Advances in Bioscience and Biotechnology
Vol.3 No.4A(2012), Article ID:21872,6 pages DOI:10.4236/abb.2012.324059
Investigation of an inflammatory viral disease HBV in cardiac patients through polymerase chain reaction
![]()
1Department of Chemistry and Biochemistry, University of Agriculture, Faisalabad, Pakistan
2Health Biotechnology Division, National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan
3Department of Microbiology, University of Agriculture, Faisalabad, Pakistan
Email: *fari.farinasaher@gmail.com
Received 29 May 2012; revised 30 June 2012; accepted 12 July 2012
Keywords: HBV; Liver Inflammation; LFTs; PCR; Agarose Gel Electrophoresis
ABSTRACT
In the present study 100 cardiac patients were randomly selected from the cardiology ward, Allied Hospital Faisalabad, Pakistan. All the selected cases were analysed for different parameters like Hepatitis B surface Antigen (HbsAg), Bilirubin, Alkaline phosphatase, serum glutamic pyruvic transaminase, and serum glutamic-oxaloacetic transaminase. Out of total 16% patients were lying in the age of 21 - 30 year, 25% in the age of 31 - 40 year, 35% in the age of 41 - 50 year, 19% in the age of 51 - 60 years and 5% patients in the age of 61 - 70 years. No subject was found positive in age group 21 - 30 years patients. 35% patients have higher value of SGPT while, other 26% were with higher value of SGOT. Rest of the 32% and 24% have higher ALP and Bilirubin levels, respecttively. Assay profile revealed that ALP level was increased with increasing age, body mass index, C-reactive protein, diabetes, smoking, sex, serum uric acid, lead, cadmium, hypercholesterolemia, lesion of liver and cardiovascular disease. The serum of the eight HbsAg positive cases were tested for the presence of HBV through PCR and no sample was found positive. At the end of the study, PCR amplified samples were run on 1.5% agarose gel to confirm the case.
1. INTRODUCTION
Many infectious diseases affecting human beings are caused by viruses. Viral hepatitis is frequently fatal and one among them, which is one of commonest infection liver disease worldwide [1]. The terms, hepatitis A and hepatitis B were first introduced by MacCallum in 1947 in order to categorize infectious (epidemic) and serum hepatitis and these terms were eventually adopted by the world Health Organization Committee on the Viral Hepatitis [2].
HBV infection is a major public health problem in most and particularly related to the underdeveloped nations. Worldwide, probably 2 billion and 350 to 400 million people are chronically infected with this viral infection [3,4]. A highly resilient virus, HBV is resistant to breakdown and can easily transmit through contact with infected body fluid. Hepatitis B virus is present in blood, semen, vaginal secretions, menstrual blood, and to lesser extent, breast milk, and urine of infected individuals [5].
HBV S gene (678 dp) encodes small HBV envelope protein of 226 amino acids [6]. It is highly hydrophobic, containing four trans-membrane spanning regions. The HBsAg contains a high number of cysteines, each of which is cross-linked to one another and may also be glycosylated at Asp 146. It also contains a highly antigenic epitope which is used for the sub typing of HBV [6]. The early phase of Chronic Hepatitis B (CHB) is characterized by the presence of hepatitis B envelop antigen (HbeAg) and high serum level of HBV DNA. Following infection the immune system attempts to clear the HBV by destroying infection hepatocytes. This leads to increase the circulatory blood levels of alanine aminotransferase (ALT). High HBV DNA and ALT levels may persists in some anti-HbeAg positive patients (referred to as HbsAg negative CHB) because of the presence of an HBV variant, that is unable to produce HbeAg, HbsAg negative variant also called pre core mutant [7].
Vaccination is the tool to prevent the transmission of HBV infection. The global community must support poorer countries to strength their health care delivery system and in particular, The National Immunization Programs, so that vaccines can delivered safely to attain high coverage against this viral disease [5].
The present study was conducted to investigate the incidence/prevalence of Hepatitis B virus in cardiac patients in order to determine any possible relationship between cardiac and HBV infection by strip test, PCR (Polymerization Chain Reaction) and agarose gel electrophoresis.
2. MATERIALS AND METHODS
2.1. Sample Source
Total 100 clinically diagnosed cardiac patients were consecutively admitted to the cardiology ward in Allied Hospital, Faisalabad, recruited for the study. Patients were considered to have hepatitis B infection if their Enzyme-linked immunosorbent assay (ELISA) or screening test was positive and had raised level of alanineaminotransferase (ALT).
2.2. Blood Samples
5 mL blood was drawn using sterilized syringes with the help of venipuncture from each patient and immediately transferred into tubes containing EDTA as an anticoagulant. The samples were thoroughly mixed and finally centrifuged at 3000 g for 5 min. Separated serum fractions were transferred into autoclaved eppendorf tubes and stored at –20˚C until further use.
2.3. Laboratory Testing
Hepatitis B surface Antigen (HbsAg) was screened out by the use of Trinity Biotech Uni-Gold HbsAg test kit in vitro for qualitative detection of HbsAg in whole blood and serum. All tests were performed at the pathology laboratory, Allied hospital, Faisalabad, Pakistan.
2.4. Biochemical Analysis
Serum samples were analyzed for bilirubin (conjugated and unconjugated), Alkaline phosphatase (ALP), SGPT and serum glutamic-oxaloacetic transaminase (SGOT).
2.4.1. Bilirubin
Bilirubin reacts with diazotised sulfanilic acid to form an azo dye, which is red in neutral and blue in alkaline solution. Whereas the water-soluble bilirubin glucuronides reacts “direct” the free “indirect” bilirubin reacts only in the presence of an accelerator. The total bilirubin in serum and plasma was determined by coupling with diazotized sulfanilic acid (29 mmol/L) after the addition of caffeine (130 mmol/L), sodium benzoate (156 mmol/L) and sodium acetate (460 mmol/L). Absorbance was recorded using spectrophotometer at 578 nm. The direct bilirubin was measured as the red azo dye at 546 nm without the addition of alkali. The indirect bilirubin was calculated from the difference between the total and direct bilirubin.
2.4.2. Alkaline Phosphatase
Human Gasellschaft fur Biochemica und Diagnostica mbH (EC 3.1.3.1) kit method was used for the purpose of ALP diagnostic. Reaction principle is given below:
p-Nitrophenylphosphate + H2O ↔ phosphate + p-nitrophenol
2.4.3. SGPT Assay
Human Gasellschaft fur Biochemica und Diagnostica mbH (EC 2.6.1.2) kit method was used for the diagnostic of SGPT. Reaction principle is given below:

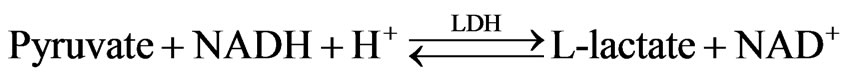
2.4.4. SGPT Assay
Human Gasellschaft fur Biochemica und Diagnostica mbH (EC 2.6.1.1) kit method was used for the diagnostic of SGOT. Reaction principle is given below:
2-Oxoglutarate + L-aspartate
↔ L-glutamate + oxaloacetate Oxaloacetate + NADH + H+ ↔ L-malate + NAD+
2.4.5. PCR, DNA Isolation
The entire reagents for the extraction were prepared and Hepatitis B virus DNA was extracted by phenol-chloroform method from plasma [8]. 50u lysis buffer (450 mM NaCl, 30 mM Tris HCl pH 8.00, 45 mM MgCl2, 1.5% Nonidet p-40 + 1.00 mg/ml protinase K) was taken in eppendorf tube followed by the addition of 100 ul serum sample and gently vortexed. Samples with lysis buffer were incubated at 37˚C for 30 min then heated at 95˚C for 10 min; finally the samples were kept at –20˚C for 10 min. Centrifuged for 5 min at 10,000 g and resulting supernatant was transferred in eppendorf tube followed by the addition of 350 ul de-ionized water. An equal volume of buffer equilibrated phenol chloroform (1:1) was added and then ccentrifuged at 10,000 g for 5 min. The upper aqueous phase was transferred to eppendrof tube followed by the addition of 1/10 volume of 3 M sodium acetate (pH 4.3) mixed and then 2.5 volume of chilled ethanol (at –20˚C) was added. The tubes were kept at –70˚C for 30 min. Centrifuged at 15,000 g for 15 min and resulting pellets were washed twice with 70% ethanol after repeating the whole step. Finally the pellets were dried under vacuumed followed by the addition of 10 ul of TE buffer to dissolve DNA and stored at –40˚C for PCR amplification.
2.4.6. Primers
Primers used in the present study are presented in the Table 1. These primers were designed using software
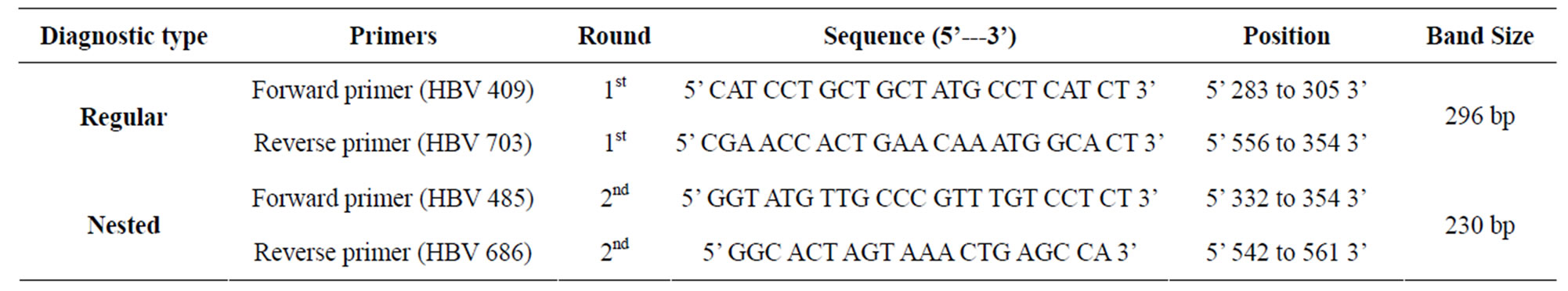
Table 1. Primer used for the amplification of target sequences of HBV-DNA.
vector NTI version 7. Positive and negative controls were included in the PCR analysis to make it more authentic and reliable.
2.4.7. Detection of HBV by Molecular Method
In this method of DNA primers targeting, the surface antigen gene region of HBV genome were utilized. PCR amplification yields 296 bp and 230 bp DNA fragments, detectable by agarose gel electrophoresis after staining with ethidium bromide. The amplified PCR product of both regular and nested PCR were run on 1.5% agarose gel in 1 × trizmabase (TBE) [5 × stock TBE : 54.0 g TBE, 27.5 g boric acid, 20 ml EDTA (0.5 M, pH 8)] following the method of Sambrook et al. [9].
3. RESULTS AND DISCUSSION
3.1. Prevalence of HbsAg in Cardiac Patients
To investigate the prevalence of HbsAg, all of the 100 clinical cardiac patients were first screened for the presence of HbsAg in the sera using the Trinity Biotech UniGold HbsAg test kit. In this regard, screening assay profile showed that total eight cases were positive among all of the 100 analyzed samples.
3.2. Level of Bilirubin, ALP, SGPT, SGOT in Cardiac Patients
3.2.1. Age Group 21 - 30 Years This age group was comprised 16% of the total patients and among those only 5% have higher Bilirubin level (1.384 ± 1.094) as compare to the normal value (1.2 mg/dl) (Figure 1). 7% patients have higher value of Alakaline phosphtase (302.5 ± 131.8) than that of the normal limits (80 - 306 U/l) (Figure 2). SGPT and SGOT test profile showed that total 6% patients have the higher value of SGPT (80.3 ± 70.7) which deviates from normal (5 - 42 U/l) and other 6% also have a higher SGOT level (115.11 ± 223) as compare to the normal value (5 - 42 U/l) (Figures 3-4). Whereas, regarding HBsAg no subject was found positive with in this age group.
3.2.2. Age Group 31 - 40 Years
This age group contained 25% patients out of the total
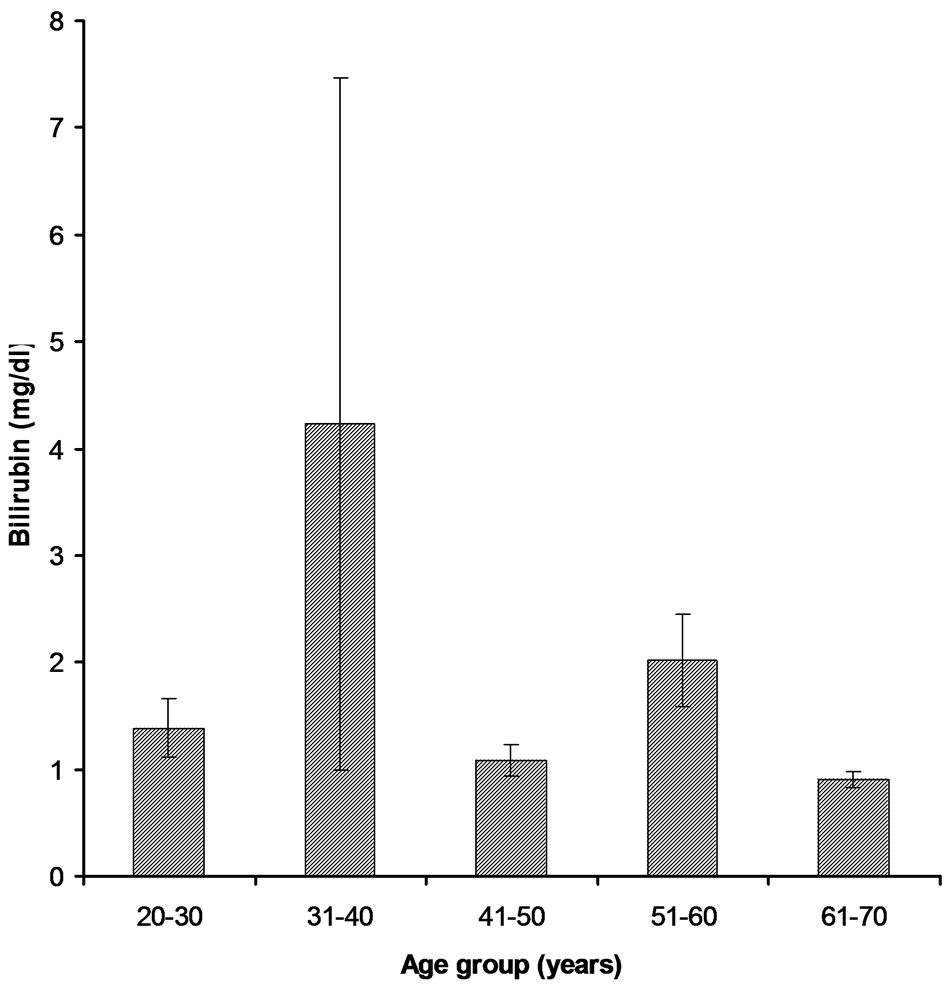
Figure 1. Bilirubin levels in different age groups of cardiac patients.

Figure 2. ALP levels in different age groups of cardiac patients.
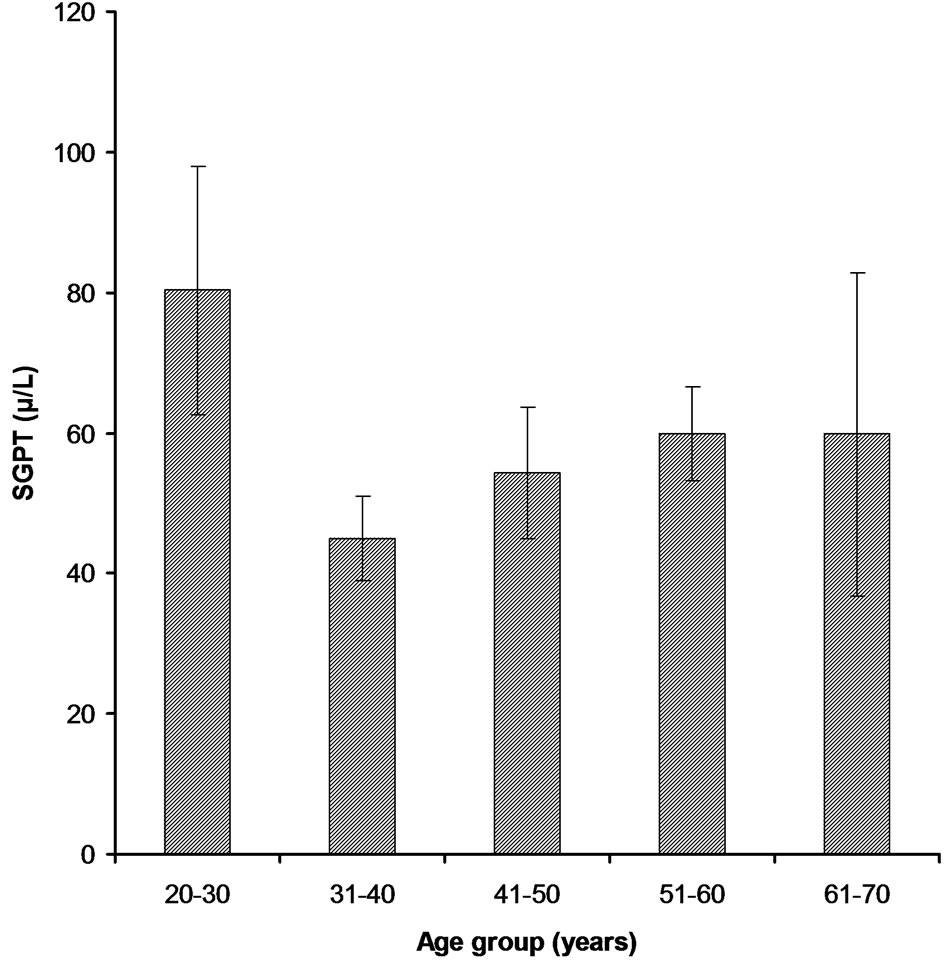
Figure 3. SGPT levels in different age groups of cardiac patients.
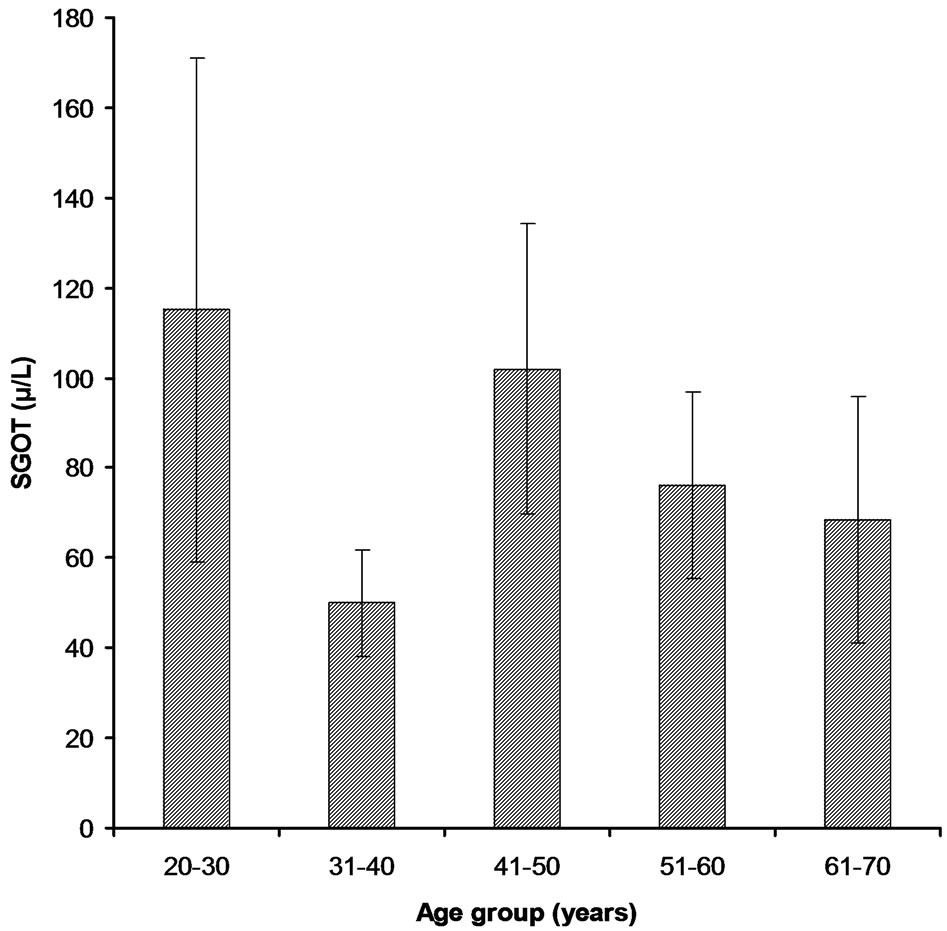
Figure 4. SGOT levels in different age groups of cardiac patients.
100 randomly selected samples. Bilirubin test assay revealed that 5% have the higher level of Bilirubin (4.23 ± 15.16) than that of the normal value while, other 3% had higher Alakaline phosphtase level (211.8 ± 97.2) (Figures 1-2). In comparison to the normal limits of SGPT and SGOT (5 - 42 U/l) out of 25 samples, 7% and 4% have the higher values of SGPT (44.95 ± 27.96) and SGOT (50.0 ± 44.3) respectively (Figures 3-4). Three subjects (case # 19, 26, and 31) were found positive for HBsAg.
3.2.3. Age Group 41 - 50 Years
In this group out of 35% patients 6% had the higher level of Bilirubin (1.085 ± 0.824) than the normal value (Figure 1). Total 13% were found to have higher value of Alakaline phosphtase (279.5 ± 141.1) than its normal limits (80 - 306 U/l) (Figure 2). Among 35% samples 12% were those having higher value of SGPT which deviates from the normal (5 - 42 U/l); mean ± SE value of SGPT was found 54.32 ± 51.56 (Figure 3). 9% cardiac patients were found to have higher SGOT (102.0 ± 151.4) level than that of the normal value (5 - 42 U/l) (Figure 4). Four subjects (case # 44, 50, 61, and 65) were found positive for HBsAg.
3.2.4. Age Group 51 - 60 Years
This age group was comprised of 19% patients and 8% were with the higher level of Bilirubin (2.016 ± 1.845) while, other 9% have higher level of Alakaline phosphtase (302.7 ± 55.5) as compare to the normal limits (Figures 1-2). Rest of the 10 and 7% has the higher value of SGPT and SGOT respectively than that of their normal values as shown in the Figures 3-4. In this group only one subject (case # 94) was found positive for HBsAg.
3.2.5. Age Group 61 - 70 Years
In this group all of the cardiac patients were found to have the level of Bilirubin within normal limits (1.2 mg/dl) (Figure 1). In comparison to all other group only 1% patients were found with higher value of Alakaline phosphtase (174.4 ± 84.7) (Figure 2). In this group only 2% and 1% patients were found to have higher value of SGPT (59.8 ± 51.5) and SGOT (68.5 ± 38.9) respectively (Figures 3-4). Unlike other groups no subject was found HBsAg positive for.
3.3. Confirmation of HBV-DNA through PCR
Blood samples of eight cardiac patients, those were found HBsAg positive were collected and later on the presence of HBV-DNA was confirmed by PCR. A part of HBV surface gene was amplified by PCR using two sets of diagnostic primers (regular: HB-409, HB-703 and nested: HB-485, HB-686). Two hundred and thirty base pairs (230 bp) DNA product was observed in nested (second round) PCR amplification. The amplified product was analyzed on 1.5% agarose gel (Figure 5).
The effects of liver cirrhosis due to viral hepatitis cardiac ventricular functions were analyzed. In the present study the value of Bilirubin was found little higher as reported in many of the previous studies. The higher value of Bilirubin indicates the biliary inflammation, which may be intrahepatic or extra hepatic. No correlation was found between Bilirubin and cardiac patients so increased value of Bilirubin is related to liver hepatitis not with heart disease [10].
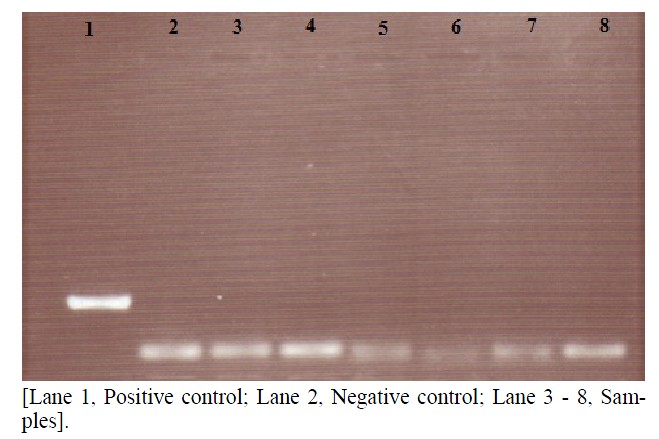
Figure 5. Agarose gel of PCR amplified samples.
Increased in both transaminases were found in liver with SGPT much higher than SGOT. Serum ALT remains the most accessible test for monitoring the chronic hepatitis B viral infection [11]. In the present study serum ALP values were found little bit higher than previous studies. Out of the total 100 samples, only eight samples were HBV positive by HBV screening kit whereas only two were positive by ELISA test for HBV. These all samples were negative for HBV-DNA PCR. Negative result for HBV/DNA on PCR indicates that these patients have no titer of viral DNA in their serum [12].
The prevalence of HBV infection was found to be higher in person with age higher than 35. Similar findings have also been reported previously by Leung [13]. Blood transfusion continues to cause hepatitis B in those countries, where donor blood is not screened for HBV [14]. The present study indicates that blood transfusion is taking part in transmission of virus infection in cardiac patients. Previously, HBV transmission by blood transfusion has also been reported in Pakistan by [15].
4. CONCLUSION
In conclusion, the increasing prevalence of HBsAg in cardiac patients was found which indicates that the cardiac patients are at higher risk of HBV while, no carrier of HBV was indicated in 100 cardiac patients by PCR. Further epidemiological studies at molecular level may be conducted by taking large sample size to measure the prevalence and risk factors of HBV infection.
5. ACKNOWLEDGEMENTS
The present work was a part of M.Sc. research work conducted by Farina Saher under the supervision of Dr. Khalil ur Rehman, Department of Chemistry and Biochemistry, UAF. On providing technical expertise and collaborative help for the present study, J.A. Qureshi and H.M.N. Iqbal are thankfully acknowledged. Authors are also grateful to the Dr. Ikram-ul-Haq, Head of Pathology Department, Allied Hospital Faisalabad, for his valuable guidance, immense moral support and masterly advice.
REFERENCES
- Liu, B., Li, J., Han, Y., Liu, Y., Kong, L., Cao, Y. and Huang, Z. (2010) Dynamic analysis of lymphocyte subsets of peripheral blood in patients with acute self-limited hepatitis B. Health, 2, 736-741. doi:10.4236/health.2010.27112
- WHO (1997) Emerging and other communicable diseases (EMC). Weekly Epimiological Report (WER), 72, 5487- 5494.
- Noah, D.N., Njouom, R., Bonny, A., Pirsou., Meli, J. and Sida, M.B. (2011) HBs antigene prevalence in blood donors and the risk of transfusion of hepatitis B at the central hospital of yaounde, Cameroon. Open Journal of Gastroenterology, 1, 23-27.
- Badar, N., Farooq, U., Ali, S., Nisar, N., Abubakar, M. and Qureshi, J.A. (2012) A molecular approach for genotyping of hepatitis B virus using restriction pattern analysis of samplicon in Pakistan. Open Journal of Medical Microbiology, 2, 16-23. doi:10.4236/ojmm.2012.21003
- Lavenchy, D. (2004) Hapetitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of Viral Hepatitis, 11, 97-107. doi:10.1046/j.1365-2893.2003.00487.x
- Schirmbeck, R., Zheng, X., Roggendorf, M., Geisserler, M., Chiisari, F.V., Reimann, J. and Lu, M. (2001) Targeting immune responses to selected T cells or antibody defined determinants of hepatitis B virus surface antigen by plasmid DNA vaccines encoding chimeric antigen. Journal of Immunology, 166, 1405-1413.
- Carman, M.H., Chen, C.J. and Lai, M.S. (1997) Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in childhood hepatona study group’s. England Journal of Medicine, 336, 1855-1859.
- Kao, J.H., Chen, P.J., Lai, M.Y. and Chen, D.S. (2000) Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gasteroenterology, 118, 554-559. doi:10.1016/S0016-5085(00)70261-7
- Sambrook, J., Fritsch, E.F. and Maniatis, T. (1999) Molecular cloning, a laboratory mannual. 2nd Edition, Gold Spring Harbor Laboratory Press, Cold spring Harbour, New York.
- Khan, A.A., Parveen, N., Mahaboob, V.S., Rajendraprasad, A., Ravindraprakash, H.R., Venkateswarlu, J., Rao, P., Pande, G., Narusu, M.L., Khaja, M.N., Pramila, R., Habeeb, A. and Habibullah, C.M. (2008) Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: A case report. Transplant Proceedings, 40, 1153-1155. doi:10.1016/j.transproceed.2008.03.110
- Tsang, P.S., Trinh, H., Garcia, R.T., Phan, J.T., Ha, N.B., Nguyen, H., Nguyen, K., Keeffe, E.B. and Nguyen, M.H. (2008) Significant prevalence of histologic disease in patients with chronic hepatitis B and mildly elevated serum alanine aminotransferase levels. Clinical Gastroenterology and Hepatology, 6, 569-574. doi:10.1016/j.cgh.2008.02.037
- Chopra, C.G.S., Gupta, L.C.P.K., Anand, C.A.C., Varma, C.P.P., Nai, C.V. and Rai, L.G.R. (2005) Real time-PCR HBV-DNA analysis: Significance and first experience in armed forces. MJAFI, 61, 234-237.
- Leung, N. (2002) Transmission of hepatitis B: Case selection and duration of therapy. Journal of Gastroenterology and Hepatology, 17, 409-414. doi:10.1046/j.1440-1746.2002.02767.x
- Fleming (2000) The origin and evolution of hepatitis virus in humans. Journal of General Virology, 82, 693- 712.
- Rahman, M., Akhtar, G.N. and Lodi, Y. (2002) Transfusion transmitted HIV and HBV infection in Punjab, Pakistan. Pakistan Journal of Medical Science, 18, 18-25.
NOTES
*Corresponding author.

