Advances in Bioscience and Biotechnology
Vol.5 No.7(2014), Article
ID:46570,3
pages
DOI:10.4236/abb.2014.57068
Basidiocarp Production of Stropharia melanosperma (Bull.) Gillet in Nutrient Agar Media
Anatoly Trostanetsky
Institute for Postharvest and Food Sciences, Agricultural Research Organization, The Volcani Center, Bet Dagan, Israel
Email: anatoly@volcani.agri.gov.il
Copyright © 2014 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 15 April 2014; revised 16 May 2014; accepted 26 May 2014
ABSTRACT
The present work studies the fruiting body production of S. melanosperma (Bull.) Gillet using pairing test in nutrient agar media. Fourteen monokaryotic strains from single fruit body were chosen for the pairing test. Ninety-one crossings were made and fruit bodies were found in 20 crossings. Fully mature basidiocarps were produced in a ring-like fashion in one or both colonies. The patterns of the fruit body production in crossing single spore isolates are similar to those common in heterothallic tetrapolar species. The conducted work shows that S. melanosperma can serve as a model organism for the study of developmental processes in the basidiomycetes.
Keywords:Fruiting Body Formation, Mushroom, Heterothallic, Tetrapolar

1. Introduction
Worldwide production of edible mushrooms amounts to above 7 million tons annually [1] . Fruiting bodies contain substances of various kinds that are highly valued as medicines, flavorings and perfumes. A major problem in mushroom industry is the poor understanding of biological processes which lead to the initiation of fruiting body development. A thorough knowledge of these processes is important for the understanding of many ecological processes. Achieving such knowledge is possible by studying fruiting in controlled conditions in axenic culture on nutrient agar media [2] . However, getting most of the mushrooms to fructification in such conditions is a difficult task and only few species of fungi form basidiocarp in axenic culture [2] -[6] . One of such species is Stropharia melanosperma (Bull.) Gillet. The influence of the nutrient agar medium and light on the fructification was demonstrated [7] . The present work studies the fruiting body production of S. melanosperma using pairing test in nutrient agar media.
2. Materials and Methods
The culture of S. melanosperma was isolated from basidiocarps collected at the Kiryat Gat Grain Storage Bins, Israel. The mushroom was identified by Prof. S. Wasser from Haifa University. S. melanosperma was cultivated on mushroom blocks in plastic boxes in which high humidity was maintained and inflow of fresh air was provided [8] . The spore print from a single fruit body was used for obtaining a spore suspension. Spores were washed by a 0.02% Tween-20 solution and 0.5 ml of the spore suspension was spread on potato dextrose agar (PDA) plates. Petri dishes were incubated at 20˚C. In 2 - 4 days of incubation, well-separated germinating spores were picked up cautiously and transferred to fresh PDA plates for isolate growth. After 12 days of growing, fourteen single spore isolates were chosen for a pairing test.
3. Results
None of the isolates showed fruiting during the experiments. Cutting pieces of agar were placed in pair, 2 - 3 cm apart from each other in Petri dishes with PDA in all possible combinations. After 7 - 14 days of growing, the mycelia touched each other. Primordia usually emerged in 10 - 20 days of inoculation. 91 crossings were made and fruit bodies were found in 20 crossings (Table 1). Fully mature basidiocarps were produced in a ring-like fashion in one or both colonies (Figure 1).
4. Discussion
Mating is an essential step in the reproduction of fungi. Most of the basidiomycetes are heterothallic, i.e. two
Table 1. Pairing test between single spore isolates from S. melanosperma.
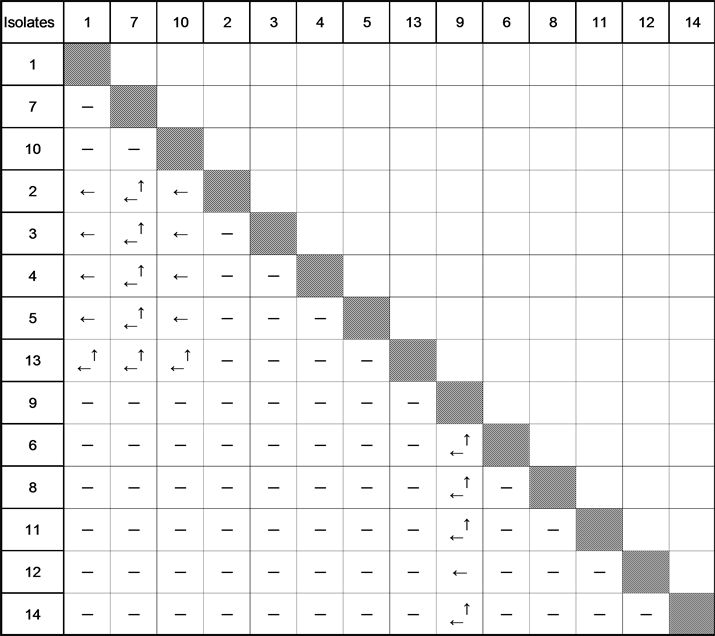
―: Absence of fruit bodies; ← and ↑: Fruit bodies form on one of the cultures crossed, the arrow indicates the number of the culture on the mycelium of which fruit bodies form; ←↑: Both cultures produce fruit bodies.
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. Fruit body formation in crossing S. melanosperma single spore isolates. (a) Absence of fruit bodies on the two cultures (cultures 2 and 6); (b) Fruiting on single culture (cultures 1 and 3); (c) Fruiting on both cultures (cultures 9 and 14).
haploid mycelia of different mating type specificity have to fuse in order to give rise to dikaryotic mycelium. The mating types in heterothallic species are determined by either one (bipolar) or two (tetrapolar) chromosomal mating type loci which each have at least two alleles. Two mating type loci of tetrapolar higher basidiomycetes are signed as A and B. Fruit body of tetrapolar species produce four different mating-type specificity basidiospores (A1B1, A1B2, A2B1 and A2B2). In favorable conditions basidiospores germinate by forming haploid mycelium. A compatible mating and dikaryotic mycelium formation take place when mates have different alleles of genes at both mating type loci, i.e. A1B1 × A2B2 or A1B2 × A2B1. Fruiting bodies usually develop on the dikaryotic mycelium [2] [5] [9] [10] . Our experiment shown, that the patterns of the fruit body production in crossing single spore isolates of S. melanosperma are similar to those common in heterothallic tetrapolar species. Although some model higher basidiomycetes produce fruit body on sterile agar media, such clear patterns of fructification were not seen. This has to do with the difficulties of getting to fructification in axenic culture and that monokaryotic strains also can produce fruit bodies [2] [5] [10] [11] .
5. Conclusion
The conducted work shows that S. melanosperma may serve as a model organism for the study of developmental processes in the basidiomycetes.
References
- Food and Agriculture Organization of the United Nations (2014) FAO Statistical Databases, FAOSTAT. http://faostat.fao.org/
- Kües U. and Liu, Y. (2000) Fruiting Body Production in Basidiomycetes. Applied Microbiology and Biotechnology, 54, 141-152. http://dx.doi.org/10.1007/s002530000396
- Natarajan, K., Narayanan, K. and Kumaresan, V. (2002) Basidiocarp Production in Agaricus heterocystis Heinem and Gooss in Nutrient Agar Media. Current Science, 82, 1084-1085.
- Srivilai, P. and Loutchanwoot, P. (2009) Coprinopsis cinerea as a Model Fungus to Evaluate Genes Underlying Sexual Development in Basidiomycetes. Pakistan Journal of Biological Sciences, 12, 821-835. http://dx.doi.org/10.3923/pjbs.2009.821.835
- Sykhomlyn M.M. (2001) Sexual Breading of the Higher Basidiomycetes, (in Ukrainian). DonNU, Donezk, 173 p.
- Stamets P. and Chilton, J.S. (1983) The Mushroom Cultivator. A Practical Guide to Growing Mushrooms at Home. Agaricon Press, Olympia, Washington DC, 415 p.
- Trostanetsky A. (2003) Fruit-Body Production in Stropharia melanosperma (Bull.: Fr.) Gillet in Nutrient Agar Media. Proceedings of the 14th Congress of European Mycologists, Crimea, 22-27 September 2003, 111.
- Trostanetsky A. (2005) Minicultivation of Medical Mushrooms. International Journal of Medicinal Mushrooms, 7, 477.
- Casselton, L.A. and Olesnicky N.S. (1998) Molecular Genetics of Mating Recognition in Basidiomycete Fungi. Microbiology and Molecular Biology Reviews, 62, 55-70.
- Kües, U. James, T.Y. and Heitman, J. (2011) Mating Type in Basidiomycetes: Unipolar, Bipolar and Tetrapolar Patterns of Sexuality. In: Esser, K., Ed., The Mycota: Evolution of Fungi and Fungal-Like Organisms, Springer-Verlag, Berlin, Heilderberg, 97-160.
- Labarere J. and Noël, T. (1992) Mating Type Switching in the Tetrapolar Basidiomycete Agrocybe aegerita. Genetics, 131, 307-319.

