Green and Sustainable Chemistry
Vol.3 No.1(2013), Article ID:27969,7 pages DOI:10.4236/gsc.2013.31001
Use of Solid-Supported Reagents towards Synthesis of 2-Arylbenzoxazole, 3,5-Diarylisoxazole and 1,3,5-Triarylpyrazole
Department of Chemistry, Dnyanprassarak Mandal’s College of Arts, Science and Commerce, Mapusa, India
Email: *vidchem@gmx.net
Received December 2, 2012, revised January 5, 2013; accepted January 15, 2013
Keywords: Schiff’s Bases; 3,5-Diarylisoxazoles; 2-Arylbenzoxazoles; 1,3,5-Triarylpyrazoles; Solid-Supported Reagents
ABSTRACT
Herein we report a convenient and efficient synthesis of 2-arylbenzoxazole from the Schiff’s bases of 2-aminophenol, 3,5-Diarylisoxazole from α, β-unsaturated ketoxime and 1,3,5-triarylpyrazole from 2-pyrazoline and N-Arylhydrazone by using milder, less toxic and less expensive-NBS-SiO2, KMnO4-Al2O3, PCC-SiO2 and ACC-Al2O3 as solid-supported oxidizing agents at room temperature. Within the framework of Green Chemistry, the use of solid supported reagents has many advantages such as 1) they are easy to remove from reactions by filtration 2) excess reagents can be used to drive the reaction without introducing any difficulties in purification 3) such solid-supported reagents react differently, mostly more selectively, than their unbound counterparts and 4) toxic, noxious and explosive chemicals are handled more safely when contained on solid support.
1. Introduction
In the recent years heterocyclic compounds are playing important role in the synthetic organic chemistry. Among these heterocycles the bicyclic heterocycles-benzoxazoles [1-3] and five membered heterocycles pyrazoles [4,5] and isoxazoles [6-8] are known to exhibit various bioactive properties. Arylbenzoxazole possesses the important biaryl pharmacophore thus exhibiting a variety of biological and pharmacological activities such as antitumor [9], anti-viral [10] and many more. Isoxazoles act as intermediates in the synthesis of natural products and as building blocks for construction of new molecular systems [11]. It exhibits wide range of medicinal properties [12]. On the other hand 1,3,5-triarylpyrazole occupy an important position in agrochemicals and pharmaceutical industries due to its wide range of bioactive properties such as anti-inflammatory, anti-arrythmic etc. [13,14].
Lot of research has been carried out and various methods have been reported towards the synthesis of 2-arylbenzoxazoles, 3,5-diarylisoxazole and 1,3,5-triarylpyrazoles in the recent years. One of such synthesis of 2- arylbenzoxazole includes oxidative cyclization of phenolic Schiff’s bases (derived from 2-aminophenols and aromatic aldehydes) using various oxidizing agents like PhI(OAc)2 [15], Mn(OAc)3 [16], Ba(MnO4)2 [17], NiO2 [18], Pb(OAc)4 [19] and DDQ [20]. Despite of these intensive works, the development of more effective and convenient method still remains challenging, since many of the known methods require severe reaction conditions and workup procedures. There are several routes by which 3,5-diaryl isoxazoles can be synthesized. The [5 + 0] route involving oxidative cyclization of α, β-unsaturated ketoximes using oxidizing agents like DDQ [21], MnO2 [22], I2/KI in NaHCO3 [23], are some of the few known. Our method depicts use of less toxic reagent as well as good yields of product. On the other side, to carry out aromatization of 2-pyrazoline and oxidative cyclization of phenylhydrazone, limited reports are there in the literature which include reagents like Pb(OAc)4 [24], PhI(OAc)2 [25], Pd/C [26] and Zr(NO3)4 [27]. Our method stands out to be more feasible compared to literature known methods due to its versatility in variety of substrates being subjected to oxidation with good yields of the product.
In our work we report a simple, convenient and efficient procedure for the synthesis of 2-arylbenzoxazoles by oxidative cyclization of phenolic Schiff’s bases using NBS-SiO2, synthesis of 3,5-diarylisoxazole by oxidative cyclization of α, β-unsaturated ketoxime using ACCAl2O3 and synthesis of 1,3,5-triarylpyrazoles by oxidation of 2-pyrazolines using KMnO4-Al2O3, PCC-SiO2 and oxidative cyclization of phenylhydrazones using ACC-Al2O3 and KMnO4-SiO2 as solid supported oxidizing reagents.
With our growing interest in developing greener routes towards the synthesis of these heterocycles we thought of using older and stronger oxidizing agents but adsorbed on a solid support. These solid supported oxidizing agents are chosen to be as greener reagents due to the fact that they are mild, have good selectivity, easy to handle, easily removed after completion of reaction and reduced toxicity of the reagents.
2. Experimental Details
2.1. Typical Procedure for the Synthesis of 2-Arylbenzoxazole
All the reactions were carried out in 25 mL round bottom flask and the stirring was carried out using a magnetic stirrer. In a typical procedure the phenolic Schiff’s basse 1a (0.15 g, 0.8 mmol) was dissolved in dichloromethane (5 mL) and kept for stirring at room temperature. To this stirring mixture, NBS-Silica (0.27 g, 1.6 mmol) was added and further stirred for 30 minutes. The reaction was continuously monitored on TLC. After the completion of reaction, the mixture was filtered and the filtrate was evaporated on hot water bath. The residue that remained after evaporation was purified by column chromatogramphy over silica gel. Yields indicated are obtained after purification.
Selected product: 2-phenyl benzoxazole (2a), white solid, melting point: 98˚C - 100˚C, yield = 84% H1NMR (300 MHz, CDCl3): δ 8.20 (dd, 2H), 7.75 (m, 1H), 7.48 - 7.52 (m, 4H), 7.34 - 7.388 (m, 2H). C13NMR (75 MHz, CDCl3): δ 164.0, 152.7, 143.87, 131.29, 130.89, 127.59, 126.79, 122.06, 112.6. IR (cm−1): 1050, 1250, 1450, 1600, 3050.
Selected product: 2-(4’-nitrophenyl) benzoxazole (2d), pale yellow solid, melting point: 270˚C, yield = 64% 1HNMR (300 MHz, CDCl3): δ 8.4 (dd, 2H), 7.82 (m, 1H), 7.43 - 7.62 (m, 4H), 7.39 - 7.42 (m, 2H). 13CNMR (75 MHz, CDCl3): δ 190.24, 167.0, 151.0, 141.96, 132.84, 128.43, 126.24, 125.2, 120.26, 110.6. IR (cm−1): 1050, 1250, 1450, 1630, 3050.
Selected product: 2-(2’hydroxynaphthyl) benzoxazole (2i), white solid, melting point: 152˚C, yield = 84% 1HNMR (300 MHz, CDCl3): δ 9.08(s, 1H), 7.88 - 7.95 (m, 4H), 7.76 (s, 1H), 7.34-7.388 (m, 6H). 13CNMR (75 MHz, CDCl3): δ 164.0, 152.7, 143.87, 131.29, 130.89, 127.59, 126.79, 122.06, 112.6. IR (cm−1): 1050, 1250, 1450, 1630, 3400.
2.2. Typical Procedure for the Synthesis 3,5-Diarylisoxazole
All the reactions were carried out in 25 mL round bottom flask and the stirring was carried out using a magnetic stirrer. Ketoxime 7a (1 mmol), ACC-Alumina (1 mmol) and dichloromethane were placed in a 25 mL round bottom flask. The reaction was stirred for three hours and frequently monitored using T.L.C. After completion of the reaction, the reaction mixture was filtered and filtrate obtained was then evaporated to give the product 8a.
Selected product: 3,5-diphenyl isoxazole (8a) White solid, melting point = 140˚C H1NMR (300 MHz, CDCl3): -δ 6.8 (s, 1H), 7.21 - 8.05 (m, 10H). IR (cm−1): 1250, 1435, 3010.
Selected product: 3-(4’-bromophenyl)-5-(4”-methoxyphenyl) isoxazole (8c) White solid, melting point = 130˚C H1 NMR (300 MHz, CDCl3): -δ 5.3 (s, 3H), 6.83(s, 1H), δ 7.34 - 7.86 (m, 10H). IR (cm−1): 690, 1250, 1500, 1600, 3010.
Selected product: 3-(4’-bromophenyl)-5-phenyl isoxazole (8d) White solid, melting point = 178˚C 1H NMR (300 MHz, CDCl3): -δ 6.814 (s, 1H), 7.19 - 7.25 (m, 5H), 7.45 - 8.07(m, 4H). 13C NMR (75 MHz, CDCl3): 142, 135.2, 134.7, 134.09, 133.644, 133.05, 132.17, 130.157, 128, 127.646, 116.9, 95.26. IR (cm−1): 685, 1250, 1510, 1600, 3010.
2.3. Typical Procedure for the Synthesis of 1,3,5-Trisubstituted Pyrazoles Using
2.3.1. KMnO4-Alumina
All the reactions were carried out in 25 mL round bottom flask and the stirring was carried out using a magnetic stirrer. In a typical procedure the 2-pyrazoline 3a (0.15 g, 0.4 mmol) was placed in round bottom flask containing magnetic needle/pellet. To this mixture dichloromethane (5 mL) was added and kept for stirring at room temperature. To this stirring mixture, KMnO4-Alumina (0.206 g, 0.8 mmol) was added and further stirred for four hours. The progress of the reaction was continuously monitored on TLC. After the completion of reaction, the mixture was filtered and the filtrate was evaporated on hot water bath. After evaporation, a pale yellow solid pyrazole is obtained which was purified by recrystallization using ethanol as solvent.
Selected product: 1,3,5-triphenylpyrazole (4a), pale yellow solid, melting point: 144˚C, yield = 66% 1H NMR (300 MHz, CDCl3): δ 6.8 (s, 1H), 7.21 - 7.45 (m, 13H), 7.92 (d, 2H) IR (cm−1): 1495, 1500, 1600, 3010.
2.3.2. PCC-Silica
All the reactions were carried out in 25 mL round bottom flask and the stirring was carried out using a magnetic stirrer. In a typical procedure the 2-pyrazoline 5b (0.15 g, 0.4 mmol) was placed in round bottom flask containing magnetic needle/pellet. To this mixture dichloromethane (5 mL) was added and kept for stirring at room temperature. To this stirring mixture, PCC-Silica (0.546 g, 2 mmol) was added and further stirred for five hours. The progress of the reaction was continuously monitored on TLC. After the completion of reaction, the mixture was filtered and the filtrate was evaporated on hot water bath. After evaporation, a pale yellow solid pyrazole is obtained which was purified by recrystallization using ethanol as solvent.
Selected product: 5-(4’-chlorophenyl)-1,3-diphenyl pyrazole (6b), pale yellow solid, melting point: 116˚C, yield = 51% 1H NMR (300 MHz, CDCl3): -δ 6.83(s, 1H), 7.20 - 7.29 (m, 5H), 7.31 - 7.42 (m, 5H), 7.92 (d, 2H), 7.45 (d, 2H) IR (cm−1): 670, 1490, 1600, 3015.
2.3.3. KMnO4-Silica
All the reactions were carried out in 25 mL round bottom flask and refluxing was carried out using a heating mantle. In a typical procedure the parent phenylhydrazone 9a (0.15 g, 0.33 mmol) was placed in round bottom flask. To this mixture dichloromethane (5 mL) was added and kept for stirring at room temperature. To this stirring mixture, KMnO4-Silica (0.546 g, 0.33 mmol) was added and further refluxed for three hours. The progress of the reaction was continuously monitored on TLC. After the completion of reaction, the mixture was filtered and the filtrate was evaporated on hot water bath. After evaporation, a pale yellow solid pyrazole is obtained which was purified by recrystallization using ethanol as solvent.
Selected product: 5-(4’-chlorophenyl)-1,3-diphenyl pyrazole (6b), pale yellow solid, melting point: 116˚C, yield = 63% 1H NMR (300 MHz, CDCl3): -δ 6.83 (s, 1H), 7.212 - 7.45 (m, 12H), 7.31 - 7.42 (m, 5H), 7.92 (d, 2H), IR (cm−1): 670, 1500, 1577, 3010.
2.3.4. ACC-Alumina
All the reactions were carried out in 25 mL round bottom flask and refluxing was carried out using a heating mantle. In a typical procedure the 2,4-dinitro phenylhydrazone 11b (0.15 g, 0.26 mmol) was placed in a round bottom flask. To this mixture dichloromethane (5 mL) was added and kept for stirring at room temperature. To this stirring mixture, ACC-Alumina (0.546 g, 0.78 mmol) was added and further refluxed for three hours. The progress of the reaction was continuously monitored on TLC. After the completion of reaction, the mixture was filtered and the filtrate was evaporated on hot water bath. After evaporation, a pale yellow solid pyrazole is obtained which was purified by recrystallization using ethanol as solvent.
Selected product:
3-(p-Bromophenyl)-5-phenyl-1-(2’,4’-dinitrophenyl)- pyrazole (12a), light yellow solid mp 142˚C; yield = 65% IR (KBr): 3100, 1620, 1550, 1500, 680 cm−1. 1H NMR (300 MHz, CDCl3): δ 6.7 (s, 1H, 4-H), 7.1 - 7.4 (m, 5H), 7.76 - 7.83 (m, 4H), 7.88 (d, 1H), 8.75 (d, 1H), 9.056 (d, 1H).
13C NMR (75 MHz, CDCl3): δ 116, 117, 126,127, 127.6, 128.3, 128.4, 128.7, 129.05, 129.11, 129.45, 129.46, 129.53, 129.6, 129.91, 130.0, 130.19, 130.39, 132.61, 137.469, 141.3, 156.1, 157.5.
Selected product:
5-(p-methoxyphenyl)-3-(p-nitrophenyl)-1-(2’, 4’-dinitrophenyl) pyrazole (12b), yellow solid, melting point: 166˚C, yield = 73% IR (cm−1): 1248, 1290, 1490, 1600, 3050 1H NMR (300 MHz, CDCl3): δ 3.88 (s, 3H), 6.90 (s, 1H), 7.25 - 7.44 (m, 4H), 7.78 (d, 2H), 7.35 (d, 2H) 7.79 (d, 2H), 8.74 (d, 1H).
Selected product:
5-(p-chlorophenyl)-3-(p-methoxyphenyl)-1-(2’, 4’-dinitrophenyl) pyrazole (12c), yellow solid, melting point: 132˚C; yield = 50% IR (cm−1): 1255, 1280, 1520, 1620, 3070 1H NMR (300 MHz, CDCl3): δ 3.85 (s, 3H), 6.87 (s, 1H), 7.22 - 7.48 (m, 4H), 7.70 (d, 2H), 7.40 (d, 2H) 7.82 (d, 2H), 8.80 (d, 1H).
3. Results and Discussion
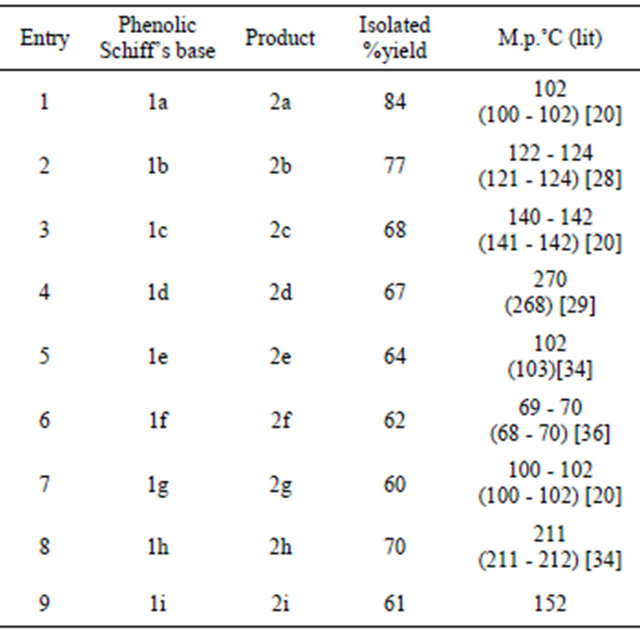
Initially, we prepared nine phenolic Schiff’s bases by reacting aromatic aldehydes with 2-aminophenol. Once the Schiff’s bases were prepared, we carried out oxidative cyclization on it. Here the parent Schiff’s base, prepared from benzaldehyde and 2-aminophenol, was dissolved in dichloromethane to which NBS-silica was added and kept for stirring at room temperature for 30 minutes. After that the reaction mixture was filtered so as to remove the solid support. The filtrate was concentrated and purified using column chromatography. The product, 2-phenyl benzoxazole was obtained in 84% yield after recrystallization. To check the feasibility of this reaction the remaining Schiff’s bases (those prepared earlier) were also tried for similar oxidative cyclization using NBS-silica as the oxidizing agent. In all nine 2-arylbenzoxazoles were prepared in moderate-good yields (60% - 84%) after chromatographic separation and purification (Scheme 1, Table 1).
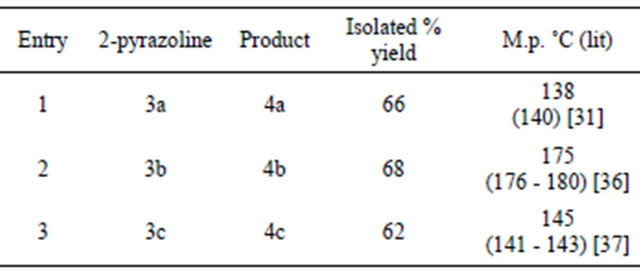
Next task was to synthesize 1,3,5-trisubstituted pyrazoles from the corresponding 2-pyrazolines. In this case the parent pyrazoline, which was synthesized from phenyl hydrazine and chalcone was dissolved in dichloromethane and to this solution KMnO4-Alumina was added. The reaction mixture was stirred for four hours at room temperature. After the completion of reaction, the mixture was filtered and the filtrate was evaporated to give a pale yellow solid pyrazole as the product which was purified by recrystallization using ethanol. In all three 1,3,5- trisubstituted pyrazoles were prepared in good yields
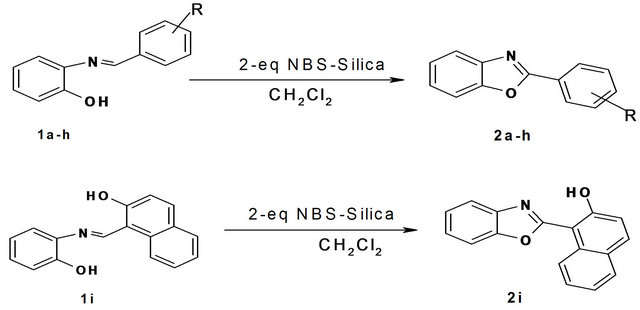
Scheme 1. Synthesis of 2-arylbenzoxazoles 1a: R = H, 1b: R = 2-OH, 1c: R = 4-Cl, 1d: R = 4-NO2, 1e: R = 2-NO2, 1f: R = 2-Cl, 1g: R = 4-OMe, 1h: R = 3-NO2.
Table 1. Synthesis of 2-arylbenzoxazoles.
after purification (Scheme 2, Table 2).
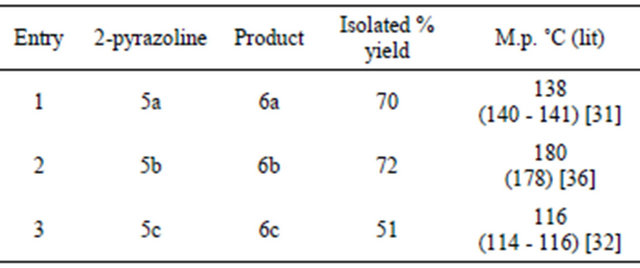
We also used another reagent i.e. PCC-Silica for the oxidation of 2-pyrazolines to the corresponding 1,3,5- trisubstituted pyrazoles. Here the parent 2-pyrazoline was dissolved in dichloromethane and then subjected to oxidation by adding PCC-Silica. The reaction mixture was stirred for five to six hours at room temperature. After the completion of reaction, the mixture was filtered and the filtrate was evaporated to give a pale yellow solid pyrazole as the product which was purified by recrystallization using ethanol. In all three 1,3,5-trisubstituted pyrazoles were prepared in good yields after purification (Scheme 3, Table 3).
In addition, we also prepared 3, 5-diarylisoxazole from the corresponding α, β-unsaturated ketoxime. The α, β-unsaturated ketoxime was initially prepared by reacting α, β-unsaturated chalcones with hydroxylamine hydrochloride under weakly basic conditions. Once the α, β-unsaturated ketoxime were prepared, we carried out oxidative cyclization on it. Here the parent α, β-unsatu-
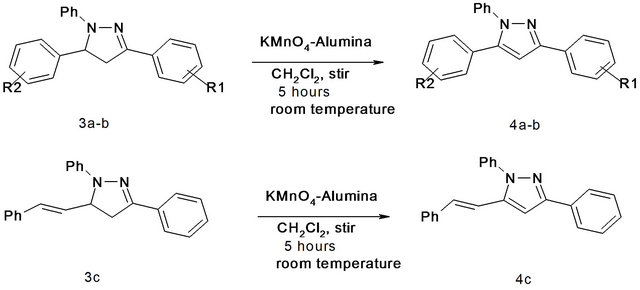
Scheme 2. Synthesis of 1,3,5-trisubstituted pyrazoles using KMnO4-Alumina 3a: R1 = H, R2 = H; 3b: R1 = 4-OMe, R2 = 4-NO2.
Table 2. Synthesis of 1,3,5-trisubstituted pyrazoles using KMnO4-Alumina.
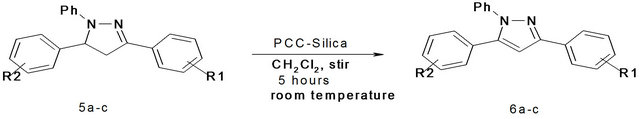
Scheme 3. Synthesis of 1,3,5-trisubstituted pyrazoles using PCC-Silica 5a: R1 = H, R2 = H; 5b: R1 = 4-OMe, R2 = 4-NO2; 5c: R1 = H, R2 = 4-Cl.
Table 3. Synthesis of 1,3,5-trisubstituted pyrazoles using PCC-Silica.
rated ketoxime was dissolved in dichloromethane to which ACC-Alumina was added and kept for stirring at room temperature for three hours. After that the reaction mixture was filtered so as to remove the solid support. The filtrate was concentrated and purified by recrystallization using absolute ethanol as the solvent. To check the feasibility of this reaction the remaining α, β-unsaturated ketoxime were also tried for similar oxidative cyclization using ACC-Alumina as the oxidizing agent. In all five 3, 5-diarylisoxazole were prepared in moderate-good yields after purification (Scheme 4, Table 4).
Since we were also successful in synthesizing 1,3,5-
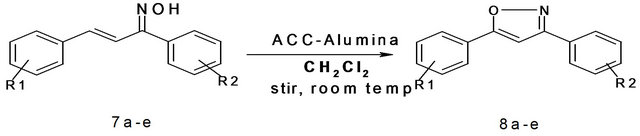
Scheme 4. Synthesis of 3,5-diarylisoxazole using ACC-Alumina. 7a: R1 = H, R2 = H; 7b: R1 = 4-Cl R2 = 4-OMe; 7c: R1 = 4-OMe R2 = Br; 7d: R1 = H, R2 = Br, 7e: R1 = H, R2 = 4-NO2
Table 4. Synthesis of 3,5-diarylisoxazole using ACC-Alumina.
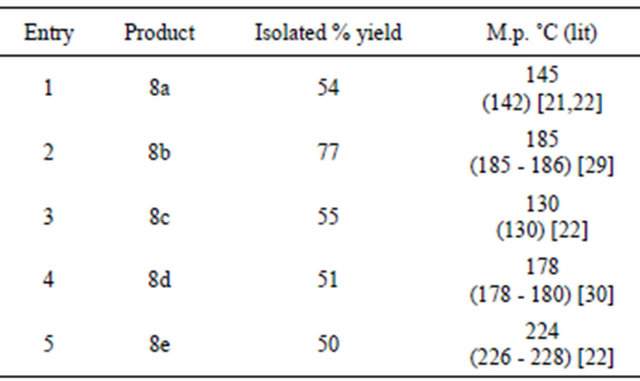
trisubstituted pyrazoles from 2-pyrazolines, we thought of preparing 1,3,5-trisubstituted pyrazoles from the corresponding N-Arylhydarzone and 2,4-dinitrophenylhydrazone via oxidative cyclization. For which we used KMnO4-silica and ACC-alumina as the oxidizing agents. Here the parent phenyl hydrazone was initially synthesized from phenyl hydrazine and chalcone under acidic conditions. This phenyl hydrazone was dissolved in dichloromethane and then subjected to oxidative cyclization by adding KMnO4-silica. The reaction mixture was refluxed for three hours. After the completion of reaction, the mixture was filtered and the filtrate was evaporated to give a pale yellow solid pyrazole as the product which was purified by recrystallization. In all three 1,3,5-trisubstituted pyrazoles were prepared in good yields after purification (Scheme 5, Table 5).
Similarly the 2,4-dinitrophenylhydrazone was dissolved in dichloromethane and then subjected to oxidative cyclization by adding ACC-alumina. The reaction mixture was refluxed for three hours. After the completion of reaction, the mixture was filtered and the filtrate was evaporated to give a pale yellow solid pyrazole as the product which was purified by recrystallization. In all three 1,3,5-trisubstituted pyrazoles were prepared in good yields after purification (Scheme 6, Table 6).
4. Conclusion
In conclusion, we have been successful in developing a convenient, efficient and greener method for the synthesis of 2-arylbenzoxazoles from phenolic Schiff’s bases, 3,5-diarylisoxazole from α, β-unsaturated ke-
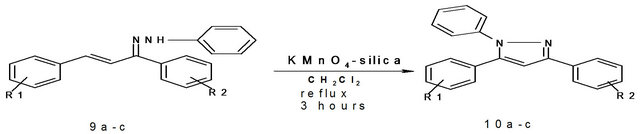
Scheme 5. Synthesis of 1,3,5-trisubstituted pyrazoles using KMnO4-silica 9a: R1 = H, R2 = H; 9b: R1 = 4-OMe, R2 = 4-NO2; 9c: R1 = H, R2 = 4-Cl.
Table 5. Synthesis of 1,3,5-trisubstituted pyrazoles using KMnO4-Silica.
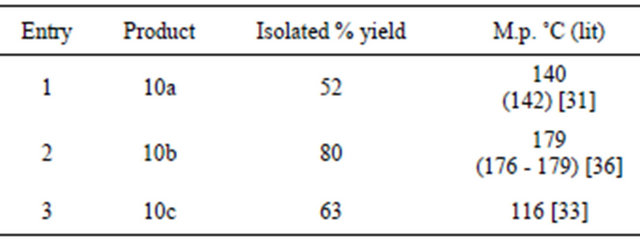
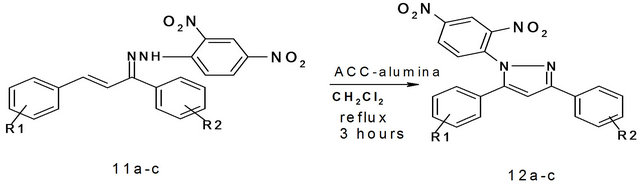
Scheme 6. Synthesis of 1,3,5-trisubstituted pyrazoles using ACC-alumina. 11a: R1 = H, R2 = Br; 11b: R1 = 4-OMe R2 = 4-NO2; 11c: R1 = 4-Cl R2 = 4-OMe.
Table 6. Synthesis of 1,3,5-trisubstituted pyrazoles using ACC-alumina.
toxime and 1,3,5-trisubstituted pyrazoles from the 2-pyrazolines and N-Arylhydrazones respectively. All the reactions were carried out at room temperature and the conditions used were quite mild. The product yields The products are obtained in moderate-good yield, which is compatible with literature known methods and moreover our methodology is eco-friendly. This methodology also shows greater versatility of using solid-supported reagents towards synthesis of variety of heterocycles. Use of solid supported reagents in our reaction has reduced the toxicity of the reagent which makes it much safer and easier to handle.
5. Acknowledgements
The authors are grateful to the Department of Science and Technology-Government of Goa for the financial support. Also, grateful to Professor Santosh G. Tilve, Goa University for constant encouragement.
REFERENCES
- R. J. Perry, B. D. Wilson and R. J. Miller, “Synthesis of 2-Arylbenzoxazoles via the Palladium Catalysed Carbonylation and Condensation of Aromatic Halides and OAminophenols,” Journal of Organic Chemistry, Vol. 57, No. 10, 1992, pp. 2883-2887. doi:10.1021/jo00036a025
- T. Kondo, S. Yang, K.-T. Huh, M. Kobayashi, S. Kotachi and Y. Watanabe, “Ruthenium Complex-Catalysed Facile Synthesis of 2-Substituted Benzoxazoles,” Chemistry Letters, Vol. 20, No. 7, 1991, pp. 1275-1278. doi:10.1246/cl.1991.1275
- M. I. E. Sheikh, A. J. Marks and E. R. Biehl, “Investigation of the Synthesis of Benzoxazoles via Aryne Reaction,” Journal of Organic Chemistry, Vol. 46, No. 16, 1981, pp. 3256-3259. doi:10.1021/jo00329a022
- T. L. Gilchrist, “Heterocyclic Chemistry,” 3rd Edition, Addison-Wesley Longman, Ltd., London, 1998.
- D. Azarifar and M. Shaebanzadeh, “Synthesis and Characterisation of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Anti-Microbial Properties,” Molecules, Vol. 7, No. 12, 2002, pp. 885-895. doi:10.3390/71200885
- A. Barco, S. Benitti, G. P. Pollini and D. Simoni, “Synthesis of Natural Products via Isoxazoles,” Synthesis, Vol. 1987, No. 10, 1987, pp. 857-869.
- Y. Kondo, D. Uchiyama, T. Sakamoto and H. Yamanaka, “Synthesis and Reactions of 5-(Tributylstannyl)isoxazoles,” Tetrahedron Letters, Vol. 30, No. 32, 1989, pp. 4249-4250. doi:10.1016/S0040-4039(01)80702-4
- O. Morriyo, H. Nakamura, T. Kageyama and Y. Urata, “Synthesis of Isoxazolines and Isoxazoles from Aldoximes by the Use of Sodium Bromite with Organotin Halide,” Tetrahedron Letters, Vol. 30, No. 30, 1989, pp. 3987-3990. doi:10.1016/S0040-4039(00)99302-X
- O. Temiz, I. Rren, E. Sener, I. Yalcin and N. Ucartuk, “Synthesis and Microbiological Activity of Some Novel 5-and 6-Methyl-2-(2,4-disubstitutedphenyl) benzoxazole Derivatives,” Farmaco, Vol. 53, No. 5, 1998, pp. 337-341. doi:10.1016/S0014-827X(98)00030-5
- S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. Mathews, T. D. Bradshaw and A. D. Westwell, “Synthesis and Biological Properties of Benzothiazoles, Benzoxazoles and Chromene-4-One Analogues of the Potent Antitumor Agent 2-(3,4-Dimethoxyphenyl)-5-fluorobenzothiazole(PMX610, NSC 721648),” Journal of Medicinal Chemistry, Vol. 51, No. 16, 2008, pp. 5135-5139. doi:10.1021/jm800418z
- E. Dominguez, E. Ibeus, E. M. de Margart, J. K. Palacius and E. San Marka, “A Convenient One-Pot Preparative Method for 4,5-Diaryl Isoxazoles involving Amine Exchange Reaction,” Journal of Organic Chemistry, Vol. 61, No. 16, 1996, pp. 5435-5439. doi:10.1021/jo960024h
- J. W. Wang, J. Jai, H. M. Li and C. Wang, “Synthesis of Novel Isoxazole-Contained Analogues of Losartan,” Chinese Chemical Letters, Vol. 11, No. 11, 2000, pp. 961- 962.
- H. Katayama, T. Oshiyama, “Preparation and Bioactivity of Pyrazole Derivatives as Potential Cross-Linking Agents,” Canadian Journal of Chemistry, Vol. 75, No. 6, 1997, pp. 913-919. doi:10.1139/v97-109
- H. M. Faid and H. M. Mokhtar, “Pyrazole Derivatives with Possible Hypoglycemic Activity,” Indian Journal of Chemistry, Vol. 27B, No. 3, 1988, pp. 245-249.
- R. S. Varma, R. K. Saini and O. Prakash, “Hypervalent Iodine Oxidation of Phenolic Schiff’s Bases: Synthesis of 2-Arylbenzoxazoles,” Tetrahedron Letters, Vol. 38, No. 15, 1997, pp. 2621-2622. doi:10.1016/S0040-4039(97)00444-9
- R. S. Varma and D. Kumar, “Manganese Triacetate Oxidation of Phenolic Schiff Bases: Synthesis of 2-Arylbenzoxazoles,” Journal of Heterocyclic Chemistry, Vol. 35, No. 6, 1998, pp. 1539-1540. doi:10.1002/jhet.5570350656
- R. G. Shrivastava and P. S. Venkataramani, “Barium Manganate Oxidation in Organic Synthesis: Part III: Oxidation of Schiff’s Bases to Benzimidazoles, Benzoxazoles and Benzthiazoles,” Synthetic Communications, Vol. 18, No. 13, 1988, pp. 1537-1544. doi:10.1080/00397918808081311
- K. Nakagawa and H. Onoue, “Oxidation with Nickel Peroxide. IV. The Preparation of Benzoxazoles from Schiff’s Bases,” Chemical and Pharmaceutical Bulletin, Vol. 12, No. 10, 1964, pp. 1135-1138. doi:10.1248/cpb.12.1135
- F. F. Stephens and J. D. Bower, “The Preparation of Benzimidazoles and Benzoxazoles from Schiff’s Bases. Part I,” Journal of Chemical Society, Vol. 71, No. 9, 1949, pp. 2971-2972. doi:10.1039/jr9490002971
- J. Chang, K. Zhao and S. Pan, “Synthesis of 2-Arylbenzoxazoles via DDQ Promoted Oxidative Cyclization of Phenolic Schiff’s Bases—A Solution Phase Strategy for Library Synthesis,” Tetrahedron Letters, Vol. 43, No. 6, 2002, pp. 951-954. doi:10.1016/S0040-4039(01)02302-4
- V. G. Desai and S. G. Tilve, “A Novel and Convenient Method for the Synthesis of 3,5-Diarylisoxazoles,” Synthetic Communications, Vol. 29, No. 17, 1999, pp. 3017- 3020. doi:10.1080/00397919908086477
- R. F. Kurangi, R. Kawthankar and V. G. Desai, “Convenient Synthesis of 3,5-Disubstituted Isoxazoles,” Synthetic Communications, Vol. 37, No. 4, 2007, pp. 585-587. doi:10.1080/00397910601055107
- G. Buchi and J. C. Vedera, “Interchange of Functionality Compounds through Isoxazoles,” Journal of American Chemical Society, Vol. 94, No. 26, 1972, pp. 9128-9131. doi:10.1021/ja00781a023
- S. P. Singh, D. Kumar, O. Prakash and R. P. Kapoor, “Hypervalent Iodine Oxidation of 1,3,5-Trisubstituted Pyrazolines: A Facile Synthesis of 1,3,5-Trisubstituted Pyrazoles,” Synthetic Communications, Vol. 27, No. 15, 1997, pp. 2683-2689. doi:10.1080/00397919708004136
- I. Bhatnagar and M. V. George, “Oxidation with Metal Oxides-II: Oxidation of Chalcone Phenylhydrazones, Pyrazolines, O-Aminobenzylidine Anils and O-Hydroxybenzylidine Anils with Manganese Dioxide,” Tetrahedron, Vol. 24, No. 3, 1968, pp. 1293-1298. doi:10.1016/0040-4020(68)88080-9
- W. A. F. Gladstone and R. O. C. Norman, “Reactions of Lead Tetra-Acetate Part VII: Some Reactions Leading to Pyrazoles,” Journal of Chemical Society (C), 1966, pp. 1536-1540. doi:10.1039/j39660001536
- G. Sabitha, G. S. K. Kumar Reddy, Ch. S. Reddy, N. Fatima and J. S. Yadav, “Zr(NO3)4: A Versatile Oxidizing Agent for Aromatization of Hantzsch 1,4-Dihydropyridines and 1,3,5-Trisubstituted Pyrazolines,” Synthesis, No. 8, 2003, pp. 1267-1271.
- C. Wei-hua and Yi. Pang, “Efficient Synthesis of 2-(2’- Hydroxyphenyl)benzoxazole by Palladium(II)-Catalyzed Oxidative Cyclization,” Tetrahedron Letters, Vol. 50, No. 48, 2009, pp. 6680-6683. doi:10.1016/j.tetlet.2009.09.084
- K. H. Park, K. Jun, S. R. Shin and S. W. Oh, “2-Arylbenzoxazoles from Phenolic Schiffs Bases by Thianthrene Cation Radical,” Tetrahedron Letters, Vol. 37, No. 49, 1996, pp. 8869-8870. doi:10.1016/S0040-4039(96)02070-9
- M. Kidwai, S. Kukreja and R. Thakur, “K2CO3-Mediated Regioselective Synthesis of Isoxazoles and Pyrazolines,” Letters of Organic Chemistry, Vol. 3, No. 2, 2006, pp. 135-139. doi:10.2174/157017806775224170
- S. Tang, J. He, Y. Sun and X. She, “Efficient and Regioselective One Pot Synthesis of 3-Substituted and 3,5- Disubstituted Isoxazoles,” Organic Letters, Vol. 11, No. 17, 2009, pp. 3982-3985. doi:10.1021/ol901626n
- S. Morrocohi, A. Ricca and A. Zanarotti, “New Features of the Reaction between Nitrilimines and Arylacetylenes,” Tetrahedron Letters, Vol. 11, No. 37, 1970, pp. 3215-3218. doi:10.1016/S0040-4039(01)98434-5
- D. Azarifar, M. A. Zulfigol and B. Maleki, “Silica-Supported 1,3-Dibromo-5,5-dimethylhydantoin (DBH) as a Useful Reagent for Microwave-Assisted Aromatization of 1,3,5-Trisubstituted Pyrazolines under Solvent-Free Conditions,” Synthesis, No. 11, 2004, pp. 1744-1746. doi:10.1055/s-2004-829149
- S. Tariq and S. Wakode, “Synthesis through Microwave Irradiation, Characterisation and Evaluation of Antimicrobial Activity of 2-Phenyl-1,3-benzoxazole Derivatives,” International Research Journal of Chemistry, Vol. 3, No. 9, 2012, pp. 213-217.
- C. Praveen, A. Nandakumar, P. Dheenkumar, D. Murlidharan and P. T. Perumal, “Microwave Assisted One Pot Synthesis of Benzothiazole and Benzoxazole Libraries as Analgesics Agents,” Journal of Chemical Society, Vol. 124, No. 3, 2012, pp. 609-624.
- B. C. Bishop, K. M. J. Brands, A. D. Gibbs and D. J. Kennedy, “Regioselective Synthesis of 1,3,5-Substituted Pyrazoles from Acetylenic Ketones and Hydrazines,” Synthesis, No. 1, 2004, pp. 43-52. doi:10.1055/s-2003-44376
- O. A. Ignatenko, A. N. Blandov and M. A. Kuznetsov, “Oxidative Addition of N-Aminophthalimide to Alkenyl- 4,5-Dihydropyrazoles and Alkenylpyrazoles Synthesis of Aziridinylpyrazoles,” Russian Journal of Organic Chemistry, Vol. 41, No. 12, 2005, pp. 1793-1801. doi:10.1007/s11178-006-0039-3
NOTES
*Corresponding author.

