-PBII. Based on the results, the following conclusions can be made:
1) In the present process, even when the partial pressure of CH4 was low in the mixed gas, a carbon-rich boron carbide film with a low concentration of B could be deposited.
2) When the CH4 partial pressure was 10% or higher, the deposited film dominantly had a DLC structure. When the flow ratio was 6.7% or lower, the concentration of B in the film exceeded 10%, and the film had distinct B-C bonds different from that of the DLC-based film.
3) Even when the concentration of B in the film was varied, the hardness of the film was not largely affected, and the hardness was high ranging 15 - 20 GPa.
4) The friction coefficient of the film depends on the concentration of B. When it was 10% or lower (CH4 flow ratio: 6.7% or higher), the coefficient of friction decreased to 0.2 or lower. In this concentration range of B, the specific wear rate also decreased to the order of 10−7 mm3/Nm, and excellent wear resistance was displayed.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Chin, H., Yoshida, S. and Watanabe, S. (2018) Tribological Behavior of Binary B-C Films Deposited by Sputtering-PBII Hybrid System. Materials Sciences and Applications, 9, 723-731. https://doi.org/10.4236/msa.2018.99052
References
- 1. Ahmad, A.A., Ianno, N.J., Hwang, S.-D. and Dowben, P.A. (1998) Sputter Deposition of High Resistivity Boron Carbide. Thin Solid Films, 335, 174-177. https://doi.org/10.1016/S0040-6090(98)00876-1
- 2. Lousa, A., Martinez, E., Esteve, J. and Pascual, E. (1998) Effects of Ion Bombardment on the Properties of B4C Thin Films Deposited by RF Sputtering. Thin Solid Films, 355/356, 210-213. https://doi.org/10.1016/S0040-6090(99)00506-4
- 3. Lee, K.W. and Harris, S.J. (1998) Boron Carbide Film Grown from Microwave Plasma Chemical Vapor Deposition. Diamond and Related Materials, 7, 1539-1543. https://doi.org/10.1016/S0925-9635(98)00233-7
- 4. Hu, T., Steihl, L., Rafaniello, W., Fawcett, T., Hawn, D.D., Mashall, J.G., Rozeveld, S.J., Putzig, C.L., Blackson, J.H., Cermignani, W. and Robinson, M.G. (1998) Structures and Properties of Disordered Boron Carbide Coatings Generated by Magnetron Sputtering. Thin Solid Films, 332, 80-86. https://doi.org/10.1016/S0040-6090(98)01019-0
- 5. Pascual, E., Martinez, E., Esteve, J. and Lousa, A. (1999) Boron Carbide Thin Films Deposited by Tuned-Substrate RF Magnetron Sputtering. Diamond and Related Materials, 8, 402-405. https://doi.org/10.1016/S0925-9635(98)00274-X
- 6. Li, S., Zeng, B., Feng, Z., Liu, Y. and Zhang, L. (2010) Effects of Heat Treatment on the Microstructure of Amorphous Boron Carbide Coating Deposited on Graphite Substrates by Chemical vapor Deposition. Thin Solid Films, 519, 251-258. https://doi.org/10.1016/j.tsf.2010.08.099
- 7. Bao, R. and Chrisey, D.B. (2010) Chemical States of Carbon in Amorphous Boron Carbide Thin Films Deposited by Radio Frequency Magnetron Sputtering. Thin Solid Films, 519, 164-168.
- 8. Castillo, H.A., Restrepo-Pasrra, E., Velez, J.M. and de la Cruz, W. (2011) Substrate Temperature Influence on Boron Carbide Coatings Grown by the PLD Technique. Surface and Coatings Technology, 205, 3607-3612. https://doi.org/10.1016/j.surfcoat.2010.12.043
- 9. Zhang, L.L., Yang, Q., Tang, Y., Yang, L. and Cui, X. (2015) Synthesis and Characterization of Boron Incorporated Diamond-Like Carbon Thin Films. Thin Solid Films, 589, 457-464. https://doi.org/10.1016/j.tsf.2015.05.067
- 10. Eckardt, T., Bewilogua, K., van der Kolk, G., Hurkmans, T., Trinh, T. and Fleischer, W. (2000) Improving Tribological Properties of Sputtered Boron Carbide Coatings by Process Modifications. Surface and Coatings Technology, 126, 69-75. https://doi.org/10.1016/S0257-8972(00)00525-9
- 11. Cuong, P.D., Ahn, H.-S., Yoon, E.-S. and Shin, K.-H. (2006) Effects of Relative Humidity on Tribological Properties of Boron Carbide Coating against Steel. Surface and Coatings Technology, 201, 4230-4235. https://doi.org/10.1016/j.surfcoat.2006.08.093
- 12. Jagannadham, K., Watkins, T.R., Lance, M.J., Riester, L. and Lemaster, R.L. (2006) Laser Physical Vapor Deposition of Boron Carbide Films to Enhance Cutting Tool Performance. Surface and Coatings Technol, 201, 4230.
- 13. Qian, J.C., Yan, C., Zhang, W.J., Li, D.J., Martinu, L., Klemberg-Sapieha, J.E., Zhou, Z.F., Li, K.Y., Descartes, S., Chromik, R. and Bello, I. (2015) Tailoring the Mechanical and Tribological Properties of Sputtered Boron Carbide Films via the B1-Cx Composition. Surface and Coatings Technology, 267, 2. https://doi.org/10.1016/j.surfcoat.2014.10.003
- 14. Bhowmick, S., Sun, G. and Alpas, A.T. (2016) Low Friction Behavior of Boron Carbide Coatings Sliding against Ti-6Al-4V. Surface and Coatings Technology, 308, 316-327. https://doi.org/10.1016/j.surfcoat.2016.05.092
- 15. Rao, P.N., Gupta, R.K., Saravanan, K., Bose, A. and Rai, S.K. (2018) Investigation of Composition of Boron Carbide Thin Films by Resonant Soft X-Ray Reflectivity. Surface and Coatings Technology, 334, 536-542. https://doi.org/10.1016/j.surfcoat.2017.12.010