Advances in Bioscience and Biotechnology
Vol.5 No.5(2014), Article ID:44476,9 pages DOI:10.4236/abb.2014.55049
Evaluation of Quality of Life Related to I-131 Therapy in Patients with Well-Differentiated Thyroid Cancer and Emphasis in Salivary Morbidity: A Follow up Study after Treatment
Lucélia Garcia Corrêa1, Sônia Marta Moriguchi2, Érica Boldrini3, André Lopes de Carvalho4, José Humberto Tavares Guerreiro Fregnani4, Euclides Timóteo da Rocha1*
1Department of Nuclear Medicine, Barretos Cancer Hospital, Barretos, Brazil
2Department of Nuclear Medicine, Medical School, Sao Paulo State University (UNESP), Botucatu, Brazil
3Department of Pediatrics, Barretos Cancer Hospital, Barretos, Brazil
4Department of Oncological Surgery, Barretos Cancer Hospital, Barretos, Brazil
Email: *euclidestimoteo@uol.com.br
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 17 January 2014; revised 28 February 2014; accepted 18 March 2014
ABSTRACT
Goal: To evaluate the impact of iodine-131 therapy received during childhood and adolescence and correlate it with the quality of life in these patients. Methods: We studied 19 patients diagnosed with cancer in childhood or adolescence who underwent thyroidectomy and supplemental therapy with I-131. We also recruited a control group of healthy subjects with the same demographic parameters. All patients were subjected to a scintigraphy examination of the salivary glands, and were also asked to complete a questionnaire in order to assess their overall quality of life. In addition, a more specific questionnaire for patients with head and neck cancer was also given to all study participants. Results: The quantitative and qualitative analyses of the salivary glands showed functional deficits with greater involvement of the parotid gland for volume, concentration and excretion. The right submandibular gland showed significant changes for volume in the patient group. The questionnaires made it possible to observe significant differences between the patient and control groups for symptoms such as thick saliva, dry mouth and speech problems. Conclusion: In spite of being very effective and widely used, iodine radionuclide therapy is correlated with a lower quality of life in young people.
Keywords:Scintigraphy of the Salivary Glands; Thyroid Cancer; Iodine Radionuclide

1. Introduction
In general, differentiated thyroid carcinoma (DTC) is a condition less frequent in children or adolescents than in adults, ranging from 0.3% to 3% of malignant neoplasia [1] . In 90% of all cases there is involvement of cervical lymph nodes at diagnosis, which by itself is not the worst prognosis [1] -[5] . In spite of cervical lymph node involvement and frequent detection of metastases at diagnosis, children with DTC have a better prognosis than adults. There are reports of patients reaching 90% of their life expectancy after 15 to 20 years of follow up [6] .
The initial treatment for DTC is surgical, with total thyroidectomy, with or without dissection of the associated lymph node. Complementary therapy with radioactive iodine is an almost universal approach because it is efficient, accessible and well tolerated [2] [3] .
Salivary glands concentrate iodine by substituting iodine as a substrate for the Na+/K+/Cl− co-transport system. This ability to concentrate iodine and radioactive iodine makes the salivary glands potential targets for the diagnostic or therapeutic use of these substances. However, as a consequence, sialadenitis, taste disturbances, xerostomia and an increase in the number of dental caries are short and long-term side effects of radioiodine therapy (RIT) [7] -[10] . As important as the cancer treatment itself is the evaluation of the lasting effects of this treatment and the implications for social aspects of the disease.
The quality of life assessment has been used in health programs as an indicator of the effectiveness and impact of some treatments. This measurement has enhanced the diagnostic evaluation of the nature and severity of diseases, and is used for prognostics, therapeutic efficacy and etiological factors [11] [12] .
Instruments that measure quality of life have been developed and assessed, since they allow for the identification of problems in areas such as emotional state, general physical state and social interaction, as well as the design of appropriate intervention programs, enabling the modification of variables that could negatively impact the multidisciplinary follow-up of cancer patients [13] [14] .
Finally, due to the paucity of studies on the effects of radioactive iodine therapy in children and adolescents on quality of life, further studies are needed. Thus, the goal of this study was to investigate these potential effects on those patients in terms of quality of life with emphasis in salivary morbidity.
2. Materials and Methods
2.1. Data from the Patient Group
This was a case-control study which examined the medical records of 60 patients who had a diagnosis of DTC during childhood or adolescence, up to 18 years of age. This study included patients with a histopathologic diagnosis of DTC who had undergone total thyroidectomy with supplementary iodine radionuclide therapy from 1998 to 2008. Of the 60 subjects identified on medical records, 19 were recruited with a mean age at diagnosis of 13.5 years (SD ± 3.2), ranging from 7.6 to 18 years. During recruitment the mean age was 19.4 years (SD ± 3.92), ranging from 15 to 28 years. The group was comprised of 3 males and 16 females who accepted an invitation to participate. The remaining subjects were not found or refused the invitation. All patients answered two questionnaires to assess quality of life―one to collect general information (EORTC QLQ-C30) and the other specific to the head and neck (EORTC QLQ-H&N35). Moreover, scintigraphies of the salivary glands were also performed in order to detect abnormalities resulting from iodine therapy. This study was approved by our institutional research ethics committee and all patients or their parents gave their informed consent.
2.2. Data from the Control Group
As basis for a comparative analysis, healthy individuals were actively recruited to take part in the study. We excluded those who underwent ionizing radiation procedures in the head and neck or those who had a history of infectious disease in the oral cavity, including the salivary glands. We recruited 19 subjects with similar demographic characteristics to the patient group, including age and gender. The group had a mean age of 19.2 (SD ± 3.6), ranging from 15 to 27 years. They were all subjected to a scintigraphy examination of the salivary glands and also responded to the quality of life questionnaires. Before treatment for thyroid cancer none of them had problems with speech or eating.
2.3. Protocol for Salivary Gland Scintigraphy
After venous puncture in a superficial vein of the arm, dynamic salivary gland scintigraphy was obtained immediately after intravenous administration of 370 MBq of 99 mTc-pertechnetate. Blood flow was measured and uptake and excretion were verified. The acquisition of images was performed in a dual head gamma camera equipped with low-energy and high resolution collimators (Millennium VG, General Electric Medical System, USA). A standard protocol was used with image acquisition every 3 seconds in the first minute, and every 30 seconds for the next 29 minutes, with a matrix of 128 × 128 pixels and magnification of 1.5 times. Stimulation with lemon juice was carried out 15 minutes into the study to stimulate salivary secretion.
At the workstation computer, regions of interest were drawn by hand on the salivary glands (parotid and submandibular salivary glands). As a measure of salivary gland function, the uptake of 99 mTc-pertechnetate was calculated as a percent of the activity injected. In order to better understand salivary gland function and compare this function between groups, the ejection flow was also calculated. The ejection flow is the excretion fraction in relation to a determined time and was expressed as the elimination percentage per minute (%/min).
2.4. Quality of Life Questionnaire Assessment
Patients and the control group completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) and a specific questionnaire for head and neck cancer (EORTC QLQ-H&N35).
The scores, which range from 0 to 100 points, were calculated according to the EORTC QLQ-C30 manual. A high score on the functional scale indicates good quality of life, while a high score on the symptoms scale indicates mild disease with symptoms and problems. The specific questionnaire (H&N35) consists of issues relevant to the problems reported in the thyroid tumor and its associated treatment [15] [16] .
The EORTC questionnaire is considered an integrated health assessment system related to the quality of life of cancer patients, being validated already in Brazil [16] . The QLQ-H&N35 is widely used in assessing the quality of life in head and neck cancer patients, and is a specific module that was applied in conjunction with the QLQ-C30 questionnaire to assess the overall quality of life [11] .
2.5. Statistical Analyses
The Fisher’s test was used for statistical analysis of categorical variables and the Mann-Whitney test was used for continuous variables, since the distribution of some variables was not normal. For comparisons, a p < 0.05 was considered statistically significant. Data were tabulated and analyzed using SPSS software for Windows® version 17.
3. Results
3.1. Demographics
Out of the 19 patients selected, 16 were female (84%) and 3 were male (15.8%); in relation to diagnosis, 84% were due to papillary and 15.8% to follicular carcinoma. At the time of the study, 68.4% (13/19) were alive without disease, and 31.6% (6/19) were alive with disease or being monitored.
3.2. Scintigraphy of the Salivary Glands―Qualitative Analysis
3.2.1. Volume
The findings regarding volume changes were more evident on the right parotid gland in 52.4% of patients; however, the left parotid gland displayed alterations in 71.4% of patients. A slight change was observed in 40% of the control group. Changes were observed in the right submandibular gland in 28.6% of patients (p = 0.03), whereas in the control group there were no abnormal findings. On the left submandibular gland, abnormalities were observed in 33.3% of patients (p = 0.052), however, none of the alterations were categorized as severe deficits or absences. Mild abnormalities were seen in 5% of the control group (Table 1).
3.2.2. Concentration
Table 2 shows that the parotid glands displayed a decreased concentration of radiotracer―regardless of the degree—in 52.4% of patients in the right parotid gland (p = 0.004) and 66.6% of patients in the left parotid gland (p = 0.001).
3.2.3. Excretion
With regard to excretion, Table 3 shows that excretions from the right parotid gland decreased by 52.4% in patients and by 15% in the control group. A higher proportion of affected cases, 67%, was seen in the left parotid gland.
3.3. Scintigraphy of the Salivary Glands―Quantitative Analysis
Quantitative analysis showed that the parotid glands displayed significant differences between groups upon measuring the excretion fraction (EF%) with reduced salivary excretion, considered at p < 0.05. The analysis showed no difference for half-life (T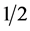 ) nor for salivary excretion expressed as a percent age per minute (EF%/min). For the submandibular gland, the analysis showed no significant difference between groups (Table 4).
) nor for salivary excretion expressed as a percent age per minute (EF%/min). For the submandibular gland, the analysis showed no significant difference between groups (Table 4).
3.4. Quality of Life Assessment
Table 5 shows that the groups displayed significant differences in the results, including global health field (p =
Table 1. Qualitative Analysis―saliva volume assessment (%).
Table 2. Qualitative analysis―saliva concentration assessment (%).
Table 3. Qualitative analysis―saliva excretion assessment (%).
Table 4. Quantitative analysis of the largest glands in the case and control group.
SD = Standard Deviation; *significant value < 0.05.
0.003), functional domain (p = 0.016), dry mouth (p = 0.008), thick saliva (p = 0.043) and speech problems (p = 0.003), assuming a value of p <0.05.
4. Discussion
We enrolled 19 young adults who underwent total thyroidectomy for DTC and were referred for additional iodine radionuclide therapy in childhood and adolescence. There was a predominance of females, 16/19 subjects, equivalent to 84% of the group. Moreover, the distribution of histological types showed that 84% of individuals were affected by papillary carcinoma. Finally, all are alive, and the majority (68%), without active disease.
It is undeniable that the introduction of iodine-131 therapy for DTC treatment changed the natural history of this disease, bringing increased survival. This data is valid both for adults as well as children and adolescents [2] [10] . However, despite the therapeutic effectiveness, a decrease in the quality of life of a portion of this population has been noticed. This has had an impact on patients’ lives, especially because it deals with people re-integrated into society, as survivors, with the challenge of having to re-adapt to their daily routine.
Table 5. Quality of life comparative analysis―overall health assessment and head and neck assessment.
SD = Standard Deviation; *significant value < 0.05.
The scintigraphic assessment of salivary gland function is a widely known and documented technique, and is non-invasive, informative and easy to carry out. The scintigraphic assessment based on qualitative parameters showed a clear functional impairment of the salivary glands. The parotid gland showed a significant reduction in volume, with a proportionately greater involvement of the left parotid gland in 71.4% of the subjects. The submandibular glands were also compromised, but comparatively less. The parotid gland also showed a greater reduction in concentration and excretion, affecting particularly the right gland.
The aggressive treatment of a relatively nonlhetal cancer requires a considerable explication of the very serious issues of the overtreatment and potential morbidities. Almost inevitably, the salivary glands and oral mucosa are affected. So, sialoadenites and xerostomia have been considered as transient side effects of radioiodine therapy, but long term xerostomia have been described [17] . These findings are interesting, and it happens because of the higher concentration of iodine in the parotid. Rosario et al. (2005) assessed a group of young people regarding the safety of therapy with I-131. The authors found that the presence of xerostomia was related to radioiodine. The data was supported by assessment of the salivary gland [18] using scintigraphy. We found that out of 19 subjects treated with iodine-131, about 50% presented some type of abnormalities. The parotid glands dysfunction developed more frequently and more severely than submandibular glands after radioiodine ablation. This can be explained by the deleterious effects of I-131 that are concentrated in impairment of the salivary glands, particularly the parotid glands, impairing saliva production and affecting swallowing function [19] [20] .
A recent study evaluated the long-term side effects caused by radioiodine therapy. Age was considered a relevant factor, and patients older than 45 years showed less non-stimulated and stimulated salivary flow. Patients undergoing radioiodine therapy had difficulty in excreting saliva, mainly in the parotid glands [21] . Our study replicated the findings mentioned above, for the juvenile population, in which the main effect of iodine-131 on the salivary glands correlated with a difficulty to excrete saliva produced in normal amounts by ductal constriction induced by radiation [8] .
Furthermore, it should be stressed that these changes impact quality of life, such as complaints related to swallowing, distorted taste, and tooth decay. Moreover, one must take into consideration that iodine therapy in the juvenile population does not completely destroy the ability of the salivary glands to produce saliva, as the stimulus-response is still possible [21] [22] . Following the same line of reasoning, the measurements obtained by quantitative analysis showed, for the parotid glands, a significant difference between groups in excretion fraction (EF%), with reduced salivary excretion. There was no significant difference in relation to the submandibular glands. The data are of interest, because this assessment, carried out in an objective way, brings strength to the involvement of the parotid glands, found in visual analysis.
Upon the assessment of the head and neck domains, the patient group displayed xerostomia related to iodine therapy. As it happened to adult patients, the growing advances in the treatment of children and adolescents with chronic diseases brought up the concern regarding the quality of life of such population [23] ; these long-term effects of the iodine therapy have been reported in adults [24] , representing an impact on quality of life, which has also been detected by this study in the juvenile population. The resulting oral sequelae may cause substantial problems during and after radiation therapy and are major factors in determing the patient’s quality of life.
Quality of life is a distinct and important emerging healt focus, guiding practice and research. The routine of life evaluation in clinical, economic and epidemiological studies promises a better quality of life and improved health optimization particularly in oncological patients. Thus, Table 5 shows meaningful data related to global health perception, speech problems, dry mouth and thick saliva. Furthermore, even if not significant, the notion regarding the existence of eating disorders and weight loss points toward a significant detriment in the quality of life. Therefore, among the many healthy problems encountered after radiation therapy there are a few that may interfere directly with the quality of life as showed in Table 5. Alterations of taste sensation occurs as a result of the direct effect of radiation on the taste buds and due to change in the saliva [21] [25] . In most instances, tastes returns gradually to normal or near normal levels in the first year after therapy. Since taste loss can result in weight loss, the importance of dietary counseling should be stressed [26] . Depression and anxiety are particularly worrisome and have been reported in patients with DTC and are correlated with quality of life and social support.
One must consider as well that xerostomia may be influenced by psychological and somatic states, and by the hydration status of the patient, among other factors [11] [27] . However, such concerns shall be related to the consequences they entail, such as speech problems, identified through the application of QLQ-H&N35, and greater chance of tooth decay and consequently infections, compromising oral health.
In general terms, concern for patients’ health after therapy has increased. Some authors point out the great repercussions on the lives of children and adolescents, as well as their families. Many topics have been covered, such as the impact of cancer diagnosis on children, but many of these show the impact on patients’ families, with concerns regarding co-existence, integration, and the challenges facing these children in society. Given the importance of the topic, other studies in this direction and with a larger number of patients are welcome [28] [29] . The small sample size is the major limitation of this study. However, the instrument used QLQ-C30 questionnaire which is widely-used in quality of life research have well established norms derived from adult cancer population. It is important to emphasize that the patients allocated for this study came from various states in Brazil, of which many were more than one thousand kilometers from the study center. We believe that studies with larger populations will be necessary.
5. Conclusion
In conclusion, this study indicates that despite the safety and effectiveness of radioactive iodine therapy in children, damage arising from this treatment leads to a poorer perception of quality of life through QLQ-C30 and H&N35. Considering that this is a young population, these health problems can have great impact on their lives.
References
- McClellan, D.R. and Francis, G.L. (1996) Thyroid Cancer in Children, Pregnant Women, and Patients with Graves’ Disease. Endocrinology Metabolism Clinics of North America, 25, 27-48. http://dx.doi.org/10.1016/S0889-8529(05)70311-X
- Robbins, R.J. and Schlumberger, M.J. (2005) The Evolving Role of 131I for the Treatment of Differentiated Thyroid Carcinoma. Journal of Nuclear Medicine, 46, 28S-37S.
- O’gorman, C.S., Hamilton, J., Rachmiel, M., Gupta, A., Ngan, B.Y. and Daneman, D. (2010) Thyroid Cancer in Childhood: A Retrospective Review of Childhood Course. Thyroid, 20, 375-380. http://dx.doi.org/10.1089/thy.2009.0386
- Newkirk, K.A., Ringel, M.D., Wartofsky, L. and Burman, K.D. (2000) The Role of Radioactive Iodine in Salivary Gland Dysfunction. Ear, Nose & Throat Journal, 79, 460-468.
- Gorlin, J.B. and Sallan, S.E. (1990) Thyroid Cancer in Childhood. Endocrinol Metab Clin North Am, 24, 649-62.
- Caglar, M., Tuncel, M. and Alpar, R. (2002) Scintigraphic Evaluation of Salivary Gland Dysfunction in Patients with Thyroid Cancer after Radioiodine Treatment. Clinical Nuclear Medicine, 27, 767-771. http://dx.doi.org/10.1097/00003072-200211000-00003
- Helman, J., Turner, R.J., Fox, P.C. and Baum, B.J. (1987) 99mTc-Pertechnetate Uptake in Parotid Acinar Cells the Na+/K+/Cl− co-Transport System. Journal of Clinical Investigation, 79, 1310-1313. http://dx.doi.org/10.1172/JCI112954
- Newkirk, K.A., Ringel, M.D., Wartofsky, L. and Burman, K.D. (2000) The Role of Radioactive Iodine in Salivary Gland Dysfunction. Ear, Nose & Throat Journal, 79, 460-468.
- Mandel, S.J. and Mandel, L. (2003) Radioactive Iodine and the Salivary Glands. Thyroid, 13, 265-271. http://dx.doi.org/10.1089/105072503321582060
- Alexander, C., Bader, J.B., Schaefer, A., Finke, C. and Kirsch, C.M. (1998) Intermediate and Long-Term Side Effects of High-Dose Radioiodine Therapy for Thyroid Carcinoma. Journal of Nuclear Medicine, 39, 1551-1554.
- Taïeb, D., Baumstarck-Barrau, K., Sebag, F., Fortanier, C., De Micco, C., Loundou, A., et al. (2011) Heath-Related Quality of Life in Thyroid Cancer Patients Following Radioiodine Ablation. Health and Quality of Life Outcomes, 13, 33.
- Campolina, A.G. and Ciconelli, R.M. (2006) Qualidade de Vida e Medidas de Utilidade: Parâmetros Clínicos Para as Tomadas de Decisão em Saúde. Revista Panamericana de Salud Pública/Pan American Journal of Public Health, 19, 128-136. http://dx.doi.org/10.1590/S1020-49892006000200013
- Prebianchi, H.B. (2005) Medidas de Qualidade de Vida Para Crianças: Aspectos Conceituais e Metodológicos. Psicologia: Teoria e Prática, 5, 57-69.
- Gough, I.R. (1994) Quality of Life as an Outcome Variable in Oncology and Surgery. Australian and New Zealand Journal of Surgery, 64, 227-235. http://dx.doi.org/10.1111/j.1445-2197.1994.tb02190.x
- European Organization for Research and Treatment of Cancer (EORTC). http://www.eortc.be/home/qol
- Vartanian, J.G., Carvalho, A.L., Furia, C.L.B., Castro, Jr., G., Rocha, C.N., Sinitcovisky, I.M.L., et al. (2007) Questionnaries Validated in Brazilian Population for Evaluation of the Quality of Life in Patients with Head and Neck Cancer. Revista Brasileira de Cirurgia de Cabeça e Pescoço, 36, 108-115.
- Jeong, S.Y., Kim, H.W., Lee, S.W., Ahn, B.C. and Lee, J. (2013) Salivary Gland Function 5 Years after Radioactive Iodine Ablation in Patients with Differentiated Thyroid Cancer: Direct Comparison of Preand Posablation Scintigraphies and Their Relation to Xerostomia Symptoms. Thyroid, 23, 609-616. http://dx.doi.org/10.1089/thy.2012.0106
- Rosario, P.W.S., Cardoso, L.D., Barroso, A.L., Padrao, E.L., Rezende, L.L. and Purisch, S. (2005) Safety of Radioiodine Therapy in Patients with Thyroid Carcinoma Younger than 21 Years. Arquivos Brasileiros de Endocrinologia & Metabologia, 49, 241-245.
- Roach, M.C., Turkington, T.G., Higgins, K.A., Hawk, T.C., Hoang, J.K. and Brizel, D.M. (2012) FDG-PET Assessment of the Effect of Head and Neck Radiotherapy on Parotid Gland Glucose Metabolism. International Journal of Radiation Oncology Biology Physics, 82, 321. http://dx.doi.org/10.1016/j.ijrobp.2010.08.055
- Olmos, R.A.V., Keus, R.B., Takes, R.P., van Tinteren, H., Baris, G., Hilgers, F.L.M., et al. (1994) Scintigraphic Assessment of Salivary Function and Excretion Response in Radiation-Induced Injury of the Major Salivary Glands. Cancer, 73, 2886-2893. http://dx.doi.org/10.1002/1097-0142(19940615)73:12<2886::AID-CNCR2820731203>3.0.CO;2-K
- Dingle, F., Mishoe, A.E., Nguyen, S.A., Overton, L.J. and Gillespie, M.B. (2013) Salivary Morbity and Quality of Life Following Radioactive Iodine for Well-Differentiated Thyroid Cancer. Otolaryngology—Head and Neck Surgery, 148, 746-752. http://dx.doi.org/10.1177/0194599813479777
- Almeida, J., Vartanian, J.G. and Kowalski, L.P. (2009) Evaluation of Adjuvant Radioactive Iodine Therapy Side Effects on Salivary Glands in Patients with Well-Differentiated Thyroid Carcinoma. Archives of Otolaryngology—Head and Neck Surgery, 135, 342-346. http://dx.doi.org/10.1001/archoto.2009.16
- Richter, R., Oskay-Oezcelik, G., Chekerov, R., Pilger, A., Hindenburg, H.J., Sommer, H., et al. (2012) Health-Related Quality of Life during Sequential Chemotherapy with Carboplatin Followed by Weekly Paclitaxel in Advanced Ovarian Cancer: A Multicenter Phase II Study of the North Eastern German Society of Gynecological Oncology. Anticancer Research, 32, 3969-3976.
- Bushnell, D.L., Boles, M.A., Kaufman, G.E., Wadas, M.A. and Barnes, E. (1992) Complications, Sequela and Dosimetry of Iodine-131 Therapy for Thyroid Carcinoma. Journal of Nuclear Medicine, 33, 2214-2221.
- Vissink, A., Jansma, J., Spiikervet, F.K., Burlage, F.R. and Coppes, R.P. (2003) Oral Sequelae of Head and Neck Radiotherapy. Critical Reviews in Oral Biology & Medicine, 14, 199-212. http://dx.doi.org/10.1177/154411130301400305
- Mendoza, A., Shaffer, B., Karakla, D., Mason, M.E., Elkins, D. and Goffman, T.E. (2004) Quality of Life with Well-Differentiated Thyroid Cancer: Treatment Toxicities and Their Reduction. Thyroid, 14, 133-140. http://dx.doi.org/10.1089/105072504322880373
- Braz, D.S.A., Ribas, M.M., Dedivitis, R.A., Nishimoto, I.N. and Barros, A.P.B. (2005) Quality of Life and Depression in Patients Undergoing Total and Partial Laryngectomy. Clinics, 60, 135-142. http://dx.doi.org/10.1590/S1807-59322005000200010
- Challinor, J.M., Miaskowski, C.A., Franck, L.S., Slaughter, R.E., Matthay, K.K., Kramer, R.F., et al. (1999) Somatization, Anxiety and Depression as Measures of Health-Related Quality of Life of Children/Adolescents with Cancer. International Journal of Cancer. Supplement, 12, 52-57. http://dx.doi.org/10.1002/(SICI)1097-0215(1999)83:12+<52::AID-IJC10>3.0.CO;2-J
NOTES

*Corresponding author.


