Journal of Modern Physics
Vol.10 No.02(2019), Article ID:90457,11 pages
10.4236/jmp.2019.102008
Structure and Photoluminescence Properties of Pr3+ Ion-Doped BaY2ZnO5 Phosphor Prepared Using a Sol-Gel Method
Hung-Rung Shih1, Mu-Tsun Tsai2, Lay-Gaik Teoh3, Yee-Shin Chang4*
1Department of Mechanical and Computer-Aided Engineering, National Formosa University, Taiwan
2Department of Materials Science and Engineering, National Formosa University, Taiwan
3Department of Mechanical Engineering, National Pingtung University of Science and Technology, Taiwan
4Department of Electronic Engineering, National Formosa University, Taiwan

Copyright © 2019 by author(s) and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 7, 2019; Accepted: February 9, 2019; Published: February 12, 2019
ABSTRACT
The Pr3+ ion-doped BaY2ZnO5 phosphor with the orthorhombic structure was synthesized successfully using a sol-gel method in this study. The SEM images show that the BaY2ZnO5:Pr3+ phosphor particles are aggregational but have an isotropic distribution for 2 mol% Pr3+ ions doped. Under an excitation wavelength of 311 nm, the emission bands that appear in the emission spectra are due to the 3P0→3H4,5,6, 1D2→3H4 and 3P0→3F2 electron transition of Pr3+ ion, and it is the same as that for solid state reaction preparation. Comparing to the solid state reaction preparation, the intensities of the 3P0→3H4 transition were increased by about 6.5 times for sol-gel method. The enhancement in emission intensity is because the activators have more homogeneous contribution in host for the sol-gel method preparation. In addition, the color tone did not change very obviously, which located around the green light region for Pr3+ ion concentrations increasing. The color stability is better for sol-gel method than that for the solid state reaction preparation.
Keywords:
Phosphor, Rare Earth Oxides, Optical Properties

1. Introduction
Oxide phosphors have recently gained much attention for applications such as screens infield-emission displays (FEDs) [1] , plasma display panels (PDPs) [2] [3] and for white color light-emitting diodes (LEDs) [4] because the intrinsic problems such as their higher chemical stability and resistance to moisture relative to that of traditional phosphors are rare earth or transition metal activated sulphides such as ZnS, SrS, and CaS. A lot of efforts have been done to discover the novel host materials as well as activators with high photoluminescence properties including emission intensity, high quantum yield and so on for phosphor applications [5] [6] .
The emission of lanthanide ion, Pr3+ ion, in the visible region occurs because of the transition of the 3P0 level, which contains two dominant transitions from the fluorescent 3P0 level to the lower 3H6 and 3H4 states [7] . The emission spectra of Pr3+ are significantly different in different hosts and can take the form of red, green, or even blue emissions [8] [9] .
BaY2ZnO5 has the orthorhombic structure with space of Pbnm [10] , and it consists of YO7, BaO11, and ZnO5 polyhedra. BaY2ZnO5 is an excellent host for various activator ions-doped phosphors. Some previous studies investigated the effect of the size, morphology of particles, and activator concentrations on the photoluminescence properties of BaY2ZnO5:RE3+ phosphors [11] [12] [13] [14] . According to our previous study [14] , the emission spectra show that the 3P0→3H4,5,6 transitions are dominant for the Pr3+-doped BaY2ZnO5 phosphor. The color tone changes from green to greenish and finally yellow, as the Pr3+ ion concentration increases.
Many efforts have been done to enhance the emission intensities of phosphors and the improvement of particle morphologies via fluxes addition, chemical methods utilized to mix activators well or by doping with ions of different radii. Various chemical methods have been investigated for synthesizing fine-sized grains, because these have been reported to enhance the emission efficiency and intensity of phosphors [15] [16] . These methods include sol-gel [17] [18] [19] , hydrothermal [20] [21] [22] , and precipitate techniques [23] . Among these methods, the sol-gel process is an attractive route that starts from molecular precursors and forms an oxide network via inorganic polymerization reactions, and offers both product and processing advantages, such as high purity, ultrahomogeneity, and reduction of the calcining temperature, which could decrease the grain size and enhance the emission efficiency and intensity of phosphors.
In this investigation, the Pr3+ ions-doped BaY2ZnO5 phosphor was prepared using a sol-gel method to reduce the preparation parameters, improve the surface morphology, and hope to increase to the luminescence efficiency of phosphor. The influences of Pr3+ ion concentrations on the resulting structure and the photoluminescence (PL) properties of BaY2ZnO5:Pr3+ phosphor were also studied. The results indicated that the calcination conditions are lower than that of the solid state reaction, and the intensities of emission peak increased by about 6.5 times for the sol-gel method.
2. Experimental Procedure
In this study, BaY2ZnO5 compounds doped with various concentrations of Pr3+ ions were synthesized using a sol-gel method. The raw materials of barium nitride [Ba(NO3)2], yttrium acetate [Y(OOCCH3)3・4H2O], zinc acetate [Zn(OOCCH3)2] and praseodymium acetate [Pr(OOCCH3)3・xH2O] with a purity of 99.99% were supplied by Alfa Aesar. At the first, barium nitride (0.01 mole), zinc acetate (0.01 mole), yttrium acetate (0.02 - 2x mole) and praseodymium acetate (2x mole, x = 0 - 0.005) were separately dissolved in 100 ml of deionized water, respectively. Secondly, barium nitride, zinc acetate, yttrium acetate and praseodymium acetate solutions were mixed in a round bottom flask. When the precursor was completely dissolved in the solution, predetermined amounts of citric acid and ethylene glycol (equal molar ratio) were added to the solution mentioned above as a chelating agent and stabilizing agent, respectively. The amounts of citric acid and ethylene glycol were determined by the ratio of citric acid to metal cations. At last, the powders obtained were calcined in air at 1200˚C for 6 h.
Characterization
Powders were analyzed for crystal structure by X-ray diffractometry (XRD; Rigaku Dmax-33 x-ray diffractometer, Tokyo, Japan) using Cu-Karadiation with a source power of 30 kV and a current of 20 mA to identify the possible phases formed after heat treatment. The surface morphologies of phosphors were examined using high resolution scanning electron microscopy (HR-SEM, S4200, Hitachi). Optical absorption spectra were measured at room temperature using a Hitachi U-3010 UV-vis spectrophotometer. Both the excitation and emission spectra of the phosphors were measured using a Hitachi F-7000 fluorescence spectrophotometer with a 150 W xenon arc lamp as the excitation source at room temperature.
3. Results and Discussion
3.1. Structures
Figure 1 shows the X-ray diffraction patterns of the Ba(Y1-xPrx)2ZnO5(x = 0 - 0.05) powders calcined in air at 1200˚C for 6 h. The XRD results show that all of the diffraction peaks of the BaY2ZnO5:Pr3+ phosphors can be attributed to the orthorhombic structure (JCPDS 89-5856) for Pr3+ ion concentration from 0 to 5 mol%, and there is no second phase appears in the spectra, which demonstrates that the Pr3+ ion substitutes the Y3+ ion. The calcination conditions are lower than that of 1250˚C/12h for conventional ceramics processing reported by the solid state reaction [14] . When the Pr3+ content is further increased, the intensities of the diffraction peaks seem to be decrease, and the diffraction peak of the (131) shift to a lower diffraction angle. The shift of the peak position is ∆θ = 0.68˚ for the Pr3+ ion concentration is 0 and 5 mol%. It is due to the Pr3+ ion (0.99 Å, 6-coordinated) has a larger radius than that of Y3+ ion (0.90 Å, 6-coordinated), the lattice distorts and intra-stress occurs, nonuniform strain in the vicinity of the Pr3+ ions is induced when a trivalent praseodymium ion is introduced to replace trivalent yttrium ion in the BaY2ZnO5:Pr3+ system.
Figure 1. The X-ray diffraction patterns for different concentration of Pr3+ ion-doped BaY2ZnO5 phosphors prepared using sol-gel method.
Table 1 is the grain size calculated using the Scherrer’s equation for different concentrations of Pr3+ ion-doped BaY2ZnO5 phosphor. The grain sizes for BaY2ZnO5:Pr3+ phosphors are all in the nanoscale range, and increase initially then decrease for Pr3+ ion concentration increase further. The largest grain size occurs when Pr3+ ion concentration is 2 mol%. The results are in good accordance with the analysis of XRD patterns for the intensity of (131) diffraction peak is increase then decrease as the Pr3+ ion concentration increases.
3.2. Microstructures
The size of phosphor particles should be as homogeneous as possible without any aggregates or agglomerates. Moreover, the surface of the phosphor particles should also be as smooth as possible and have a high degree of crystallization to improve efficiency. Figure 2 shows the FE-SEM surface morphology of BaY2ZnO5 doped with 0, 1, 2, and 5 mol% Pr3+ ions. By the results, most of these phosphor particles have polyhedral shapes and irregular. These particles are agglomerates of smaller particles during the calcination process due to that they are on the nano-scale with large surface energies when the particles produced using this sol-gel method. When the Pr3+ ion concentration doping is 2 mol% (Figure 2(c)), the particles seem to be more uniform distribution and regular sizes. These phosphor powders with better surface morphologies will display a preferable photoluminescence property, and it will be good accordance with the optical properties measurement.
Figure 3 shows the absorption spectra of the BaY2ZnO5 doped with different Pr3+ ion concentrations phosphor calcined at 1200˚C in air for 6 h. For the host material, it shows two absorption broads in the UV region. The strong absorption band in the region from 200 to 270 nm is attributed to the band-to-band transitions, whereas the weaker broad band from 270 to 400 nm can be attributed to the tightly bound Frankel excitons, which are usually observed close to the bandgap in large-bandgap crystals [24] [25] . The absorption edge of the BaY2ZnO5 host is located at ~378 nm. When Pr3+ ion doped, a stronger broad band in the range of 250 - 350 nm and a series of small peaks between 400 and 500 nm appear in the spectrum, and that are associated with the f-f transition of the Pr3+ ion. The stronger broad band centered at 311 nm can be attributed to the 4f - 5d characteristics transition absorption of Pr3+ ion. According to the studies [26] [27] [28] , the typical Pr3+-activated oxide phosphors always demonstrate strong 4f - 5d transition band absorption at approximately 200 - 330 nm, and it is in accordance with the results for our study. In addition, there also appears a series of absorption peaks from 440 to 500 nm and 580 to 620 nm, this can be attributed to the 4f orbital characteristics transition for the 3H4→3PJ (J = 0, 1, 2) transition of the Pr3+ ion.
Table 1. The grain Size of Ba(Y1-xPrx)2ZnO5(x = 0 - 0.05) phosphor calculated using the Scherrer’s equation.


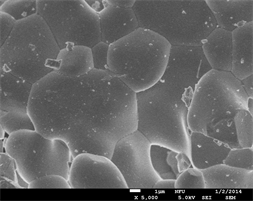
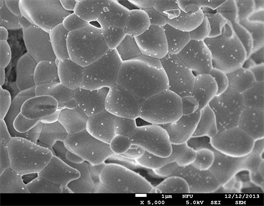
Figure 2. The FE-SEM micrographs of BaY2ZnO5 doped with different Pr3+ ion concentrations: (a) 0, (b) 1, (c) 2 and (d) 5 mol%.
Figure 3. The absorption spectra of BaY2ZnO5 doped with different Pr3+ ion phosphor prepared using sol-gel method.
Figure 4(a) shows the excitation spectra for BaY2ZnO5 doped with various Pr3+ ion concentrations calcined in air at 1200˚C for 6 h using the sol-gel method, the singles were obtained by monitoring the emission wavelength of 513 nm for the 3P0→3H4 transition. A strong broad band from 250 - 350 nm and some sharp peaks in the longer wavelength region can be observed in the excitation spectra. It is similar to the results observed in the absorption spectra, the 4f - 5d transition of Pr3+ ion centered at 311 nm was located in the broad band region. These sharp peaks centered at 458 nm, 485 nm and 495 nm in the longer wavelength region from 450 to 500 nm can be assigned to the f-f transitions of Pr3+ ion for the 3H4→3P2, 3H4→3P1 and 3H4→3P0, transition, respectively [29] [30] . The excitation intensity has a maximum value for Pr3+ ion 2 mol% doped, and then decreasing as the Pr3+ ion concentration increases which is due to concentration quenching effect.
Figure 4(b) is the comparison for excitation spectra of BaY2ZnO5:2mol%Pr3+ phosphor synthesized using the solid state reaction and sol-gel method. As can be seen, different synthesization did not change the shape of excitation curve but did change the intensities of the excitation peak. The excitation intensity of 4f - 5d transition for BaY2ZnO5:2mol%Pr3+ phosphor synthesized by the sol-gel method is 1.5 times higher than that for the solid state reaction. In Addition, The peak of the
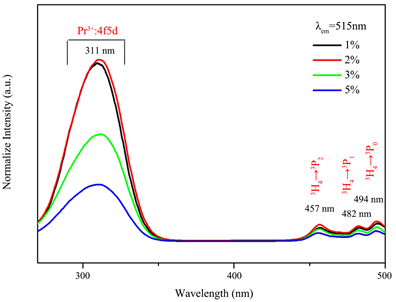
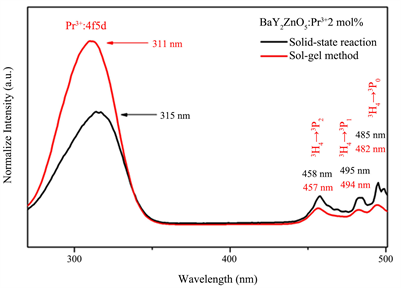
Figure 4. The excitation spectra of BaY2ZnO5 doped with different Pr3+ ion phosphor prepared using soli-gel method, and (b) the comparison of excitation spectra for BaY2ZnO5:2mol%Pr3+ phosphor prepared using sol-gel method and solid state reaction.
Figure 5(a) is the emission spectra under an excitation of 311 nm for BaY2ZnO5 doped with various Pr3+ ion concentrations prepared using the sol-gel method, and Figure 4(b) is the comparison of the emission spectra for BaY2ZnO5 doped with 2 mol% Pr3+ ions prepared by the solid state reaction and sol-gel method. As can be seen in Figure 5(a), there are several light emitting peaks in the visible light ranges for 490 - 520 nm, and 530 - 560 nm, respectively. The emission peaks at 496, 499 and 515 nm are assigned to the 3P0→3H4 transitions, those at 530, 539 and 555 nm are the 3P0→3H5 transitions. It reached a maximum when the Pr3+ concentration was 2 mol%, and decreased with the increasing Pr3+ concentration, which indicated that the concentration quenching is active when x > 0.02. Comparison to the solid state reaction (Figure 5(b)), the intensities of the 3P0→3H4 transition for BaY2ZnO5:2mol%Pr phosphor increased by about 6.5 times for sol-gel method. The enhancement in emission intensity is because the activators have more homogeneous contribution in host for the sol-gel method preparation.
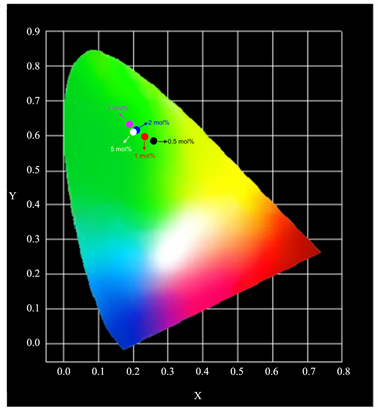
For BaY2ZnO5:Pr3+ phosphor prepared using the sol-gel method, different concentrations of Pr3+ ion-doping has no effect on the wave shape, but did change the emission peaks intensities. The Commission Internationale de I’Eclairage (CIE) color coordinates of the different color tones for Ba(Y1-xPrx)ZnO5 phosphors prepared using the sol-gel method that are excited at 311 nm are shown in Figure 6. As the Pr3+ ion concentration increasing, the color tone did not change very obviously, and which located around the green light region. It possesses a better color stability for sol-gel method synthesization, because the color tone changes from green to greenish and finally to yellow light region as the Pr3+ ion concentrations increasing for solid state reaction preparation.

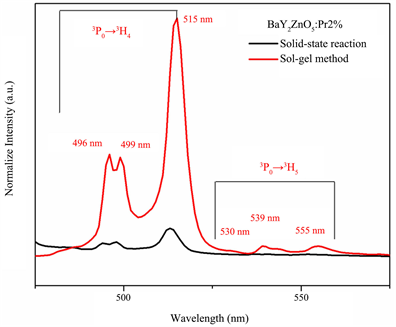
Figure 5. The emission spectra of BaY2ZnO5 doped with different Pr3+ ion phosphor prepared using soli-gel method, and (b) the comparison of excitation spectra for BaY2ZnO5:2mol%Pr3+ phosphor prepared using sol-gel method and solid state reaction.
Figure 6. CIE chromaticity diagram for BaY2ZnO5 doped with various Pr3+ ion concentration.
4. Conclusion
In this study, the orthorhombic structure for Pr3+ ion-doped BaY2ZnO5 was synthesized successfully using a sol-gel method, and the calcination conditions are lower than that for the conventional ceramics processing. The SEM images show that the BaY2ZnO5:Pr3+ phosphor particles are aggregational but have an isotropic distribution for 2 mol% Pr3+ ions doped. Under an excitation wavelength of 311 nm, the emission bands that appear in the emission spectra are due to the 3P0→3H4,5,6, 1D2→3H4 and 3P0→3F2 electron transition of Pr3+ ion, and it is the same as that for solid state reaction preparation. The optimum condition for Pr3+ ion concentration doped is 2 mol%, and the intensities of the 3P0→3H4 transition are increased by about 6.5 times for sol-gel method. The enhancement in emission intensity is because the activators have more homogeneous contribution in host for the sol-gel method preparation. In addition, the color tone did not change very obviously, which located around the green light region for Pr3+ion concentrations increasing. The color stability is better for sol-gel method than that for solid state reaction preparation.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology of the Republic of China for financially supporting this project under grant MOST 107-2622-E-150-005-CC3.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.
Cite this paper
Shih, H.-R., Tsai, M.-T., Teoh, L.-G. and Chang, Y.-S. (2019) Structure and Photoluminescence Properties of Pr3+ Ion-Doped BaY2ZnO5 Phosphor Prepared Using a Sol-Gel Method. Journal of Modern Physics, 10, 91-101. https://doi.org/10.4236/jmp.2019.102008
References
- 1. Hsu, W.T., Wu, W.H. and Lu, C.H. (2003) Materials Science and Engineering: B, 104, 40-44. https://doi.org/10.1016/S0921-5107(03)00268-X
- 2. Mauch, R.H. (1996) Applied Surface Science, 92, 589-597. https://doi.org/10.1016/0169-4332(95)00301-0
- 3. Yi, L., Hou, Y., Zhao, H., He, D., Xu, Z., Wang, Y. and Xu, X. (2000) Displays, 21, 147-149. https://doi.org/10.1016/S0141-9382(00)00046-9
- 4. Zhao, X., Wang, X., Chen, B., Meng, Q., Di, W., Ren, G. and Yang, Y. (2007) Journal of Alloys and Compounds, 433, 352-355. https://doi.org/10.1016/j.jallcom.2006.06.096
- 5. Wakefield, G., Keron, H.A., Dobson, P.J. and Hutchison, J.L. (1999) Journal of Colloid and Interface Science, 215, 179-182. https://doi.org/10.1006/jcis.1999.6225
- 6. Jung, H.K., Park, D.S. and Park, Y.C. (1999) Materials Research Bulletin, 34, 43-51. https://doi.org/10.1016/S0025-5408(98)00216-5
- 7. Pedrini, C., Bouttet, D., Dujardin, C., Moine, B., Dafinei, I., Lecoq, P., Koselja, M. and Blazek, K. (1994) Optical Materials, 3, 81-88. https://doi.org/10.1016/0925-3467(94)90010-8
- 8. Piper, W., DeLuca, J. and Ham, F. (1974) Journal of Luminescence, 8, 344-348. https://doi.org/10.1016/0022-2313(74)90007-6
- 9. Sommerdijk, J., Bril, A. and De Jager, A. (1974) Journal of Luminescence, 8, 341-343. https://doi.org/10.1016/0022-2313(74)90006-4
- 10. Kaduk, J.A., Wing, N.W., Greenwood, W., Dillingham, J. and Toby, B.H. (1999) Journal of Research of the National Institute of Standards and Technology, 104, 147-171. https://doi.org/10.6028/jres.104.011
- 11. Liang, C.H., Chang, Y.C. and Chang, Y.S. (2008) Applied Physics Letters, 93, Article ID: 211902. https://doi.org/10.1063/1.2998299
- 12. Liang, C.H., Teoh, L.G., Liu, K.T. and Chang, Y.S. (2012) Journal of Alloys and Compounds, 517, 9-13. https://doi.org/10.1016/j.jallcom.2011.11.088
- 13. Shih, H.R. and Chang, Y.S. (2017) Journal of Electronic Materials, 46, 6603-6608. https://doi.org/10.1007/s11664-017-5717-0
- 14. Shih, H.R., Tsai, Y.Y., Liu, K.T., Liao, Y.Z. and Chang, Y.S. (2013) Optical Materials, 35, 2654-2657. https://doi.org/10.1016/j.optmat.2013.08.007
- 15. Shih, H.R., Tsai, M.T., Chen, H.L., Xiang, Y.X. and Chang, Y.S. (2014) Materials Research Bulletin, 55, 33-37.
- 16. Williams, D.K., Bihari, B., Tissue, B.M. and McHale, J.M. (1998) The Journal of Physical Chemistry B, 102, 916-920.
- 17. Zhang, J., Zhang, Z., Tang, Z., Lin, Y. and Zheng, Z. (2002) Journal of Materials Processing Technology, 121, 265-268.
- 18. Li, Y., Duan, X., Liao, H. and Qian, Y. (1998) Chemistry of Materials, 10, 17-18.
- 19. Hirano, M. (2000) Journal of Materials Chemistry, 10, 469-472.
- 20. Hirano, M., Imai, M. and Inagaki, M. (2000) Journal of the American Ceramic Society, 83, 977-979. https://doi.org/10.1111/j.1151-2916.2000.tb01310.x
- 21. Tas, A.C., Majewski, P.J. and Aldinger, F. (2002) Journal of Materials Research, 17, 1425-1433.
- 22. Nedelec, J.M., Mansuy, C. and Mahiou, R. (2003) Journal of Molecular Structure, 651-653, 165-170.
- 23. Li, J. and Kuwabara, M. (2003) Science and Technology of Advanced Materials, 4, 143-148.
- 24. Hashizume, K., Matsubayashi, M., Vachal, M. and Tani, T. (2002) Journal of Luminescence, 98, 49. https://doi.org/10.1016/S0022-2313(02)00251-X
- 25. Liang, C.H., Qi, X.D. and Chang, Y.S. (2010) Journal of the Electrochemical Society, 157, 1169.
- 26. Hoefdraad, H.E. and Blasse, G. (1975) Physica Status Solidi (A), 29, K95-K97.
- 27. Donega, C.D.M., Meijerink, A. and Blasse, G. (1995) Journal of Physics and Chemistry of Solids, 56, 673-685.
- 28. Dorenbos, P. (2000) Journal of Luminescence, 91, 91. https://doi.org/10.1016/S0022-2313(00)00197-6
- 29. Lin, Y.F., Chang, Y.H., Chang, Y.S., Tsai, B.S. and Li, Y.C. (2006) Journal of the Electrochemical Society, 153, G543.
- 30. Raju, G.S.R., Park, J.Y., Jung, H.C., Balarkrishnaiah, R., Moon, B.K. and Jeong, J.H. (2011) Current Applied Physics, 11, S292-S295.
- 31. Huang, S.C., Wu, J.K., Hsu, W.J., Chang, H.H., Hung, H.Y., Lin, C.L., Su, H.Y., Bagkar, N., Ke, W.C., Kuo, H.T. and Liu, R.S. (2009) International Journal of Applied Ceramic Technology, 6, 465.