World Journal of AIDS
Vol. 2 No. 2 (2012) , Article ID: 19719 , 5 pages DOI:10.4236/wja.2012.22006
Ethambutol Induced Optic Neuritis Associated with Tenofovir Nephrotoxicity
![]()
1The Ruth M. Rothstein CORE Center, Cook County Health and Hospitals System, Chicago, USA; 2Children’s Mercy Hospitals and Clinics, Kansas City, USA; 3Collaborative Research Unit (CRU), Department of Medicine, Cook County Health and Hospitals System, Chicago, USA; 4University of Illinois at Chicago College of Pharmacy, Chicago, USA.
Email: svibhakar@cookcountyhhs.org
Received January 20th, 2012; revised February 21st, 2012; accepted March 27th, 2012
Keywords: tenofovir; nephrotoxicity; ethambutol; optic neuritis; human immunodeficiency virus (HIV)
ABSTRACT
Morbidity and mortality associated with human immunodeficiency virus (HIV) has decreased with highly active antiretroviral therapy (HAART). Tenofovir is a nucleotide reverse transcriptase inhibitor (NRTI) that is preferred by the Department of Health and Human Services (DHHS) HIV treatment guidelines and is widely used for the initial treatment of HIV. Although tenofovir is generally well-tolerated, it has been associated with rare cases of acute nephrotoxicity. HIV-infected patients frequently have co-morbidities that require treatment, thus adding another level of complexity due to drug interactions and medication adverse effects with antiretrovirals. We present a patient who suffered an acute deterioration in renal function from tenofovir, leading to an accumulation of co-administered ethambutol, thus resulting in optic neuritis.
1. Case Report
A 51 year-old woman with a past medical history of asthma and uterine fibroids was admitted to our hospital in June 2006 with fever, shortness of breath, dysphagia, and a 50 pound weight loss over the last few months. On exam, she had oropharyngeal candidiasis and was found to be HIV-positive with a CD4+ T-lymphocyte count = 1 cell/mm3. Pneumocystis jiroveci pneumonia (PCP) was suspected based on her shortness of breath, and Mycobacterium avium complex (MAC) infection was considered likely due to scattered cervical, supraclavicular, and intrathoracic lymphadenopathy noted on exam and CT scan, as well as her elevated alkaline phosphatase levels of 364 U/L (normal range 50 - 120 U/L). Lymphoma was also considered as an alternative diagnosis since she also had non-tender hepatomegaly. She was started on high dose steroids and sulfamethoxazole/ trimethoprim (SMZTMP) for empiric treatment of PCP and azithromycin and ethambutol for empiric treatment of MAC. She was discharged on azithromycin 500 mg daily and ethambutol 800 mg daily (15 mg/kg; weight ~51 kg) for empiric treatment of MAC, fluconazole 100 mg daily for oral candidiasis, and sulfamethoxazole/trimethoprim 800/160 mg daily for PCP prophylaxis.
Two days later, the patient was re-admitted for a scheduled liver biopsy and a gallium scan to exclude possible lymphomatous involvement of the liver. At the time of discharge from this second stay, MAC respiratory and blood cultures were still negative and the patient was discharged on Azithromycin 1200 mg weekly for prophylaxis, ethambutol was discontinued, and SMZ-TMP 800/160 mg daily was continued for PCP prophylaxis. During her two hospital stays, her serum creatinine (Scr) and blood urea nitrogen (BUN) had been measured a total of 13 times with ranges of 0.6 - 1.2 mg/dL (normal range 0.6 - 1.4 mg/dL) and 7 - 18 mg/dL (normal range 8 - 20 mg/dL), respectively. The patient also had low bicarbonate levels of 14 - 23 mg/dL (normal range 23 - 31 mg/dL) and two urinalysis studies were negative for protein and glucose.
Two weeks later in mid-July, the patient followed up at our HIV outpatient clinic. Blood and sputum cultures obtained during the initial hospitalization were reported to be growing MAC and she was restarted on ethambutol, now at 1200 mg daily (22.6 mg/kg; weight ~53 kg), and azithromycin 500 mg daily. Antiretroviral therapy was initiated one week later consisting of Atripla®, the fixed dose combination of tenofovir 300 mg, emtricitabine 200mg and efavirenz 600 mg daily. The patient also started gabapentin 300 mg three times daily and amitriptyline 50 mg at bedtime for peripheral neuropathy and dronabinol 2.5 mg twice daily for appetite stimulation.
Over the next several weeks, the patient discontinued gabapentin, amitriptyline, and dronabinol secondary to side effects of weakness and fatigue; however, she had no other complaints and reported excellent adherence to HAART and MAC treatment.
The patient returned monthly for follow-up visits. In August and September, she reported feeling better and stated that she was compliant with her MAC and HIV treatment. Labs were drawn at the September visit, 8 weeks after starting HAART. These labs showed an undetectable viral load (VL < 75 copies/ml) and an increase in her CD4 count to 26 cells/mm3, but her bicarbonate had decreased to 13 mg/dL and her creatinine had increased to 3.7 mg/dL with a BUN of 29 mg/dL. Her sodium, potassium, phosphorous, and serum glucose were all normal. Additionally, the urinalysis showed proteinuria and glucosuria.
One month later, approximately three months after starting HAART, the patient came in complaining of visual disturbances, reporting seeing red spots in the left eye and having decreased and blurred vision in both eyes. Of note, her weight which had increased by 3 kg had now decreased by 5 kg ~ 48 kg, making her ethambutol dose 25 mg/kg at this time, which is higher than her 15 mg/kg dose of ethambutol started at her initial hospital discharge. At this time, all medications were discontinued. An Ophthalmology consultant diagnosed the patient with possible ethambutol-induced optic neuritis manifesting with decreased visual acuity and impaired redgreen color discrimination. Our Nephrologist attributed the decrease in the patient’s renal function to acute tenofovir-associated nephrotoxicity.
The patient’s renal function began to improve following discontinuation of tenofovir. One week later her SCr decreased from 3.7 mg/dL to 3.1 mg/dL and 3 weeks after discontinuation her creatinine was 2.4 mg/dL. Her renal function continued to improve steadily over the next six months. However, her renal function has not returned to baseline, and remains stable with SCr between 1.3 mg/dL and 1.5 mg/dL (GFR ~ 50 ml/min) four years after discontinuation of tenofovir (see Figure 1). Repeat urinalysis showed continued proteinuria 30 and glucosuria 500, which resolved 5 months and 6 weeks after tenofovir discontinuation, respectively.
HAART was restarted in November with abacavir 600 mg/lamivudine 300 mg daily and efavirenz 600 mg daily. A clinical decision was made not to resume MAC treatment at this time due to the drug toxicities and the patient’s clinical status was monitored. Shortly after the discontinuation of tenofovir, her renal function began to improve, and continued to improve steadily over the next six months. The patient has had an excellent virologic and immunologic response (see Figure 1) to antiretroviral therapy, but her optic neuritis was slow to recover,
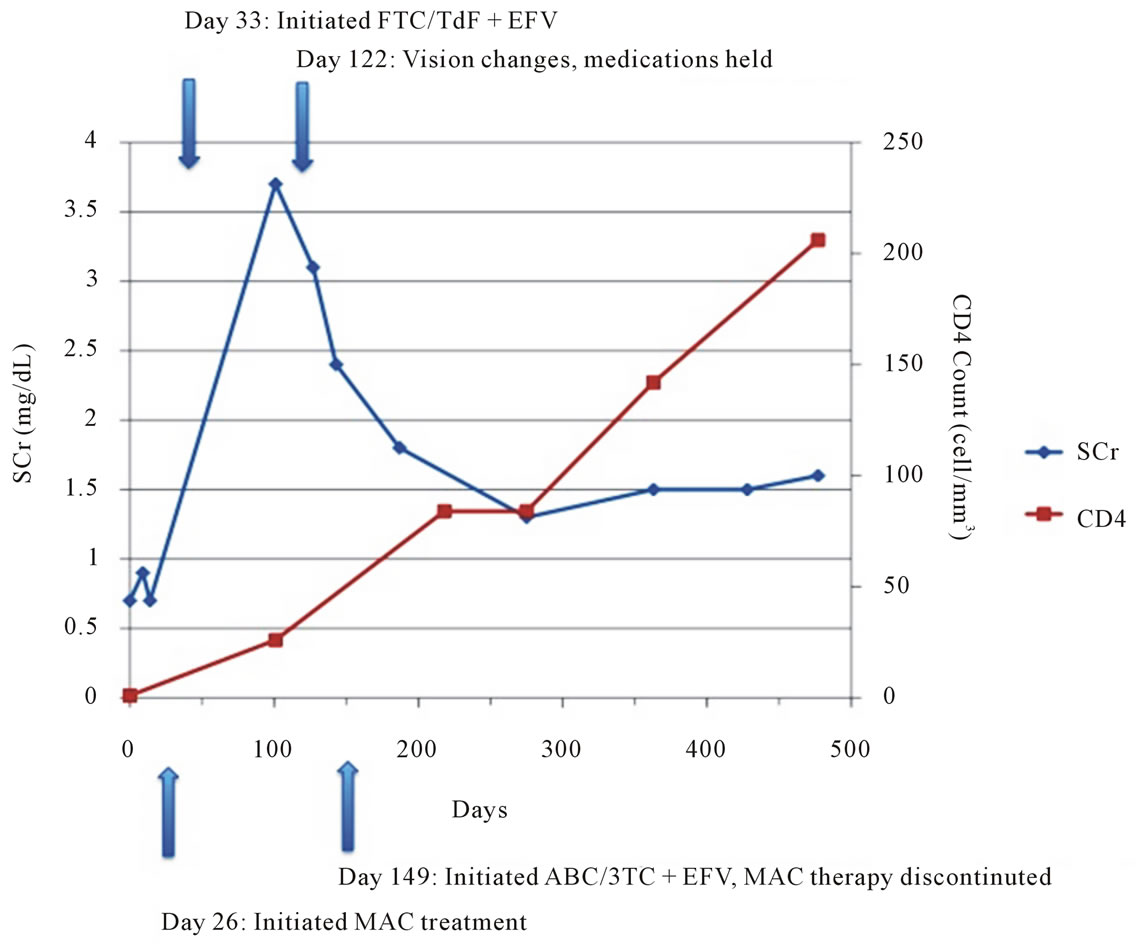
Figure 1. Timeline of laboratory parameters.
with her vision stabilizing nearly 2 years after stopping ethambutol The patient was last seen March 2010 and her kidney function and vision remain stable (See Table 1 for a summary of patient events).
2. Discussion
The Naranjo scale has shown validity in estimating the probability of adverse drug reactions to a particular drug [1]. Based on the total score of 7 on the Naranjo scale, this is a probable adverse event as a result of tenofovir nephrotoxicity [1] (see Table 2). Tenofovir is a nucleotide reverse transcriptase inhibitor (NRTI) with activity against both HIV and hepatitis B virus with favorable characteristics, such as tolerability, potency, and once daily dosing. Tenofovir 300 mg co-formulated with emtricitabine 200 mg (Truvada; Gilead, Foster City, CA) are the preferred NRTIs for the treatment of HIV [2]. Tenofovir is renally excreted by glomerular filtration and active tubular secretion. Tenofovir is a monophosphorylated nucleotide analog of adenosine and is structurally related to adefovir and cidofovir, all of which are known to cause nephrotoxicity. Data from initial clinical trials indicated that tenofovir had an excellent safety profile;
however, numerous case reports of renal impairment have been reported subsequently with tenofovir use [3-6]. Although the risk of nephrotoxicity appears to be low, product information for tenofovir contains a warning regarding the development of acute renal failure and Fanconi syndrome, which is characterized by proteinuria, glucosuria, hypophosphatemia, hypokalemia, and low serum bicarbonate [5,7,8]. The exact mechanism by which tenofovir causes nephrotoxicity has not been fully elucidated; however, it is thought to be related to an interference with the transporter proteins leading to higher intracellular drug concentrations and thus causing proximal tubular damage [4,7]. Most cases of renal toxicity due to tenofovir are mild, gradual, and reversible, requiring only discontinuation of tenofovir without further clinical intervention. However, a subset of patients with multiple risk factors for tenofovir toxicity, such as older age, low body weight, those taking concomitant nephrotoxic medications, or those that have an unknown genetic predisposition to tenofovir toxicity can develop acute renal injury [7,8]. Our patient presented with some laboratory abnormalities that were consistent with tenofovir nephrotoxicity such as low bicarbonate, normoglycemic glucosuria, and mild proteinuria.
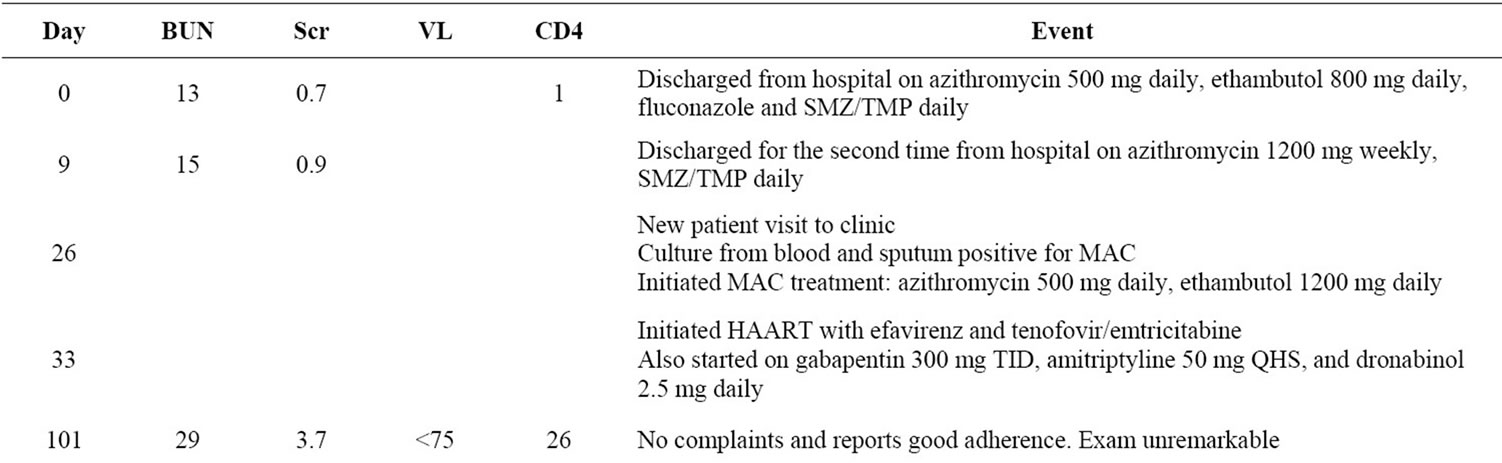
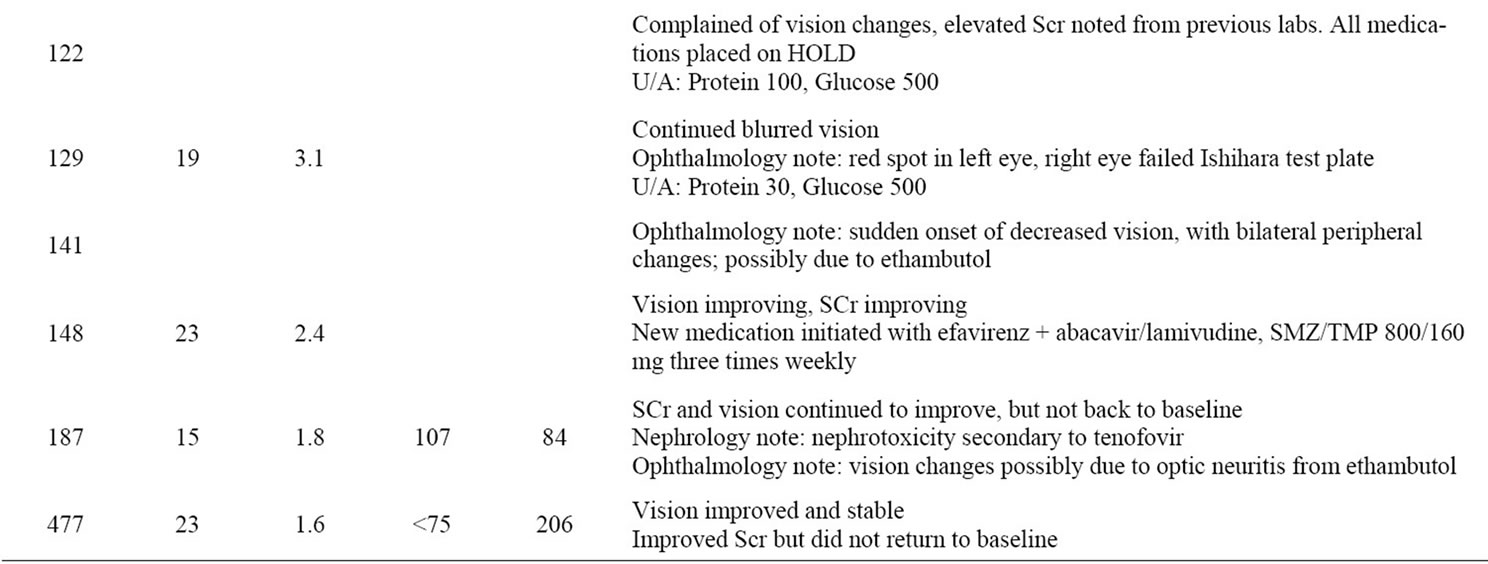
Table 1. Timeline of patient events.

Table 2. The Naranjo adverse drug reaction probability scale.
The patient’s renal function improved after discontinuation of tenofovir, suggesting tenofovir was the likely cause or contributing factor. There are cases reported in the literature of nephrotoxicity developing shortly after the initiation of tenofovir as it did in our patient, however, most of the cases report later onset (about 20 weeks) [4,7,8]. Other etiologies of renal insufficiency, such as poor renal perfusion, obstruction, infection, or concomitant nephrotoxic medications were unlikely based on the patient’s history and clinical course.
This case is unique in that while tenofovir nephrotoxicity has been well documented, to our knowledge this is the first case report that describes an adverse event from concomitant medications as a result of tenofovir nephrotoxicity that resulted in significant morbidity. In this case, not only did deteriorating renal function as a result of tenofovir toxicity lead to a higher than recommended ethambutol mg/kg dose, but an acute weight loss further increased the risk of ocular toxicity with ethambutol.
Ethambutol, in combination with a macrolide, is the recommended treatment of disseminated Mycobacterium avium complex (MAC) [9]. Although generally well tolerated, there are side effects associated with its use, such as peripheral neuropathy and optic neuritis. Classic cases of ethambutol induced optic neuritis have been described, ranging from mild cases of blurred vision and red-green color indiscrimination, both of which our patient developed, to permanent vision loss [10,11]. The mechanism by which ethambutol affects the optic nerve is unknown. Several risk factors have been identified for ethambutol-induced optic neuritis, such as extended duration of use, decreased ethambutol clearance, age, concomitant drugs that can affect vision, and other vascular diseases [11,12]. Ethambutol ocular toxicity is thought to be doserelated, with an estimated incidence of 5% to 6% at doses of 25 mg/kg and 18% at doses over 30 mg/kg. The incidence at the lowest recommended dose (15 mg/kg) has been reported to be less than 1% [12,13]. The Centers for Disease Control (CDC) Morbidity and Mortality Weekly Report (MMWR) recommends an ethambutol dose of 15 to 20 mg/kg/day, with a target dose of 15 mg/kg, for the treatment of MAC [9]. The timing of ethambutol ocular toxicity can range from as early as weeks to one year after initiation of therapy [11,12]. In our patient, acute weight loss also contributed to a higher than anticipated mg/kg dose of ethambutol, which may have increased her risk for ocular toxicity.
Clearance of ethambutol is primarily renal, approximately 50 percent is excreted in the urine unchanged [14]. In patients with impaired renal function, the half-life of ethambutol is increased which can lead to drug accumulation and increased risk of developing adverse effects. It is recommended that ethambutol be used with caution in patients with renal impairment and that appropriate dose adjustments are made. For patients with end-stage renal disease, the recommendation is to increase the dosing interval to every 48 hours [14].
In a recent article published by Talbert Estlin et al., 70 cases of ethambutol optic toxicity were reviewed [11]. Most cases of optic toxicity occur in patients with underlying kidney dysfunction from renal disease or increasing age, higher doses of ethambutol, and/or prolonged duration of use. The findings of this meta-analysis revealed that nearly two-thirds of the cases did not include any information about the patient’s renal function and no cases reported a dose adjustment for kidney disease. However, of the 25 cases that did report some data on kidney function, 88% included renal disease or significant risk factors for renal disease. Therefore, it is recommended to maintain doses close to 15 mg/kg, check patient’s weight regularly to ensure appropriate dosing, screen for renal dysfunction, and educate patients to report adverse effects promptly [11].
Fang and colleagues describe two cases of ethambutol-associated optic neuritis in hemodialysis patients [10]. Both were receiving treatment for Mycobacterium tuberculosis; the first case was a 27 y/o female patient receiving 16 mg/kg/day of ethambutol and the other a 50 y/o female patient on 22 mg/kg/day of ethambutol (onset of visual disturbances 4 months and 3 weeks, respectively). Although most reported cases of optic neuritis were reversible, both patients had irreversible damage. In our case, the patient’s sight stabilized after 20 months, but never returned to normal.
3. Conclusions
Tenofovir is one of the agents classified as preferred by the Department of Health and Human Services (DHHS) HIV treatment guidelines and ethambutol is the first line agent for MAC treatment but requires weight based dosing and dose adjustments in the presence of renal dysfunction. Practitioners should be aware of the relatively rare occurrence of renal dysfunction that can occur with Fanconi like tubular dysfunction as a result of tenofovir. Patients receiving tenofovir and ethambutol should have renal function monitored carefully in order to prevent ethambutol toxicity due to the accumulation of ethambutol that can result from declining renal function. The basis for acute nephrotoxicity with tenofovir remains unknown and needs to be further investigated. However, clinicians should be aware that co-administered renally eliminated medications may require dose adjustments to prevent unnecessary toxicities.
The authors would like to acknowledge David Barker, MD, for his contribution in revising this case report.
REFERENCES
- C. A. Naranjo, U. Busto, E. M. Sellers, et al., “A Method for Estimating the Probability of Adverse Drug Reactions,” Clinical Pharmacology and Therapeutics, Vol. 30, No. 2, 1981, pp. 239-245. doi:10.1038/clpt.1981.154
- Department of Health and Human Services, “Panel on Antiretroviral Guidelines for Adults and Adolescents, Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents,” 2011, pp. 1-166. http://www.aidsinfor.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
- J. E. Gallant, S. Staszewski, A. L. Pozniak, et al., “Efficacy and Safety of Tenofovir DF vs. Stavudine in Combination Therapy in Antiretroviral-Naive Patients: A 3- Year Randomized Trial,” The Journal of the American Medical Association, Vol. 292, No. 2, 2004, pp. 191-201. doi:10.1001/jama.292.2.191
- S. Rodriguez-Novoa, E. Alvarez, P. Labarga and V. Soriano, “Renal Toxicity Associated with Tenofovir Use,” Expert Opinion on Drug Safety, Vol. 9, No. 4, 2010, pp. 545-559. doi:10.1517/14740331003627458
- H. Peyrière, J. Reynes, I. Rouanet, et al., “Renal Tubular Dysfunction Associated with Tenofovir Therapy: Report of 7 Cases,” Journal of Acquired Immune Deficiency Syndromes, Vol. 35, No. 3, 2004, pp. 269-273. doi:10.1097/00126334-200403010-00007
- R. Cooper, N. Wiebe, N. Smith, P. Keiser, S. Naicker and M. Tonelli, “Systematic Review and Meta-Analysis: Renal Safety of Tenofovir Disoproxil Fumarate in HIV-Infected Patients,” Clinical Infectious Diseases, Vol. 51, No. 5, 2010, pp. 496-505. doi:10.1086/655681
- J. Pavie, A. Scemla, M. A. Bouldouyre, E. Pillebout, J. Verine and J. M. Molina, “Severe Acute Renal Failure in an HIV-Infected Patient after Only 2 Weeks of Tenofovir-Based Antiretroviral Therapy,” AIDS Patient Care STDs, Vol. 25, No. 8, 2011, pp. 457-459. doi:10.1089/apc.2011.0056
- A. Malik, P. Abraham and N. Malik, “Acute Renal Failure and Fanconi Syndrome in an AIDS Patient on Tenofovir Treatment-Case Report and Review Of Literature,” Journal of Infection, Vol. 51, No. 2, 2005, pp. E61-E65. doi:10.1016/j.jinf.2004.08.031
- Centers for Disease Control and Prevention, “Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents,” Morbidity and Mortality Weekly Report, Vol. 58, No. RR-4, 2009, pp. 28-31.
- J. T. Fang, Y. C. Chen and M. Y. Chang, “Ethambutol-Induced Optic Neuritis in Patients with End Stand Renal Disease on Hemodialysis: Two Case Reports and Literature Review,” Renal Failure, Vol. 26, No. 2, 2004, pp. 189-193. doi:10.1081/JDI-120038521
- K. A. T. Estlin and A. A. Sadun, “Risk Factors for Etham- butol Optic Toxicity,” International Ophthalmology, Vol. 30, No. 1, 2010, pp. 63-72. doi:10.1007/s10792-009-9293-z
- R. Y. Chan and A. K. Kwok, “Ocular Toxicity of Ethambutol,” Hong Kong Medical Journal, Vol. 12, No. 1, 2006, pp. 56-60.
- S. B. Kokkada, R. Barthakur, M. Natarajan, S. Palaian, A. K. Shhetri and P. Mishra, “Ocular Side Effects of Antitubercular Drugs: A Focus on Prevention, Early Detection and Management,” Kathmandu University Medical Journal, Vol. 3, No. 4, 2005, pp. 438-441.
- G. A. Marietta, Ethambutol (Package Insert), Versa-Pharma Inc., 2005.

