Green and Sustainable Chemistry
Vol.3 No.2(2013), Article ID:31285,6 pages DOI:10.4236/gsc.2013.32007
Ag, Hg and Cr Precipitation for Recycling Derived of Hazardous Liquid Waste
1Research Department, Universidad Popular Autónoma del Estado de Puebla (UPAEP), Puebla, México
2Technological Research División, Instituto Nacional de Investigaciones Nucleares, Ocoyoacac, México
3Division of Graduate Studies and Research, Instituto Tecnológico de Toluca, Toluca, México
4Chemical Engineering Faculty, Benemérita Universidad Autónoma de Puebla, Puebla, México
Email: *silviacristina.gutierrez@upaep.edu.mx
Copyright © 2013 Silvia C. Gutiérrez-Gutiérrez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 11, 2013; revised March 12, 2013; accepted March 20, 2013
Keywords: Hazardous Liquid Waste; Treatment for Recycling; Silver; Mercury; Chromium
ABSTRACT
Production of different compounds can generate large amounts of hazardous wastes which are dangerous to the environment and human health. The disposal or treatment of hazardous liquid waste rich in heavy metals like silver (Ag), mercury (Hg) and chromium (Cr) is difficult due to the strong acidity and toxicity which usually present in these contaminants. For this study, several research works were reviewed in order to obtain an efficient and viable treatment in time and removal efficiency. A series of chemical precipitations were evaluated for efficiency in the reduction of heavy metals in liquid waste. The precipitation of all three metals lasted 30 minutes and after treatment the wastewater presented concentrations of 0.064 mg·L−1 Ag, 0.010 mg·L−1 Hg and 0.048 mg·L−1 Cr, with a standard pH (7.5 - 8.5); with removal efficiencies of 94.31% for Ag, 99.99% for Hg and 98.17% for Cr.
1. Introduction
Progress in the chemical industry has allowed the development of a wide variety of substances serving as products or raw materials for many goods consumed worldwide.
While these advances have significantly improved the standard of living for many people, they unfortunately exert an important pressure on human health and the environment [1]{}. The use of chemical substances and dangerous materials in industrial processes or in domestic applications can generate hazardous waste.
Hazardous waste is dangerous or potentially harmful to human and animal health or the environment, and can come in many forms: liquids, solids, gases, or sludges [2]. Hazardous waste can also be defined to possess one or more of the following characteristics: corrosivity, reactivity, explosivity, toxicity, flammability, or containing infectious agents [3]. Therefore, when a residue qualifies as a hazardous waste it must be safely handled and disposed. This can be achieved in different ways: minimization of their generation by recycling and reusing prior to treatment and disposal, treatment which reduces their danger and adequate confinement [1].
An important class of hazardous wastes is those which contain heavy metals. The term “heavy metal” has no strict scientific or chemical definition but it is generally accepted that these metals have a specific gravity more than about 5.0 which are considered as priority pollutants [4].
However, contact with a heavy metal does not necessarily mean that adverse health effects will result in. Health effects depend upon the amount, form and its method of exposure.
Silver (Ag) can be found as hazardous waste in the forms of AgNO3, AgCl, Ag2S and Ag2O mixed with soil or water. Ag is used to make jewelry, silverware, electronic equipment, and dental fillings, and photographers use AgNO3 or AgCl to reveal photographs. These materials are the major source of Ag that is released into the environment [5].
Associated health effects with Ag exposure are argyria, argyrosis, abdominal pain, respiratory irrigation, allergic response and others [6], while in animals it can cause brain damage, abnormal heart size and damage to reproductive tissues [5].
Mercury (Hg) is a metal element that occurs naturally in the environment where we can find it in compounds such as Hg2Cl2, HgCl2, Hg(O2CCH3)2, HgSO4, CH3HgCl, (CH3)2Hg and C8H8HgO2 [7]. Common Hg emissions are due to the burning of fossil fuels, smelting metal ores, Hg mining, industry, waste incinerators and crematoriums [8].
Some health effects from Hg toxicity are neurological damage, irritability, paralysis, blindness, insanity, chromosome breakage and birth defects [7].
Chromium (Cr) is another important heavy metal that is used for chrome plating, dyes and pigments, leather tanning in the form of Cr2(SO4)3, and wood preserving in the form of Cu2Cr2O7. The highest potential exposure occurs in the metallurgy and tanning industries, where workers may be exposed to high concentrations in air. Cr is known to be a human carcinogen and also can affect the immune, urinary and respiratory systems [9].
The purpose of this study was to test treatment protocols to easily, efficiently, and rapidly reduce the quantity of heavy metals, specifically Ag, Hg and Cr, present in industrial hazardous liquid waste down to levels define as safe by Mexican law. This was accomplished through a series of chemical precipitations that were tested in order to obtain an effluent without hazardous characteristics that would harm the environment or human/animal health, and could be easily disposed.
2. Experimental
Several research and methodologies that offer a treatment for Ag, Hg or Cr were reviewed in order to formulate an improved method that would treat this hazardous waste.
Theexperiments were conducted with a sample obtained from the mixture of the close-reflux COD (Chemical Oxygen Demand) waste and industry waste-water rich in heavy metals, both from the Green Chemistry laboratory at the Universidad Popular Autónoma del Estado de Puebla.
A 1 L sample was tested for baseline measurements. The analysis of the sample was performed by atomic absorption spectrophotometry and showed concentrations of 1.12 mg·L−1 Ag, 5171 mg·L−1 Hg and 2.72 mg·L−1 Cr. The pH values were below 0.01 at room temperature and pressure conditions.
Prior to the experiments, the wastewater sample was filtered to eliminate suspended solids that could interfere with the reactions. An apparatus for vacuum filtration was installed using a glass Büchner funnel (Kimble & Chase, no. 284003503, fine porosity) with glass microfiber filter paper (Whatman, 934-AH 24 mmÆ, pore size 1.5µm) and a Kitasato flask. An evaporation of the 10% of the volume of the liquid was performed to concentrate the sample.
Due to the metals contained in the wastewater, the selected precipitation reactions were performed in the following order to increase the selectivity of the reactions and avoid interferences or unwanted reactions: Ag, Hg, and Cr [10].
2.1. Ag Precipitation, AgCl
2.00 g·L−1 of NaCl (J.T Baker CAS: 7647-14-5, 99.9% purity) was added to the sample, keeping it in constant agitation for 10 minutes using a 1.5 cm magnetic stirrer and a stirring hot plate (Thermolyne model SP131325) at medium speed. Two consecutive precipitations were required to precipitate the high quantity of Ag in the waste. The solution was filtered in vacuum using a Gooch crucible and glass microfiber filter paper (1.5 µm).
Forreactions 1 to 6 reported in this paper, a parenthesis nomenclature is used to indicate the reaction between different compounds with the wastewater and others assistant reactants. Reaction 1 describes the Ag precipitation into AgCl.
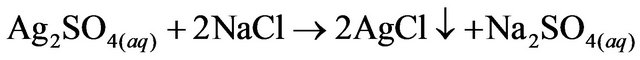 (1)
(1)
2.2. Hg Precipitation, HgS
A sulphidation device was installed in a fume hood to avoid accidental exposure to the gases produced in the reaction.The remaining liquid from the previous cyclewas placed in a flask in a water bath with constant stirring using the Thermolyne plate. A second flask was set with 50 g of FeS (Golden Bell no. 28610, 99.9% purity) and 300 mL of HCl (37.3%, J.T Baker CAS: 764701-0). The two flasks were connected to a scrubber system, which was filled with distilled water to 1/3 of the volume.
The solutions were left in contact for 15 minutes and the solution from the first flask was processed with vacuum filtration as described for the AgCl precipitate. The precipitation steps are shown in Reactions 2, 3, and 4.
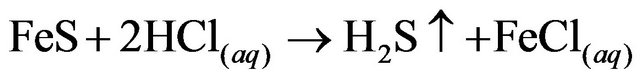 (2)
(2)
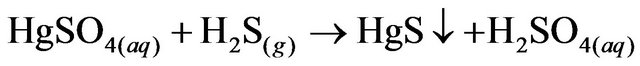 (3)
(3)
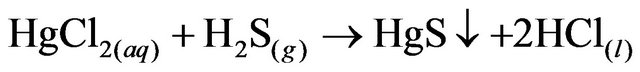 (4)
(4)
2.3. Cr Precipitation, Cr(OH)3
The samplewas diluted to a concentration of 1:2 with distilled water. To increase the alkalinity, 30 mg·L−1 of Na3PO4 (Golden Bell no. 26497, 99.9% purity) was added to the solution remaining from the Hg precipitate process. The pH of this mixture was adjusted dropwise with a 50% solution of NaOH in distilled water (pellets, J.T Baker CAS: 1310-73-2, 98.4% purity) to obtain a pH 8.5 ± 0.5, the optimum value for the formation of Cr(OH)3.
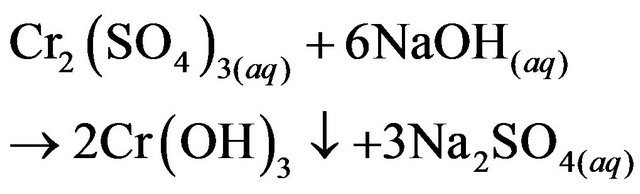 (5)
(5)
The solution was centrifuged (IEC HN SII Centrifuge, Thermo Electron) for 60 minutes at 7500 rpm to separate the phases. The solid phase form was stabilized with H2SO4 (J.T Baker CAS: 7664-93-0, 97.9% purity), to obtain Cr2(SO4)3.
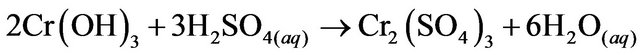 (6)
(6)
2.4. Assays
Metal assays for all runs were performed by atomic absorption (AA) spectrophotometry according to the Mexican standard NOM-117-SSA1-1994 [11], which establishes the method for determining metals by atomic absorption spectrometry. The tests were performed in a certified laboratory by the Mexican Accreditation Entity, where five replicates were carried out to obtain an accurate and precise value with a maximum 5% deviation.
The degree of purity of the precipitated metal salts was determined by X-ray fluorescent (XRF) spectrometry. The fluorescent X-rays derived from the sample were detected with a solid-state lithium-drifted silicon detector of 20 mm2 front area and cooled with liquid nitrogen. The energy resolution of the Si(Li) detector was 140 eV for Mn Ka and its Be window was 8 µm thick (ItalStructures, 2000). The signal from the detector was processed in a counting chain (preamplifier, amplifier, analogue to digital converter) attached to the digital system. All samples were excited for 500 s.
With the purpose of confirming the chemical stability of the precipitated salts, a CRIT analysis was made in the National Institute of Nuclear Investigations (ININ from its acronym in Spanish); this procedure is composed of tests for corrosiveness, reactivity, flammability and toxicity (CRIT). CRIT analysis was performed under the Mexican standards NOM-052-SEMARNAT-2005 [12] and NOM-053-SEMARNAT-1993 [13] which establish the procedure to determinate if waste possesses any hazardous characteristics.
3. Results and Discussion
Table 1 shows a comparison of the heavy metal concentrations at the beginning and end of the treatment process with the contamination limits established in the relevant standards. The results indicate that the remaining liquid meets with the Mexican standards, NOM-001-SEMARNAT-1996 [14] and NOM-002-SEMARNAT-1996 [15], which establish the maximum permissible limits of pollutants in dischargesto sewer systems as well as in national waterways, in terms of pH and Ag, Hg and Cr concentrations.
These results (Table 1) indicates that the treatment process offers a removal efficiency of 94.31% for Ag, 99.99% for Hg and 98.17% for Cr, as well as acceptable pH, generating an effluent that would not need further treatment before being safely and legally dispose.
Comparison of the previous results shows that we obtained excellent outcomes precipitating over 94% of the metal all three metals tested. The duration of treatment for Ag, Hg and Cr was a total time of 30 minutes. Duration is an advantage over other treatments [17-19] that last more than 3 hours which made them not as economically viable for industries to apply. Table 2 shows a comparison among several different treatments for Ag, Hg and Cr.
Ag precipitation with NaCl was employed by Mañunga et al., Agudelo et al. and in this research, different quantities are used but NaCl is an excellent reagent to precipitate Ag in a short time with good removal efficiencies.
According to Mañunga et al. Hg precipitation is with 10 g·L−1 of FeS in constant agitation for 1h. Agudelo et al. propose Hg precipitation with NaOH with a contact time of 2 h while in the treatment procedure developed in this research it can be achieved with fewer reagents in 15
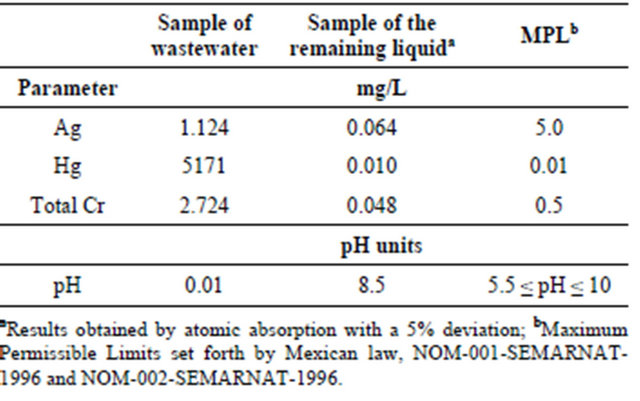
Table 1. Comparison between heavy metal concentrations at the beginning and end of the treatment process.
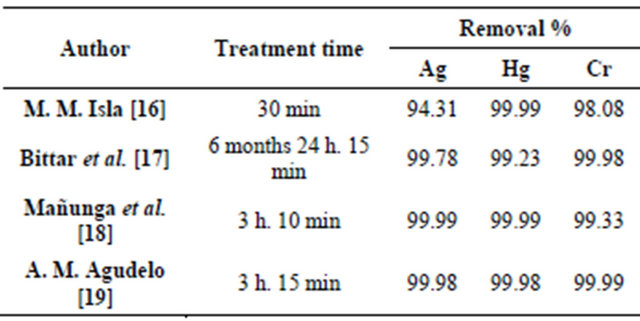
Table 2. Comparison of different treatments for the removal of Ag, Hg and Cr in liquids waste.
minutes. As presented in Reaction 3 in this paper, H2S is produced in order to Hg precipitate in the form of HgS. H2S is a flammable and colorless gas with a characteristic smell of rotten eggs and it is known as a pollutant that affects human health [20]. However, H2S contamination during the treatment does not occur because H2S is contained by the sulphidation device and is produced in necessary and sufficient quantities, according to stoichiometric calculation, to react with Hg. On a larger scale, further precise stoichiometric calculations will be required to secure the correct amount of H2S that is produce agreeing with the amount of Hg in waste. Security measures can also be introduce in case of a H2S leak by adapting a removal system of this gas, number of physicochemical and biological methods are available for remove H2S from gas streams [21,22].
For Cr precipitation, Mañungaet al. made a dilution of the sample but at a concentration 1:4 and used a 50% solution of NaOH after they reduced Cr6+ with glucose in 1h. In the method proposed in our current research an excellent removal percentage was reached with a lower dilution to maintain a proper concentration of Cr, so the reaction with the 50% solution of NaOH could precipitate as much Cr possible. No preview reduction of Cr6+ with glucose were needed, reduction of Cr6+ to Cr3+ occurred during Hg precipitation by using H2S [23].
To appreciate the efficiency of the precipitation method developed in this research, the salts we obtained were analyzed by X-ray fluorescence (XRF) spectrometry to determine their purity (Table 3).
The AgCl and HgS samples had a purity of 89.41% and 90.8%, respectively. The Cr(OH)3 precipitate obtained had a high concentration of iron, sulfur, chlorine and sodium, which were presumed to be unreacted precipitant reagents due to the presence of other pollutant substances in the wastewater sample that interfered with the efficiency of the reaction. The precipitated salts presented a chemical stability based on their corrosivity, reac-
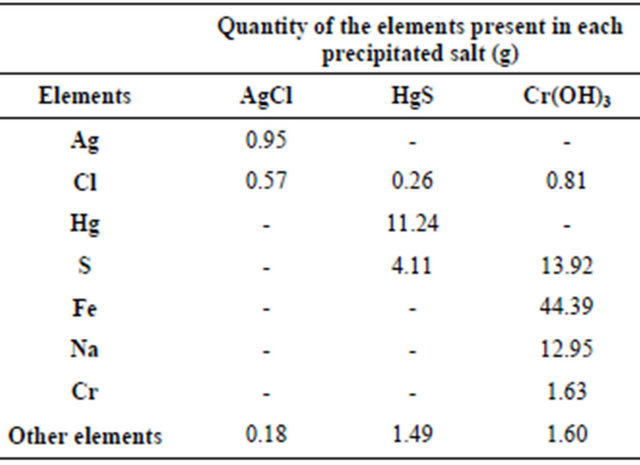
Table 3. Analysis results of the elements present in the precipitated solids of the reactions.
tivity, flammability and toxicity characteristics, which facilitates their management and storage or disposal.
For each liter of treated wastewater we obtained approximately 0.5, 2.5 and 150 cm3 of AgCl, HgS and Cr(OH)3 respectively, therefore the volume of hazardous substances to be managed was reduced by approximately 85%.
During the precipitation of the metals we observed several changes of color; this was an important indicator because it signaled that the reactions were satisfactorily completed. In the precipitation of Ag, there was a change of color from orange-amber to light orange and the formation of a white precipitate, from 0.00112 gthe recovery of Ag was 1.7 g as AgCl. In the Hg precipitation we appreciated color changes in the solution from green to orange ending with a blue-green hue. The generated solid changed from white to black, indicating the end of the reaction and the recovery of Hg from 5.171 g resulted in 19.0 g in the form of HgS. The precipitation of Cr presented a color change from blue-green to orange-brown and ended with very dark green hue. A dark green gelatinous solid precipitate was formed during the procedure; the recovery of Cr from 0.00272 g was 75.3 g as Cr(OH)3.
4. Conclusions
A simpler and economically-viable industrial wastewater treatment was achieved that removed heavy metal concentrations using common reactants, materials and equipment in a 30 minute sequential reaction. Ag, Hg and Cr present in wastewater were reduced to levels below those required by law [14,15] rendering safe discharge in sewers possible. A removal efficiency of 94.31% for Ag, 99.99% for Hg and 98.17% for Cr was achieved with a pH of 8.5. The effluent obtained from the treatment didn’t exhibit any significant danger to human health or the environment with respect to corrosivity, reactivity, explosivity, toxicity, flammability or infectious agents. The volume of substances that must be stored and/or disposed was reduced by approximately 85%, and their purity and stability also means they could be reused by other processes.
This treatment method is been scaled up to an industrial process in the laboratory of Sistemas de Ingeniería Ambiental (SIA) S.A. de C.V. The method will treat the hazardous liquid waste obtained from the determination of Chemical Oxygen Demand parameter in the municipal wastewater treatment plant of the city Puebla, Pue, Mexico. This method will be used on this industrial scale to test the removal of Ag, Hg and Cr as hazardous waste and whether the precipitates can be reused as raw material in other industrial process.
It is noteworthy that the method development can be applied to other types of liquid waste containing Ag, Hg or Cr, as each one of the stages are sequential, but independent. Several preliminary trials were performed in other wastewater which contained Ag, Hg and/or Cr in order to prove that this treatment can apply to industrial wastewater, and the preliminary results showed the expected precipitates. We believe the green chemistry potential of this process is great, and warrants investment in future study.
5. Acknowledgements
The authors thank the Research Department for financial support and Integrated Technology University Services of UPAEP for the technical work of the different characterization techniques. Also thank Isla-López, M for her contribution in this work with her graduate thesis and to SIA S.A. de C.V. enterprise for allowed us to use their laboratory.
REFERENCES
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “Chapter 7. Residues,” Report of the State of the Environment in Mexico, Mexico, 2009, pp. 326-357. http://www.semarnat.gob.mx/informacionambiental/Documents/pdf/cap_7_residuos.pdf
- US Environmental Protection Agency, “Hazardous Waste,” 2012. http://www.epa.gov/osw/hazard/
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “General Law for the Prevention and Management of Waste,” Federal Ecological Agenda, ISEF Editorial, Mexico, 2010.
- US: Environmental Protection Agency, “Priority Pollutants,” 2012. http://water.epa.gov/scitech/methods/cwa/pollutants.cfm
- Agency for Toxic Substances and Disease Registry (ATSDR), “Public Health Statement Silver,” Department of Health and Human Services, Atlanta, 1990.
- P. L. Drake and K. J. Hazelwood, “Exposure-Related Health Effects of Silver and Silver Compounds: A Review,” Oxford University Press, Vol. 9, No. 7, 2005, pp. 575-585.
- Agency for Toxic Substances and Disease Registry (ATSDR), “Public Health Statement Mercury,” Department of Health and Human Services, Atlanta, 1999. http://www.atsdr.cdc.gov/ToxProfiles/tp46-c1-b.pdf
- T. W. Clarkson, “Mercury: Major Issues in Environmental Health,” Environmental Health Perspectives, Vol. 100, 1993, pp. 31-38. doi:10.1289/ehp.9310031
- Agency for Toxic Substances and Disease (ATSDR), “Public Health Statement Chromium,” Department of Health and Human Services, Atlanta, 2012. http://www.atsdr.cdc.gov/ToxProfiles/tp7-c1-b.pdf
- R. L. Rangel, “Fundamentals of Analytical Chemistry,” 2nd Edition, Limusa, Mexico City, 1991, pp. 69-92.
- Ministry of Health (SSA in Spanish), “Test Method for the Determination of Cadmium, Arsenic, Lead, Tin, Copper, Iron, Zinc and Mercury in Food, Drinking Water and Purified Water by Atomic Absorption Spectrometry,” Official Journal of the Federation, Mexico, 1994. http://200.77.231.100/work/normas/noms/1995/117-ssa1.pdf
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “Establishes the Characteristics of Hazardous Waste and the Listing of Them and the Threshold above Which Make a Waste Hazardous Because Its Toxicity to the Environment,” Official Journal of the Federation, Mexico, 2005. http://www.aguascalientes.gob.mx/proespa/pdf/NOM-SEMARNAT-052%20RESIDUOS%20PELIGROSOS.pdf
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “Establishes the Procedure for Conducting the Extraction Test to Determine Constituents That Make a Waste Hazardous Because Its Toxicity to the Environment,” Official Journal of the Federation, Mexico, 1993. http://www.spabc.gob.mx/views/files/tmp/NOM-052.pdf
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “Establishes the Maximum Permissible Limits of Pollutants in Wastewater Discharges into National Waters,” Official Journal of the Federation, Mexico, 1996. http://www.hgm.salud.gob.mx/descargas/pdf/noticias/programa_mercurio/marco/norma_001.pdf%20
- Secretariat of Environment and Natural Resources (SEMARNAT in Spanish), “Establishes the Maximum Permissible Limits of Pollutants in Wastewater Discharges to Sewers,” Official Journal of the Federation, Mexico, 1996. http://www.aguascalientes.gob.mx/proespa/pdf/NOM-SEMA-NAT-002%20DESCARGA%20DE%20AGUAS%20RESIDUALES%20AL%20ALCANTARILLADO.pdf
- M. M. I. López, “Stabilization and Disposal of Liquid Waste from the Determination of Chemical Oxygen Demand (in Spanish),” Bachelor Thesis, UPAEP University, Mexico, 2012.
- M. Bittar, S. Llamas M. C. Quiroga and L. E. Ferrer, “Treatment and Disposal of Liquid Waste Generated in the Chemical Oxygen Demand (in Spanish),” 3rd Iberoamerican Waste Engineering Seminar, Castellón de la Plana, 2010.
- T. Mañunga, H. M. Gutiérrez, J. A. Rodríguez and A. VillarealDíaz, “COD Waste Treatment Generated in Environmental Laboratories Engineering and Research (in Spanish),” Redalyc, Vol. 30, No. 2, 2010, pp. 87-95.
- A. M. Agudelo, “Proposal for Treating Mercury, Chromium and Silver Metals Present in the Waste Generated by the Chemical Oxygen Demand,” Bachelor Thesis, Pereira University, Colombia, 2010.
- Agency for Toxic Substances and Disease (ATSDR), “Hydrogen Sulfide,” Department of Health and Human Services, Atlanta, 2006.
- T. J. Bandosz, “On the Adsorption/Oxidation of Hydrogen Sulfide on Activated Carbons at Ambient Temperatures,” Journal of Colloid and Interface Science, Vol. 246, No. 1, 2002, pp.1-20.
- S. M. Zicari, “Removal of Hydrogen Sulfide from Biogas Using Cow-Manure Compost,” Master Thesis, Cornell University, New York, 2003.
- C. Kim, Q. Zhou, B. Deng, E. C. Thornton and H. Xu, “Chromium(IV) Reduction by Hydrogen Sulfide in Aqueous Media: Stoichiometry and Kinetics,” Environmental Science and Technology, Vol. 35, No. 11, 2001, pp. 2219- 2225. doi:10.1021/es0017007
NOTES
*Corresponding author.

