Advances in Bioscience and Biotechnology
Vol.2 No.6(2011), Article ID:8896,6 pages DOI:10.4236/abb.2011.26057
Studies on antibacterial activity and biochemical/biophysical properties of phytocystatin purified from Catharanthus roseus (Madagascar Periwinkle): an evergreen subshrub commonly found in district Bijnor (U.P.)
![]()
1Department of Botany, SahuJain Degree College, M. J. P. Rohilkhand University, Bareilly, India;
2Department of Biochemistry, Aligarh Muslim University, Aligarh, India.
Email: *garima15s@yahoo.com
Received 7 January 2011; revised 1 June 2011; accepted 20 July 2011.
Keywords: Plant Cystatins; FTIR; Fluorescence Spectroscopy; Protease Inhibitor; Proteins
ABSTRACT
In the present study two phytocystatins (thiol protease inhibitors) have been isolated and purified to homogeneity form Catharanthus roseus by a simple two step procedure using ammonium sulphate fractionation and gelfiltration chromatography on Sephacryl- 100HR.The two inhibitors were named as CRCI and CRCII (Catharanthus roseus cystatin I and II). CRCI and CRCII were purified with a fold purification of 1333.3, 1348.5 and percent yield of 18.18 and 16.35% respectively. The molecular weight of purified phytocystatins were 19.1 kDa and 16.9 kDa respectively, as determined by SDS-PAGE and mass spectrometry. Effect of denaturants like ureaon CRCI and II was analysed by Fluorescence spectroscopy. Results suggest an unfolding of CRCI and II. FTIR results show that structurally CRCI is different from CRCII. Hydrophobic interactions are observed over a longer timescale (5 - 150 min). Furthermore, fluorescence spectroscopy results show quenching of fluorescence intensity of CRC I and II, although to different extent, due to perturbations of the environment of aromatic residues in the protein. Both the cystatins showed strong inhibitory/antibacterial activity against E. coli and S. aureus.
1. INTRODUCTION
Catharanthus roseus (Madagascar Periwinkle) is a species of Catharanthus native and endemic to Madagascar. English names occasionally used include Cape Periwinkle, Rose Periwinkle, Rosy Periwinkle, and “Oldmaid” wild, it is an endangered plant; the main cause of decline is habitat destruction by slash and burn agriculture [1].
The cystatins upper family includes three families. type I cystatin (the stefins), type II cystatins (class II cystatins) and type III cystatins (the kininogens) [2]. Type I cystatins are single chain proteins with molecular weight of 11,000 and contain no disulfide bonds or carbohydrate content. Type II cystatins contain single chain proteins with two disulfide bonds towards the carboxyl terminus, with a molecular weight of 13,000 [2,3]. The third cystatin family, the kininogens are large molecules with three type II like domains and bound carbohydrates. During last two decades, a fourth group belonging to cystatinsuper family has emerged, that is the plant cystatin specifically name as phytocystatins [4]. Homology searches show that some plant cystatins resemble family II cystatins of animal origin while others resemble family I cystatins of the mammalian system.
Phytocystatins, have been identified and studied in many plant sources such as rice [5], maize [6], soyabean [7], cowpea potato, Chinese cabbage [8] and carrot [9]. Thiol protease inhibitors present in the plant system perform a variety of functions, their regulation is performed by phytocystatins. They are important in a variety of ways, including their role in storage proteins [10], as regulators of endogenous proteolytic activity [11] and as participants in the mechanism of programmed plant cell death [12]. Apoptosis has been implicated in several plant processes such as xylogenesis, some forms of senescence and in the pathogens attack response [13]. Furthermore, proteinase inhibitors are expressed in abiotic stress [14] and in plant defense processes against insect attacks [15]. Phytocystatins present in cereal seeds like rice and maize have been used to prevent certain types of cancer [16].
Rice contains three species of cysteine proteinase inhibitors. Ozα, Ozβ and Ozλ which play a role inmaturation of storage proteins like glutenin, rice seed ripening and proteolysis of storage proteins during germination [17]. Furthermore, purified sugercane cystatin is found to inhibit the growth of filamentous fungus Trichoderma reesi [18], which is a potential biotechnological application of phytocystatins.
Most of the studies give details of cystatins purified from mammalian system and very few studies shed light on cystatins purified from plant sources, moreover, lacuna exist in the characterization of cystatins from Catharanthus roseus. Therefore the aim of the study was to have an indepth knowledge about this cystatin. This study reports the purification and some characteristic sofa phytocystatin purified from this new source, Catharanthus roseus. The purified phytocystatin has been Characterized on the basis of molecular weight and the secondary structure analysis by FTIR and fluorescence spectroscopy.
2. EXPERIMENTAL
2.1. Materials
All the materials used in the study were of analytical grade.
2.2. Methods
2.2.1. Purification of CRC I and II
CRCI and II were purified by the modification of the method of Juwen and Haard (Juwen and Haard, 2000). 100 gms. of CRC seeds were soaked in 25 mM sodium phosphate buffer (pH 7.0) containing 0.15 M sodium chloride. This preparation was kept overnight at 4˚C. Seeds were homogenized and subjected to centrifugation in a Sigma cooling centrifuge (Japan) at 8000 rpm for 20 min. at 4˚C. Supernatent was then collected and saturated with ammonium sulfate to a final concentration of 40%, this solution was kept for 4 hr and centrifuged at 10,000 rpm for 15min at 4˚C, the supernatent thus obtained was made 70% saturated with ammonium sulfate. After 4 hr, the pellet was recovered by centrifugation and dissolved in 10 ml. of 25 mM sodium phosphate buffer (pH 7.0). This pellet was extensively dialysed against several changes of the same buffer to remove ammonium sulfate. Dialysed sample was then loaded on a sephacryl S-100 gel filtration column. Five mililitre fractions were collected and assayed for cystatin inhibitory activity and protein concentration.
2.2.2. Mass Spectrometry of CRCI and II: Matrix Assisted Laser Desorption Time of Flight Mass Spectrometry (MALDI-TOF Analysis)
To further check the purity, cystatin 1 and 2 were again loaded on sephacryl S-100 gel filtration column and it was eluted with sodium phosphate buffer (25 mM, pH 7.0). Samples were then freeze dried, desalted and prepared for analysis on a Voyager Bioworkstation (Perspective Biosystems). The samples were dissolved in 1.0% trifluoroacetic acid and the matrix sinapinic acid (asaturated solution dissolved in acetonitrile: 0.1% TFA, 1:1, V/V, Sigma chemicals) was added. This preparation was then vortexed and 1.2 ml (1 mg/ml) of each cystatin 1 and 2 was applied on the sample plate. The spectrophotometer equipped with delayed extraction system accessory, was operated in a linear mode. Sample ions were evaporated using a N2 laser at 330 nm wave length and accelerated at a potential of 20 Kv with a delay of 134 ns. Around150 shots of 3 ns pulse width laser light were required to ionize the sample. Finally, the signal was digitize data rate of 480 MHz and averaged data was presented to the data system for correction.
2.2.3. Fourier Transform Infrared Spectroscopy
Infrared spectroscopy was done to see the secondary structure components present in CRCI and II. The spectra was truncated between 1740 and 1520 cm−1 and baseline corrected. The equipment used was NICOLET (ESP) 560 spectrophotometer (USA) equipped with a transmission OMNIC ESP 5.1 software and a DTGS detector, data was analysed and quantitated using Grams 32 software. CRC solutions (0.15 mg/ml) were prepared in sodium phosphate buffer (25 mM, pH 7.0) and original spectra of native CRCI and II were taken with a resolution of 4 cm−1 and 128 scans.
2.2.4. Antibacterial Activity of CRC I and II
The antibacterial activity of CRCI andII was checked by determination of inhibition zone diameter. Different bacterial strains (Staphylococcus aureus, Escherichia coli, Bacillus subtilis) were allowed to grow overnight in nutrient broth at 37˚C, then 0.3 ml of the fresh culture was overlayed with soft agar on nutrient agar plates to aid the formation of bacterial lawns.
Whatman filter discs were placed in 25 μg/ml, 50 μg/ml and 1 mg/ml of the inhibitor to be absorbed on discs. After 2 hr discs were placed on the bacterial lawn and the inhibition zone diameter was determined.
3. RESULTS AND DISCUSSION
3.1. Purification of the Inhibitor
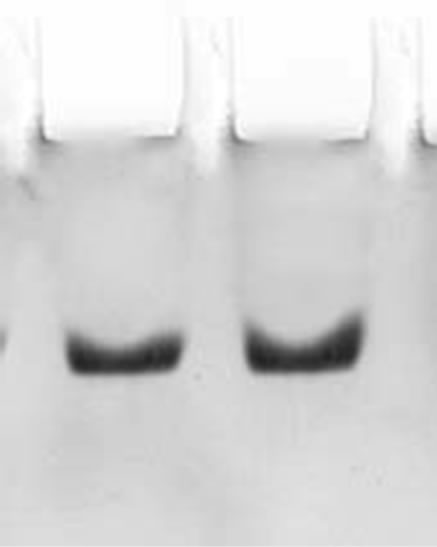
The two step procedures described in the present study for the purification of CRC I and II from Catharanthus roseus is simple and convenient than that reported earlier for other cystatins [19,20]. The precipitate obtained between 40% - 70% saturation was retained and subsequently chromatographed on S-100 gel filtration column. The retained protein was obtained as two peaks, peak I and II, the papain inhibiting fractions with the highest inhibitory activity in both the peaks were pooled and checked for their homogeneity. Pooled fractions of both the peaks when subjected to native PAGE, yielded single bands, thus the preparation was homogenous on the basis of charge as shown in Figure 1(a) (lane a and b). The subunit structure of CRCI and II was assessed by SDSPAGE under reducing (in the presence of β-mercaptoethanol) as well as non-reducing conditions (in the absence of β-mercaptoethanol), which also yielded a single band with an apparent molecular mass of 19.1 and 16.9 kDa for CRCI and II respectively, as shown in Figures 1(b) and (c). Figure 1(b) shows CRCI and II reduced with β-mercaptoethanol, lane a shows the marker proteins while lane b and c contain CRC II and I respectively. In a similar way Figure 1(c) shows the migration of CRC I and II in non-reducing conditions (in the absence of β-mercaptoethanol). The molecular weights of CRC I and II was also estimated from their relative mobilities in SDS-PAGE, CRC I and II when denatured and reduced with β-mercaptoethanol gave molecular mass of 17.72 and 14.7 kDa respectively (Figure not shown). Figure 2 shows the activity profile and protein content of CRC I (peak I) and CRC II (peak II) respectively, as obtained from gel filtration chromatography on S-100HR column, both the peaks showed approximately 90% inhibitory activity. The yield and fold purification was higher than that reported by Wu and Haard (2000) for phytocystatin isolated from methyl jasmonate treated tomato plants. It has also been reported that most of the phytocystatins are 6 - 12 kDa in size and contain no disulfide bonds (Barrett, 1987). The purified inhibitor is placed in the family of type IV cystatins (phytocystatins) and is found to possess properties of both type I and type II cystatins of the mammalian system.
3.1.1. MALDI-TOF Analysis
MALDI-TOF analysis is one of the most recent and sophisticated techniques through which accurate molecular weights can be determined easily in a short time period. Molecular weight of cystatin as analysed by mass spectrometry is found to be 19124.36 and 17510.22 daltons forcystatin 1 and 2 respectively which is very similar to that determined by SDS-PAGE (under reducing condition). It has been reported that molecular mass of type I and II animal cystatins are in the range of most of the phytocystatins (Fernandes et al., 1993; Kondo et al., 1990). A cysteine proteinase inhibitor isolated from the fruit bodies of Clitocypenebularis has been reported with a molecular mass of 17 kDa [21]. Figures 3(a) and (b) show the molecular weight determination by Mass Spectrometry. Figure 3(a) shows the MALDI-TOF analysis of CRCI while Figure 3(b) represents CRC II. Results are indicative of the fact that both CRC I and II are devoid of any subunit structure.
3.1.2. Fourier Transform Infrared Spectroscopy
FTIR spectroscopy was done to analyse the secondary structure of CRCI and II. In the IR spectra of proteins, the secondary structure is most clearly reflected by the amide I and amide II bands, particularly the former [22- 24], the amide I band absorbs at 1657 cm–1 (mainly C=O stretch) and the amide II band absorbs at 1542 cm–1 (C-N stretching coupled with N-H bending modes). It has also been reported that, for a native protein, the amide I component for the alpha helical structure locates at 1656 ± 2 cm–1, the band components for beta sheet structure should
 (a)
(a) 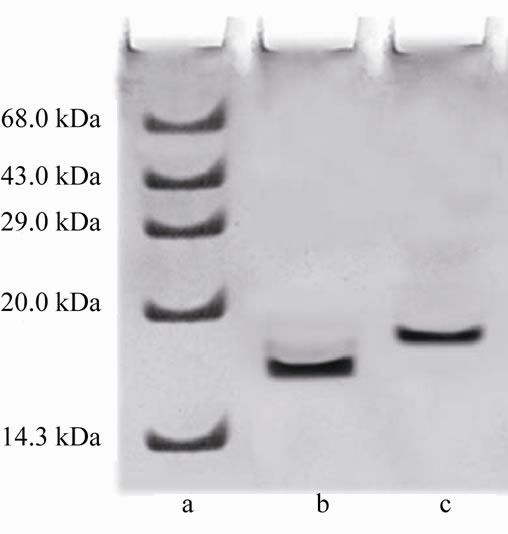 (b)
(b) 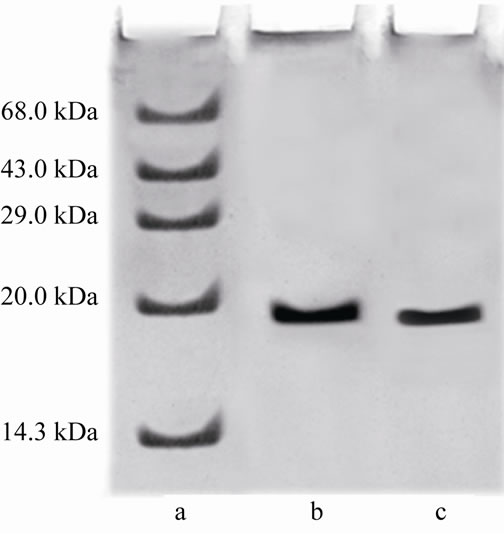 (c)
(c)
Figure 1. (a) shows the native PAGE of CRC I and II. Lane a and b represent CRC-I and II respectively; (b) shows the SDS PAGE of CRC-I and II under reducing conditions (in the presence of β-mercaptoethanol); (c) shows the SDS PAGE under non reducing conditions (in the absence of β-mercaptoethanol). In (b) and (c), Lane a shows the molecular mass of marker proteins, Lane b is CRC-II and Lane c is CRC-I.
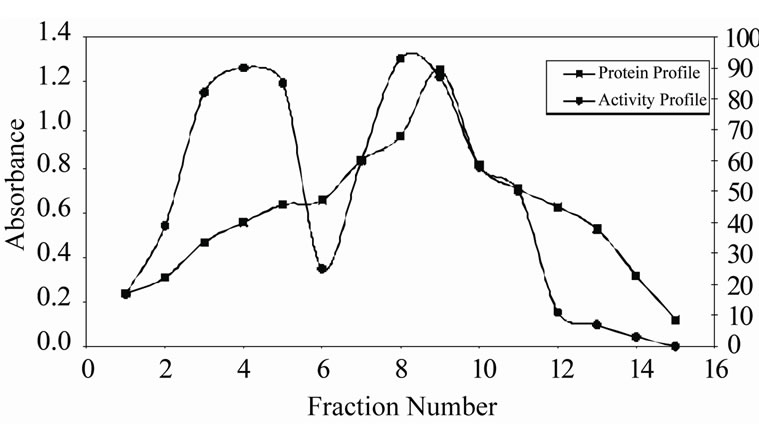
Figure 2. Gel filtration of CRC I and II on sephacryl S-100 HR (1.2 × 80 cm). The precipitate obtained from ammonium sulfate saturation (40% - 70%) was extensively dialyzed and subjected to gel filtration at a flow rate of 20 ml/hr. Fractions of 5 ml were collected and were monitored for protein concentration and papain inhibitiory activity using 2% casein as a substrate.
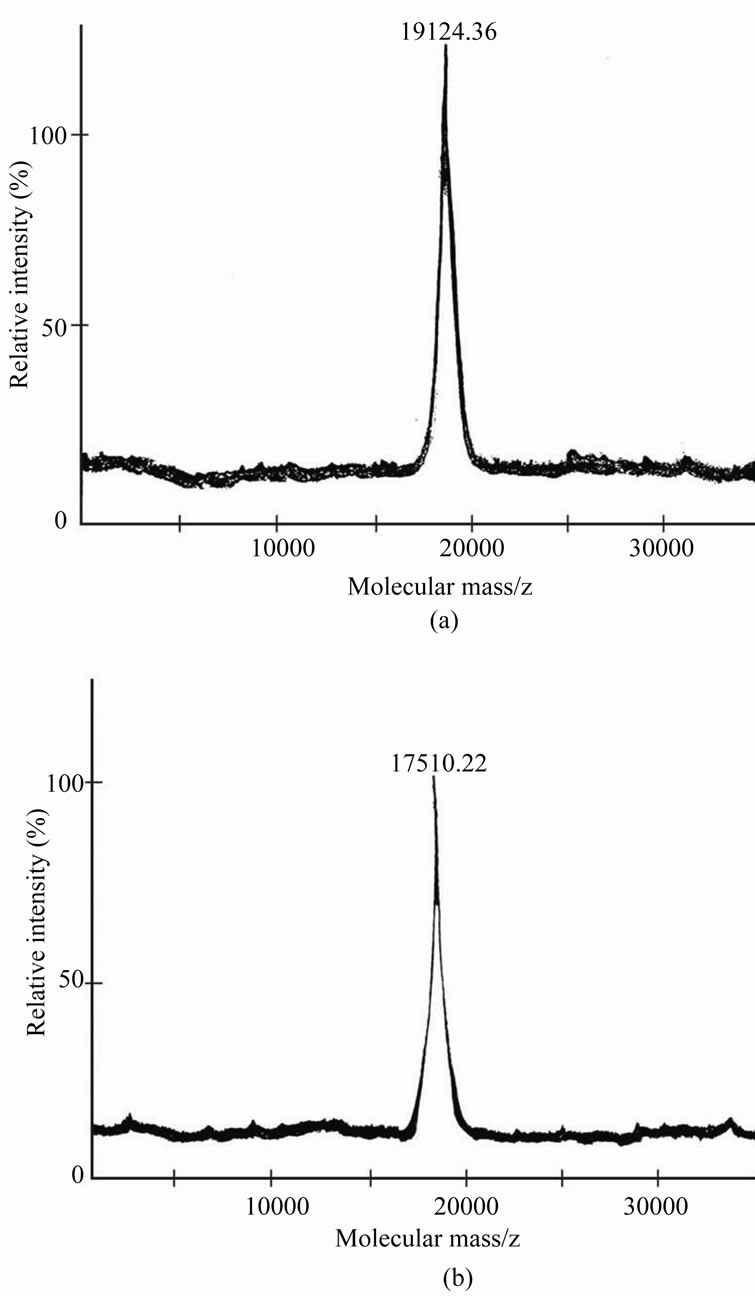
Figure 3. (a) and (b) show the molecular weights determined by mass spectrometry (MALDI-TOF). (a) represents CRC-I and (b) represents CRC-II. (Experimental details are given in methods).
locate between 1622 and 1642 cm–1 (lower wave number beta sheet bands) and between 1690 and 1698 cm–1 (higher wave number beta sheet bands) [22-25]. Figure 4 shows the original spectra of native CRC I and II. Quantitative analysis of the protein secondary structure for native CRC I and II is given in Table 1. CRC I contained the major α-helix 58%, β-sheet 27%, turn structure 8%, and β anti-parallel 7%. CRC II contained α-helix 56.5%, β-sheet 29%, turn structure 6.7%, and β-anti-paralle l7.8%.
Thus the results summarized in Figure 4 and Table 1 show the difference in the secondary structure of both the cystatins which is also evident by the peak intensities of both the proteins. The peak intensity for CRCI was recorded at 1646.22 while for CRC II it was recorded to be 1652.01. FTIR result simplicate that although CRC I and II contain α-helix as the predominant structure but CRC II showed a 1.5% decrease in alpha helical content as compared to CRC I, while there was 2% increase in β-sheet and 0.8% increase in β-anti parallel structure in CRC II as compared to CRC I, while, the turn structure in CRC II showed only a 0.2% decrease.
3.1.3. Antibacterial Activity of CRC I and II
The antibacterial effect was classified as (+), +, ++, or +++ based upon the inhibition zone diameter of 11 - 12 mm, 13 - 14 mm, 15 - 16 mm, 17 mm and more than 17 mm respectively. Results illustrated in Table 2 show that CRC I and II show strong antibacterial activity against E. coli and S. aureus which is a striking observation, while they were found to be ineffective against B. subtilis. Antifungal activity of thiol protease inhibitors like sugarcane cystatin has also been reported in earlier studies [18].
These results suggest that discovery of CRC I and II should broaden the spectrum of specific cysteine proteinase inhibitors available for potential use in human
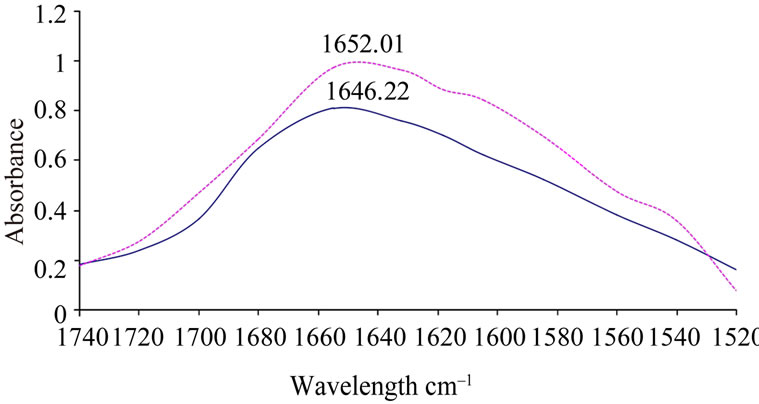
Figure 4. FTIR spectra of native (untreated) CRC I and II (0.15 mg/ml) prepared in sodium phosphate buffer (25 mM, pH 7.0), original spectra of native CRC-I and II were taken with a resolution of 4 cm–1 and 128 scans. Average of three scans was taken. Solid line represents CRC-I and dotted line represents CRC-II.
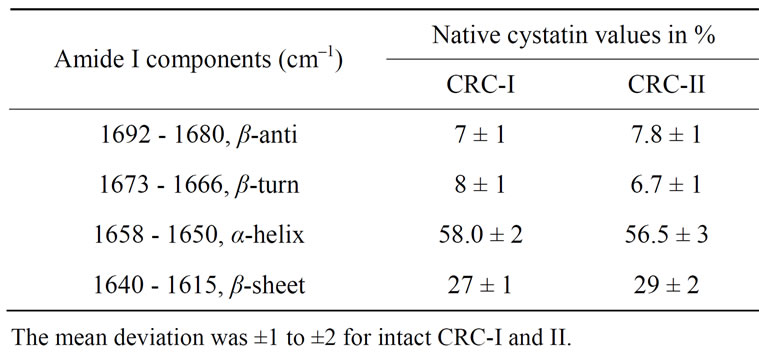
Table 1. Secondary structure determination of native CRC-I and II, as analysed by fourier transform infrared spectroscopy.
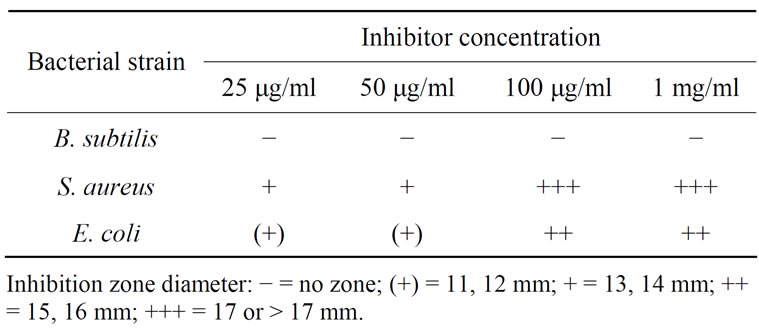
Table 2. Antibacterial effects of CRC-I and II.
medicine and in agricultural crop protection. Furthermore, a detailed conformational analysis of phytocystatins must await the results of X-ray crystallographic studies of phytocystatin-urea complex.
4. CONCLUSIONS
C. roseus species has long been cultivated for herbal medicine and a sanorna mental plant. In traditional Chinese medicine, extracts from it have been used to treat numerous diseases, including diabetes, malaria and Hodkin’s disease. The substances vinlastine and vincrisine extracted from the plant are used in the treatment of leukemia. This conflict between historical indigenous use, and recent patents on C. roseus-derived drugs by western pharmaceutical companies, without compensation, has led to accusations of biopiracy [26]. C. roseusis also used in plant pathology as an experimental host for phytoplasmas, In conclusionon the basis of these findings, it can be suggested that cystatins are abundantly present intubers, latex [27] and seeds [28] of plants, thus any sort of cystatin damage or imbalance of protease and antiprotease has also been associated to diseases like cancer, Alzheimer’s and rheumatoid arthritis [29,30], therefore keeping in view the ethnobotanical importance of Catharan thus as significant indigenous biomedicine cystatins and their presence in mammalian system and plant kingdom, the study may be used as a model system tounder stand the interaction of plant and mammalian cystatins which may further help biotechnologists and ethno biologists in elucidating the mechanism of action of C. roseus in therapeutic, diagnostic, disease treatment and other significant medicinal applications.
5. ACKNOWLEDGEMENTS
Facilities provided by IIT Delhi and DRS operative in the department of Biochemistry are thankfully acknowledged. Academic support provided by Dr. Mukesh Kumar is thankfully acknowledged.
REFERENCES
- Huxley, A. (1992) New RHS Dictionary of Gardening. Macmillan, Oxford.
- Machleidt, W., Sasaki, M. and Turk, V. (1986) Nomenclature and classification of the proteins homologous with the cysteine proteinase inhibitor chicken cystatin. Biochemical Journal, 236, 312.
- Ritonja, A., Machleidt, W. and Barrett, A.J. (1985) Amino acid sequence of the intracellular cysteine proteinase inhibitor cystatin B from human liver. Biochemical and Biophysical Research Communications, 131, 187-192. doi:10.1016/0006-291X(85)90216-5
- Brown, W.M. and Dziegelewska, K.M. (1997) Friends and relations of the cystatin super family-new members and their evolution. Protein Science, 6, 5-12. doi:10.1002/pro.5560060102
- Abe, K., Emori, Y., Kondo, H.,Susuki, K. and Arai, S. (1987) Molecular cloningofa cysteine proteinase inhibitor of rice (oryzacystatin). Homology with animal cystatins and transient expression in the ripening process of rice seeds. Journal of Biological Chemistry, 262, 16793.
- Abe, M., Abe, K., Iwabuchi, K., Domoto, C. and Arai, S. (1994) CorncystatinI expressed in Escherichiacoli: Investigation of its inhibitory profile and occurrence in corn kernels. Journal of Biochemistry, 116, 488.
- Misaka, T., Kuroda, M., Iwabuchi, K., Abe, K. and Arai, S. (1996) Soyabean cystatin,a novel cysteine proteinase inhibitor in soyabean, is distinct in protein structure and gene organization from other cystatins of animal origin and plant origin. European Journal of Biochemistry, 240, 609. doi:10.1111/j.1432-1033.1996.0609h.x
- Lim, C.H., Lee, S.I., Chung, W.S., Park, S.H., Hwang, I. and Cho, M.J. (1996) Characterization of a cDNA encoding cysteine proteinase inhibitor from Chinese cabbage (Brassica campestris L.ssp. pekinensis) flowerbuds. Plant Molecular Biology, 30, 373. doi:10.1007/BF00020124
- Ojima, A., Shiota, H., Higashi, K., Kamada, H., Shimada, Y.I., Wadamasata, P. and Satoh, S. (1997) Anextracellular in soluble inhibitor of cysteine proteinases in cell cultures And seeds of carrot. Plant Molecular Biology, 34, 99. doi:10.1023/A:1005842719374
- Xavier-Filho, J. (1992) Sementes e suasdefesas contra insetos. Projeto Multinacional de Biotecnologia e Alimentos. Organizacao dos Estados Americana, 1, 31
- Ryan, C.A. (1989) Proteinase inhibitor gene families: Strategies for transformation to improve plant defenses against herbivores. Bioessays, 10, 20. doi:10.1002/bies.950100106
- Solommon, M., Belenghi, B., Delledonne, M., Menachen, E. and Levine, A. (1999) The involvement of cysteine proteinase and proteinases inhibitor genes in the regulation of programmed cell death inplants. Plant Cell, 11, 431.
- Ceros, M. and Carbonell, J. (1993) Purification and characterization of thiol-protease induced during sense cence of unpollinated ovaries of Pisum sativum. Physiology Plant, 88, 267-274. doi:10.1111/j.1399-3054.1993.tb05498.x
- Franco, O.L. and Melo, F.R. (2000) Osmoprotectants-A plant strategy in response to osmotic stress Russ. Journal of Plant Physiology, 47, 137-144.
- Ryan, C.A. (1990) Protease inhibitors in plants: Genes for improving defenses against insects and pathogens. Annual Review of Phytopathology, 28, 425. doi:10.1146/annurev.py.28.090190.002233
- Schelp, F.P. and Pongpaew, P. (1988) Protection against cancer through nutritionally induced increase of endogenous thiol proteinases inhibitor-a hypothesis. International Journal of Epidemiology, 17, 287-292. doi:10.1093/ije/17.2.287
- Abe, K., Kondo, H., Watanabe, H., Emori, Y. and Arai, S. (1991) Oryzacystatins as the first well-defined cystatins of plant origin and their target proteinases in rice seeds. Biomedica Biochimica Acta, 50, 637-641.
- Soares-Costa, A., Beltramini, L.M., Theimann, O.H. and Henrique-Silva, F. (2002) A sugarcane cystatin: Recombinant expression, purification andantifungal activity. Biochemical and Biophysical Research Communications, 296, 1194-1199. doi:10.1016/S0006-291X(02)02046-6
- Pernas, M., Sanchez-Monge, R., Gomez, L. and Salcedo, G. (1998) Antifungal activity of a Plant Cystatin. Plant Molecular Biology, 38, 1235-1242. doi:10.1023/A:1006154829118
- Ryan, S.N., McManus, M.T. and Laing, W.A. (2003) Identification and characterisation of proteinase inhibitors and their genes from seeds of apple (Malus domestica). Journal of Biochemistry, 134, 31-42. doi:10.1093/jb/mvg110
- Brzin, J., Rogelj, B., Popovic, T., Strukelj, B. and Ritonja, A. (2000) Clitocypin, a new type of cysteine proteinase inhibitor from fruit bodies of mushroom. Clitocybe nebularis. Journal of Biological Chemistry, 275, 20104-20109. doi:10.1074/jbc.M001392200
- Elliot, A. and Ambrose, E.J. (1950) Spectroscopy in the 3 region of the infrared spectrum. Nature, 165, 921- 922.
- Timasheff, S.N., Susi, H. and Stevens, L. (1967) Infrared spectra and protein confirmation in aqueous solutions. Journal of Biological Chemistry, 242, 5467-5473.
- Ruegg, M., Metzger, V. and Susi, H. (1975) Computer analysis of characteristic infrared of globular proteins. Biopolymers, 14, 1465-1471. doi:10.1002/bip.1975.360140712
- Miyazawa, T. and Blout, E.R. (1961) The infrared spectra of polypeptides invarious conformations: Amide I and Amide II bands. Journal of the American Chemical Society, 83, 712-719. doi:10.1021/ja01464a042
- Karasov, C. (2001) Who reaps the benefits of biodiversity? Environmental Health Perspectives, 109, A582- A587. doi:10.1289/ehp.109-a582
- Nallamsetty, S., Kundu, S. and Jagannadham, M.V. (2003) Purification and biochemical characterization of a high lyactive cysteine protease ervatamin a from the latex of Ervatamia coronaria. Journal of Protein Chemistry, 22, 1-12. doi:10.1023/A:1023047309023
- Oliveira, A.S., Pereira, R.A., Lima, L.M., Morais, A.H.A., Melo, F.R., Franco, O.L., Bloch Jr., C., Grossi-de-sa, M.F. and Sales M.P. (2002) Activity toward Bruchid pest of a Kunitz type inhibitor from seeds of the Algaroba tree (Prosopisjuliflora D.C.). Pesticide Biochemistry and Physiology, 72, 122-132. doi:10.1006/pest.2001.2591
- Deng, A., Irizarry, M., Nitsch, R.M., Growdon, J.H. and Rebeck, G.W. (2000) Elevation of cystatin C in susceptible neurons in Alzheimer’s disease. American Journal of Pathology, 159, 1061-1068.
- Trabandt, A., Gay, R.E., Fasbender, H.G. and Gay, S. (1991) Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum, 34, 1444. doi:10.1002/art.1780341116

