Advances in Bioscience and Biotechnology
Vol.4 No.6(2013), Article ID:33526,3 pages DOI:10.4236/abb.2013.46091
Proline-leucine polymorphism of human glutathione peroxidase 1 in Thalassemia major
![]()
1Department of Biochemistry, G.R Medical College, Gwalior, India
2Department of Medicine, G.R Medical College, Gwalior, India
3Department of Biotechnology, Gwalior, India
Email: *bhargavavishal6@gmail.com
Copyright © 2013 Vishal Bhargava et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 12 April 2013; revised 15 May 2013; accepted 28 May 2013
Keywords: GPX1; Thalassemia; SNP
ABSTRACT
Thalassemia is associated with low antioxidant enzyme deficiency especially glutathione peroxidase. GPX exists in 6 isomeric forms out of which GPX1 Single Nucleotide Polymorphism is found to be associated with Thalassemia major. In our study, the determination of the allelic frequency and phenotype of a common polymorphism in Se-dependent glutathione peroxidase 1 (GPX1) was observed in Thalassemic populations. A proline/leucine variant occurs at position 197 close to the C-terminus of the protein. The genotypes encoding Pro/Pro, Pro/Leu, and Leu/ Leu are distributed according to the Hardy-Weinberg relationship. The study has been carried out in 40 Thalassemic cases and 40 control subjects. No significant association between allele frequency and risk to get fatal was evident. Erythrocyte GPX activity was determined and no significant differences were obtained between the genotypes. It can be concluded that the Pro/Leu genetic variation does not appear to compromise the defense against oxidative stress in red blood cells or to be associated with significant pathology.
1. INTRODUCTION
Human glutathione peroxidase 1 is an abundant and widely distributed seleno-protein [1]. Mouse knock-out models have revealed that loss of the enzyme confers no obvious phenotype but the animals become more sensitive to oxidative stress [2]. Oxidative stress often has been implicated in Thalassemia [3,4] as well as aging [5]. To determine the actual impact of oxidative stress, tissue and plasma antioxidant levels are often determined. In addition, various antioxidant enzyme activities have been determined in blood. Although considerable variation in antioxidant capacity does occur in humans, the variation does not account for disease susceptibility in the majority of cases [6-9]. It must either be concluded that oxidative stress is of limited importance or that current measurements of antioxidant capacity is a blunt instrument. As intra individual variation in antioxidant capacity is well documented and influenced by nutriational status [10- 12], other measurements of antioxidant capacity involving determination of genetic profile are highly desirable.
Association studies of functional or linked surrogate marker polymorphic sites in genes offer a way to probe variant function [13]. In an effort to survey polymorphisms in genes related to oxidative stress, we recently determined a common allelic variant in human glutathione peroxidase 1 [14]. We observed the genotyping of 30 subjects and comparison to the corresponding blood glutathione peroxidase activity levels and 80 subjects where 40 had suffered from Thalassemia.
2. MATERIALS AND METHODS
2.1. Assay of Glutathione Peroxidase Activity
With informed consent from patient blood was collected from all individuals. The erythrocyte GPX activity was determined in hemolysates with a coupled spectrophotometric assay [15]. Hemoglobin was determined with a standard cyanomethemoglobin assay. Patients were not taking antioxidants including selenium and the study was approved by the research ethical committee of Gajra Raja Medical College Gwalior [16]. First ever thalassemic individuals were compared in a nested case-control design to age matched participants remaining free of oxidative stress.
2.2. Isolation of Genomic DNA
Genomic DNA was prepared from whole blood using the Nucleon DNA extraction kit, Amersham Pharmacia.
2.3. PCR and Restriction Analysis
Human genomic DNA (0.05 mg) was amplified with the following primers: Forward, 5’-GCCTGGTGGTGGGTTCGAGCC-3’; Reverse, 5’-GACAGCAGCACTGCAACTGCC-3’. Amplifications were all carried out with 0.15 mM dNTP, 20 pmol of respective primer and 0.5 U Taq Polymerase (SIGMA) in the supplied buffer. 27 cycles were used, each involving denaturation at 94˚C for 45 sec annealing at 57˚C for 45 sec, and extension at 72˚C for 1 min. The amplifications included 10% DMSO. The amplified PCR-product was cleaved with the restriction enenzyme DdeI (New England BioLabs, Beverly, MA) in the supplied buffer and analyzed in 1% agarose gels.
3. RESULTS
Above table shows GPX enzyme activity from groups of donors representing the frequency of Pro/Pro, Leu/Leu and Pro/Leu genetic variants.
3.1. Statistics
Odds ratios (OR) were calculated as estimates of relative risks for the development of Thalassemia in the study. The influence of age was adjusted by using logistic regression when calculating ORs with 95% confidence intervals.
Above table shows the allele frequency of the more common Homozygous Pro encoding variant as compared to Heterozygous Pro/Leu and Homozygous Leu among Thalassemic cases and controls along with the odds ratio of allelic frequency.
2. DISCUSSION AND CONCLUSION
We have previously identified, by a bioinformatics approach [21], a polymorphism in the human GPX1 gene where a C/T variation results in either a Pro or Leu at amino acid position 197. This polymorphism was recently confirmed by others [22] and had also been noted in a loss of heterozygosity study [23]. Here in the study, we observed allelic frequency in 80 Thalassemic cases/ controls with known erythrocyte GPX activity. As shown in Tables 1 and 2, the allele frequency of the more common Pro encoding variant is 73 and 59%, respectively, in these populations. The distributions of Pro, Leu homozygotes and heterozygotes are in Hardy-Weinberg equilibrium in both populations. When measured in erythrocytes, the GPX enzyme activity from groups of donors representing the Pro/Pro, Leu/Leu and Pro/Leu genetic variants was not significantly altered (Table 1). In fact, ac-
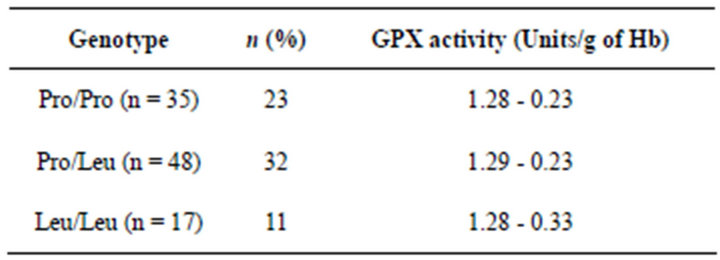
Table 1. Glutathione peroxidase 1 genotype frequencies in Thalassemia patient and corresponding GPX1 levels in erythrocytes.
tivity levels are virtually identical. It is known that changes in erythrocyte activity do occur for other polymorphic enzymes (e.g., Cu-Zn-SOD) [16] where enzyme stability is implied. Erythrocytes do not replenish proteins and are on average 60 days old. The normal GPX activity in erythrocytes from carriers of the rare allele suggests that the variant enzyme has a high stability and that normal activity also should be found in other cells of the body with faster turnover. This suggestion of course needs to be investigated separately using nucleated cells. It is also known that erythrocyte GPX activity can be upregulated under conditions of oxidative stress [24]. Therefore, if one of the variants had impaired activity, a compensatory up-regulation cannot be excluded. The Pro encoding allele is linked to a polymorphic site containing GCG triplet nucleotide repeats coding for either 5 or 7 alanines whereas the Leu encoding variant is linked to a 6 alanine encoding nucleotide repeat and base changes at 2592 and 12 [23]. The alanine stretch commences at position 7 after the N terminus. Our analysis indicates that these variants do not differ dramatically in terms of activity and stability. As an independent measure of a possible contribution of GPX1 variation to disease where oxidative stress is implicated in the etiology, we also examined whether GPX1 genotypes were associated with Thalassemia. As evident from our results, there was no difference between genotype frequencies, in Thalassemia cases (Table 2). The odds ratio of Leu/Leu vs. the combined Pro/Pro and Pro/Leu genotypes indicated no risk (OR 0.92), the 95% confidence interval was (0.37 - 2.3) (Table 2). In conclusion, this is the first study showing that the Pro and Leu variants of human GPX1 do not
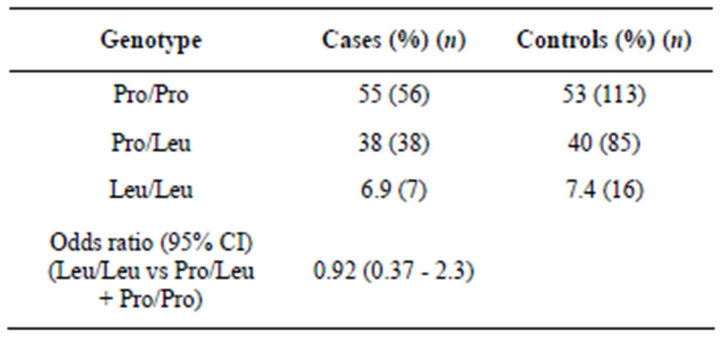
Table 2. Glutathione peroxidase 1 genotype frequencies in Thalassemic case/control population.
differ in activity and stability. At least the erythrocyte GPX capacity clearly does not vary with genotype. Also, this genetic variation is not significantly associated with an increased risk for Thalassemia.
REFERENCES
- Holben, D.H. and Smith, A.M. (1999) The diverse role of selenium within selenoproteins: A review. Journal of the American Dietetic Association, 99, 836-843. doi:10.1016/S0002-8223(99)00198-4
- Fu, Y., Cheng, W.H., Porres, J.M., Ross, D.A. and Lei, X.G. (1999) Knockout of cellular glutathione peroxidase gene renders mice susceptible to diquatinduced oxidative stress. Free Radical Biology and Medicine, 27, 605-611.
- Halliwell, B. (1999) Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radical Research, 31, 261-272. doi:10.1080/10715769900300841
- Halliwell, B. and Cross, C.E. (1994) Oxygen-derived species: Their relation to human disease and environmental stress. Environmental Health Perspectives, 102, 5-12. doi:10.2307/3432205
- Sohal, R.S. and Weindruch, R. (1996) Oxidative stress, caloric restriction, and aging. Science, 273, 59-63. doi:10.1126/science.273.5271.59
- Riemersma, R.A., Oliver, M., Elton, R.A., Alfthan, G., Vartiainen, E., Salo, M., Rubba, P., Mancini, M., Georgi, H. and Vuilleumier, J.P. (1990) Plasma antioxidants and coronary heart disease: Vitamins C and E, and selenium. European journal of clinical nutrition, 44, 143-150.
- Eichholzer, M., Stahelin, H.B. and Gey, K.F. (1992) Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke respectively: 12-year follow-up of the Prospective Basel Study. EXS, 62, 398-410.
- Foy, C.J., Passmore, A.P., Vahidassr, M.D., Young, I.S. and Lawson, J.T. (1999) Plasma chain breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. International Journal of Medicine, 92, 39-45. doi:10.1093/qjmed/92.1.39
- Yamamoto, Y., Yamashita, S., Fujisawa, A., Kokura, S. and Yoshikawa, T. (1998) Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochemical and Biophysical Research Communications, 247, 166-170. doi:10.1006/bbrc.1998.8752
- Bolzan, A.D., Bianchi, M.S. and Bianchi, N.O. (1997) Superoxide dismutase, catalase and glutathione peroxidase activities in human blood: Influence of sex, age and cigarette smoking. Clinical Biochemistry, 30, 449-454. doi:10.1016/S0009-9120(97)00047-7
- Omaye, S.T., Burri, B.J., Swendseid, M.E., Henning, S.M., Briggs, L.A., Bowen, H.T. and Ota, R.B. (1996) Blood antioxidants changes in young women following beta-carotene depletion and repletion. Journal of the American College of Nutrition, 15, 469-474.
- Krajcovicova-Kudlackova, M., Simoncic, R., Babinska, K. and Bederova, A. (1995) Levels of lipid peroxidation and antioxidants in vegetarians. European Journal of Epidemiology, 11, 207-211. doi:10.1007/BF01719489
- Brookes, A.J. (1999) The essence of SNPs. Gene, 234, 177-186. doi:10.1016/S0378-1119(99)00219-X
- Forsberg, L., de Faire, U. and Morgenstern, R. (1999) Low yield of polymorphisms from EST blast searching: Analysis of genes related to oxidative stress and verification of the P197L polymorphism in GPX1. Human Mutation, 13, 294-300.
- Hardell, L., Danell, M., Angqvist, C.A., Marklund, S.L., Fredriksson, M., Zakari, A.L. and Kjellgren, A. (1993) Levels of selenium in plasma and glutathione peroxidase in erythrocytes and the risk of breast cancer. A case— Control study. Biological Trace Element Research, 36, 99-108. doi:10.1007/BF02783168
- Andersen, P.M., Nilsson, P., Forsgren, L. and Marklund, S.L. (1998) CuZn-superoxide dismutase, extracellular superoxide dismutase, and glutathione Peroxidase in blood from individuals homozygous for Asp90Ala CuZu-superoxide dismutase mutation. Journal of Neurochemistry, 70, 715-720. doi:10.1046/j.1471-4159.1998.70020715.x
- WHO MONICA Project Principal Investigators (1998) The World Health Organization MONICA Project (Monitoring Trends and Determinants in Cardiovascular Disease): A major international collaboration. The Journal of Clinical Epidemiology, 41, 105-114.
- Stegmayr, B. and Asplund, K. (1992) Measuring stroke in the population: Quality of routine statistics in comparison with a population-based stroke registry. Neuroepidemiology, 11, 204-213. doi:10.1159/000110933
- Ahmed, E., Trifunovic, J., Stegmayr, B., Hallmans, G., and Lefvert, A.K. (1999) Autoantibodies against oxidatively modified LDL do not constitute a risk factor for stroke: A nested case—Control study. Stroke, 30, 2541- 2546. doi:10.1161/01.STR.30.12.2541
- Soderberg, S., Ahren, B., Stegmayr, B., Johnson, O., Wiklund, P.G., Weinehall, L., Hallmans, G. and Olsson, T. (1999) Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke, 30, 328-337. doi:10.1161/01.STR.30.2.328
- Forsberg, L., de Faire, U. and Morgenstern, R. (1998) To Identify Genetic Polymorphisms in the “Expressed Sequence Tag” (EST) Database. http://www.elsevier.com/locate/tto
- Emahazion, T., Jobs, M., Howell, W.M., Siegfried, M., Wyoni, P.I., Prince, J.A. and Brookes, A.J. (1999) Identification of 167 polymorphisms in 88 genes from candidate neurodegeneration pathways. Gene, 238, 315-324. doi:10.1016/S0378-1119(99)00330-3
- Moscow, J.A., Schmidt, L., Ingram, D.T., Gnarra, J., Johnson, B. and Cowan, K.H. (1994) Loss of heterozygosity of the human cytosolic glutathione Peroxidase I gene in lung cancer. Carcinogenesis, 15, 2769-2773. doi:10.1093/carcin/15.12.2769
- Saugstad, O.D. and Marklund, S.L. (1988) High activities of erythrocyte glutathione peroxidase in patientswith the Lesch-Nyhan syndrome. Acta Medica Scandinavica, 224, 281-285.
NOTES
*Corresponding author.

