World Journal of Vaccines
Vol. 2 No. 2 (2012) , Article ID: 19309 , 6 pages DOI:10.4236/wjv.2012.22008
Safety and Antibody Responses to Aerosolized MMR II Vaccine in Adults: An Exploratory Study
![]()
1Instituto Nacional de Salud Pública, Cuernavaca, México; 2Retired from Centers for Disease Control and Prevention, Atlanta, Georgia, USA; 3Instituto Nacional de Enfermedades Respiratorias, Mexico City, México.
Email: jdiaz@correo.insp.mx
Received September 7th, 2011; revised November 24th, 2011; accepted January 20th, 2012
Keywords: aerosolized MMR vaccine; Attenuvax Schwarz measles strains; Jeryl Lynn mumps strain; adult immunization; safety; immunogenicity
ABSTRACT
Background: There have been no reported studies involving aerosol immunization with 2 of the 3 components of MMR II vaccine—Attenuvax measles vaccine and Jeryl-Lyn mumps vaccine. Objective: To evaluate the safety and antibody responses to aerosolized Attenuvax measles strain, Jeryl Lynn mumps strain and RA 27/3 rubella component of an MMR vaccine in adults, before assessing the booster administration of this vaccine in children. Methods: A pilot study to evaluate safety and antibody responses of MMR II (Merch Sharp & Dhome Corp., Whitehouse Station, NJ 08889, USA) components administered by aerosol was carried out in 27 healthy adults of 21 to 38 years of age. All participants were followed-up during 28 days following immunization for detection of clinical adverse events. Immune response was evaluated by plaque reduction neutralization test for measles, and commercial ELISA kits for rubella and mumps. Results: Only mild clinical adverse events were noted. Despite high levels of baseline seropositivity to all vaccine components, seroresponses to measles, rubella and mumps occurred in 44%, 15% and 41%, respectively. Conclusions: These outcomes compare favorably with earlier studies of other MMR vaccines given by aerosol. Further evaluations on safety and booster immune response should be performed in children.
1. Introduction
Measles, rubella and mumps have previously been considered as illnesses of infancy. However, in countries now in transition to measles and rubella elimination, the greatest number of cases frequently occurs in young adults and adolescents. Measles and rubella elimination strategies implemented in Mexico include the routine administration of two doses of MMR vaccine, the first at 12 months and the second at 6 years of age. The routine program is accompanied by supplemental immunization activities of children 1 - 4 years of age, and the administration of MR vaccine to persons 13 or more years of age in catch-up campaigns. Mumps is not presently targeted for elimination in Mexico.
Globally there are 3 published studies about reactogenicity and secondary immune response to MMR vaccines when given by aerosol. One of these was performed in children of 4 - 6 years of age [1] and the others in adults [2,3]. The three studies have in common the evaluation of the memory immune response induced by the Edmonston-Zagreb measles strain (EZ), and the RA 27/3 rubella strains [1-3]. Aerosolized L-Zagreb [1,2] and Rubini [3] mumps strains were evaluated in these trials. Findings in the aerosol recipients were compared with those in recipients given the same vaccine by injection [1,2], and/or with recipients who received the MMR II vaccine given by injection [3]. The most recent publication [4] reported antibody persistence against the three antigens and the occurrence of delayed adverse events one year post-immunization following the preceding trial [3]. Antibody responses in these MMR studies comparing aerosol with injection of the same vaccines have consistently shown aerosolized EZ measles vaccine to be more immunogenic, and antibody responses to RA27/3 rubella vaccine to be equivalent or more frequent. Antibody responses to aerosolized L-Zagreb mumps vaccine were superior to injection in one study and equivalent in another. In the single study involving aerosolized Rubini mumps vaccine [3], there was little to no response following respiratory tract exposure to this very highly attenuated vaccine.
There have been no reported studies of aerosolized MMR II vaccine. Aerosol administration of two of the three antigens in MMR II vaccine, specifically the Attenuvax measles strain and the Jeryl Lynn mumps strain, have not previously been evaluated, whereas the RA 27/3 rubella component has been studied as noted above. However, the base sequence of Attenuvax is surprisingly identical to Schwarz measles vaccine [5]. There have been no recent studies of aerosolized Schwarz vaccine, but a study reported more than a decade ago documented rapid degradation of potency of Schwarz vaccine within the nebulizer of a compressed air system [6]. Thus, we opted to use a vibrating mesh device in the present study. In unpublished studies involving one of the authors (JVB), aerosols of Schwarz measles vaccine proved completely stable when produced by a vibrating mesh nebulizer. We undertook an exploratory study to evaluate the safety and antibody responses to each component of aerosolized MMR II in adults 21 - 38 years of age. This preliminary study was deemed necessary before further assessing the possible role of this vaccine in children in accord with Mexico’s current immunization policies, as noted above.
2. Methods
2.1. Recruitment
We carried out 5 information sessions with National Institute for Public Health (INSP) staff, during which attendees were informed orally and by written material about the benefits and risks of the study and the procedures for blood sampling and vaccination. At the end of each session we distributed the Informed Consent Letter (ICL) asking attendants to carefully read the letter and sign the document after a lapse of 2 - 3 days if they agreed to participate. In addition, we subsequently performed individual interviews to clinically evaluate potential participants, as well as to evaluate the inclusion and exclusion criteria. Only clinically healthy individuals were accepted into the study. Criteria for exclusion included histories of asthma, cancer, leukemia, HIV infection, untreated seizures, thrombocytopenic purpura, immuno-suppressive medications, blood transfusion or gamma globulin in the 3 previous months, or a positive test result for HIV or pregnancy. The same criteria, including signing of the ICL, were used for the recruitment of vaccine administrators. Nurses who were recruited received training on procedures used in the study. Only 27 persons of 33 interviewed were accepted into the study. We rejected six individuals due to asthma, and one person each for positive pregnancy test, receipt of corticosteroids, frequent epistaxis and hemorrhage with multiple ecchymotic areas in inferior limbs, MR vaccination in the previous 2 months, and a person who presented uncontrolled neurological manifestations (muscle spasms and hyperreflexia).
2.2. Vaccine
MMR II vaccine Lot number NJ 03070 (Merck Sharp & Dhome), with a potency evaluated by the Commission of Analytical Control and Expansion of Coverage (Mexico’s Health Secretariat) of log10 3.95 CCID50/0.5 mL for the Attenuvax strain of measles virus attenuated in chick embryo fibroblast; and log10 3.8 CCID50/0.5 mL for the strain RA 27/3 of rubella virus attenuated in human diploid cells, and of log10 4.65 CCID500/0.5 mL for Jeryl Lynn strain of the mumps virus attenuated in chick embryo fibroblast [7-10].
2.3. Nebulizer Device
We used the Aeroneb Go® nebulizer device [11]. The equipment consists of a plastic holder that has a cylindrical outlet port extending from the nebulization chamber. The outlet port allows attachment of a mask or mouthpiece for aerosol delivery. A medication chamber into which reconstituted vaccine was placed is located in the upper part of the holder. A vibrating membrane, the “generator OnQ®”, pumps fluid through small holes in the membrane. This transforms the reconstituted vaccine in the medication chamber into a small particle aerosol in the nebulization chamber with a median particle diameter of 3.6 microns. A transparent plastic lid covers the medication chamber. The control module works with three “AA” batteries, which connects to the medication device through a removable cord 1 meter in length. The assembled device has dimensions of 4.0 × 10.5 × 9.5 cm and weighs 60 g taking into account only the nebulizer, or 320 g with the control module included.
2.4. Mouthpiece
Disposable Safe-T-Check® mouthpieces were used [12]. The mouthpiece is a single use, cardboard, cylindrical, valved mouthpiece that was fitted over the outlet port. The inner diameter of the mouthpiece slightly exceeded the outer diameter of the outlet port, so a narrow strip of duct tape was wrapped around the distal surface of the outlet port in order to obtain a tight and secure fit. The default position of the valve is closed, and it opened only during inhalation and remained closed during exhalation.
2.5. Vaccine Administration
Experiments conducted at INSP showed that the Aeroneb Go nebulizer dispensed 0.2 mL of reconstituted vaccine during 30 seconds of continuous operation. Thus, ten doses of the lyophilized vaccine were reconstituted in 2 mL of diluent, in order to dispense in 30 seconds of nebulization an aerosol vaccine dose of 0.2 mL equivalent to 0.5 mL of vaccine for subcutaneous injection. The reconstituted vaccine was deposited in the nebulizer medication chamber. The device with the reconstituted vaccine was inserted into a container with crushed ice, to ensure a temperature of 4˚C to 8˚C. Each adult, while in a standing position, was exposed to aerosolized vaccine for 30 seconds after the battery powered control module was switched on. Subjects were asked to perform normal respiratory movements, breathing in through the mouthpiece and out through the nose. Subjects were asked to close their lips around the mouthpiece to limit the release of aerosol to the environment and prevent dilution of aerosol by ambient air. At the end of the 30 seconds of exposure to aerosol, the switch was turned off and the mouthpiece discarded into a plastic bag for potentially infectious materials. Immunizations were given in 6 separate sessions during April, 2009.
2.6. Laboratory Methods
Immediately before vaccination and after asepsis of the antecubital region, a 5 mL sample of blood was obtained by venipuncture and captured into a vacutainer without anticoagulant. A subsequent blood sample was similarly collected 4 weeks later. Blood samples were transported in an ice box at room temperature (not exceeding 25˚C) and centrifuged at the Laboratory of Viral Vaccines of the INSP. During the 3 - 4 hours while blood was being collected, samples were kept at −20˚C in cryotubes, and kept at that temperature until assayed for antibodies. The plaque reduction neutralization test (PRN test) was used to evaluate measles antibody levels [13,14], and immune-enzymatic assays with commercial ELISA kits (Behring) were used to determine antibody levels against rubella and mumps [15,16]. Laboratory results were given to each participant in the study. Seropositivity was defined as ≥120 mIU/mL for measles, ≥10 IU/mL for rubella, and >500 U/mL for mumps. Seroresponse was defined as a 2-fold or greater increase in antibody titer that also resulted in a post-exposure titer that was seropositive.
2.7. Clinical Follow-Up
To evaluate the health status of participants and the incidence of adverse events following immunization (AEFI), nurses visited each subject every other day during 28 days post-exposure. The medical investigator then visited participants with clinical signs and symptoms in order to collect more clinical details and to direct the person to a clinic or health center if required.
2.8. Data Analysis
Data were entered into an electronic database in EpiInfo version 6.02, and analyzed by EpiInfo and STATA 10. We obtained absolute and relative frequencies of the anthropometric and clinical characteristics as well as of the baseline and post-vaccination immunity. We calculated the proportions of seropositive preand post-vaccination for each vaccine component, seroresponse ratios and confidence intervals of 95%. We estimated the geometric mean antibody titers preand post-vaccination in seroresponders. We also estimated the proportions of AEFI and respective confidence intervals.
2.9. Biosafety and Ethical Considerations
This study was based on the Helsinki Declaration and was approved by the IRB of the INSP. Recruitment and informed consent procedures have been described earlier. Subjects were assured that the information that was collected on them would be kept in confidence. Participants could withdraw from the study at any time without any penalty or impairment of their rights. Potentially infectious material, such as cotton swabs and mouthpieces, were placed in plastic bags for disposal. For the disposal of sharps and other materials, we followed the Standard 087 for handling potentially infectious waste.
3. Results
3.1. Baseline Conditions
The average age of participants was 30 years, ranging from 21 to 38 years. Women comprised 2/3rds of the subjects. Less than half of the participants reported a history of measles (44%), rubella (19%) or mumps (26%). Twenty-four of the 27 (89%) subjects reported having received measles vaccine either as monovalent measles vaccine or MR, and no one reported receipt of MMR vaccine. About half (52%) had previously been given rubella vaccine. We found a high proportion of pre-vaccination seropositivity to measles (96%), rubella (89%), and mumps (70%), with high geometric mean antibody titers, respectively, of 1023 mIU/mL, 52 IU/mL, and 681 U/mL.
3.2. Immune Response
Seroresponses were noted in 12 for measles, 11 for mumps and only 4 for rubella. When presented by tertile of baseline antibody, a significant Chi-square for trend was noted with measles (p = 0.001) but not for the other 2 components. Seropositivity for measles and rubella changed little from their high baseline values, while mumps seropositivity increased by nearly 20 percentage points. Geometric mean titers in seroresponders increased 9-fold for mumps, 3.6-fold for measles and 2.6-fold for rubella. On logistic regression in this small data set, baseline titers were significant predictors of seroresponses to measles (p = 0.01), but not for rubella or mumps (Table 1).
3.3. Adverse Events Following Immunization (Table 2)
Only minor clinical events were detected, none of them required hospitalization, and no one was absent from work due to illness. The frequency of occurrence of one or more symptoms did not differ significantly between seroresponders to one or more of the three MMR II antigens 12/18 (67%) and non-responders 6/9 (67%). The frequencies of one or more AEFI after seroresponding to one, two or three of the MMR II components showed no significant trend in this small data set (6/10, 5/7 and 1/1, respectively.) One of the two cases with fever presented with bacterial pharyngitis that resolved satisfactorily after antibiotic treatment. One of the two adults with rash had an eruption in the folds of elbows, behind the head and on the neck above the sternum. The patient reported that he had previously had this type of rash associated with situations of emotional stress, and we considered the case to be compatible with neurodermatitis. The other case with rash occurred in a woman with a history of multiple allergies. The eruption affected the face and neck and had onset during a weekend. We were unable to observe this sign. A relatively high proportion of rhinitis was observed in the study, but clinical examination did not note inflammation, irritation, or redness of the nasal mucosa in any of the 7 cases. One of the 3 cases of conjunctivitis had recurrences for 2 years preceding immunization. The only single symptom that occurred significantly more frequently among seroresponders was headache, occurring in 8/18 seroresponders but 0/9 non-seroresponders (p = 0.02, Fisher’s). However, all cases of headache had histories of suffering chronically of this problem. Three participants experienced symptoms highly unlikely to be related to MMR vaccine: tightness in the chest and dyspnea (1 case), and epigastric pain (1 case). The third case reported headache and back pain, which was diagnosed as hypertension in the institution where she currently receives medical treatment.
4. Discussion
These three illnesses were highly endemic in Mexico during the lives of these subjects and this, coupled with frequent vaccination with measles and rubella vaccines, helps explain the high proportion of participants who had prior immunity to measles and rubella. Seropositivity to mumps could be explained in this age group as evidence of previous infection. The seroresponse to aerosolized measles component of the MMR II vaccine (44%), was higher than that obtained in another study where adults (18 - 25 years of age) received the aerosolized Triviraten vaccine (Berna Biotech®) containing the EZ measles virus (30%) and also higher than adults of the same study who received injections of MMR II (19%) or Triviraten vaccine (6%) [3,4]. Booster doses by aerosol of EZ measles vaccine, whether given as M, MR, or MMR,
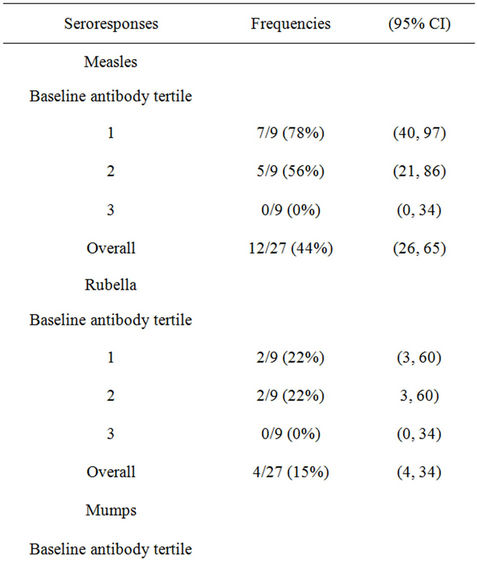
Table 1. Antibody responses to MMR II aerosol.
Table 2. Clinical manifestations in the 28 days following immunization.
have consistently proved more immunogenic than the same vaccine given by injection [1-3,6,17-19]. The above positive findings, despite very high baseline titers to measles, suggest this may also prove true for Attenuvax (Schwarz) measles vaccine.
The seroresponse to aerosolized rubella (15%), was higher than that reported for Triviraten vaccine containing the same strain of rubella vaccine given by aerosol (5%) or injection (4%) and comparable to that observed for the same virus (RA 27/3) included in the MMR II formulation administered by injection (14%) [3,4]. The response to aerosolized rubella was also less frequent than the 28% seroconversions noted in a preceding MMR study following aerosolized RA 27/3 vaccine [2]. The seroresponse to the aerosolized Jeryl Lynn strain of mumps (41%) was also higher than that obtained in the referenced study with the same mumps vaccine strain in MMR II applied by injection (15%), and comparable to the injected Rubini mumps strain in Triviraten vaccine (37%). There was virtually no immune response to Rubini strain applied by aerosol (1%) [3,4]. In a study performed in health workers, 74% of persons seroconverted following aerosolized L-Zagreb mumps vaccine in MMR from Serum Instituite of India [2]. The satisfactory response to Jeryl-Lyn is especiallly notable, since this vaccine is generally considered among the safest of mumps vaccines [7]. The above comparisons with antibody responses in the preceding trials with aerosolized MMR vaccine may not be reliably informative, since they involved different study populations with differing baseline antibody profiles, vaccines with differing potencies, and different antibody tests and aerosol administration techniques were used. Reliable comparative estimates of MMR vaccines will require head-to-head comparisons in randomized trials using the same aerosol administration techniques. No serious adverse events were noted, and most were mild and transitory, except for headache, did not occur more frequently in seroresponders than in non-seroresponders. We believe most of the adverse events are consistent with background illnesses occurring over a month of observation.
5. Conclusion
Aerosolized MMR II vaccine was safe and capable of inducing secondary immune response to the three components of the vaccine. The frequencies and types of AEFI are consistent with the mild AEFI observed in prior aerosol trials. Additional comparative evaluations of MMR aerosols seem warranted mainly in booster response in children.
6. Acknowledgements
The study was partially funded by the Consejo Nacional de Ciencia y Tecnología (Mexico) (grant Salud-2007- CO1-71241). Authorities of the INSP complemented funding.
REFERENCES
- J. V. Bennett, J. Fernández de Castro, R. Martínez Poblete, M. L. Garcia-Alcantara, E. G. Diaz, M. A. M. Angeles, R. M. Wong-Chew, E. Arias-Toledo, C. Witham and J. I. Santos-Preciado, “A New Rapid, and Promising Approach to Aerosol Immunization: Inflatable Bags and Valved Masks,” Vaccine, vol. 27, No. 34, 2009, pp. 4571-4575. doi:10.1016/j.vaccine.2009.05.086
- J. Fernández de Castro, J. V. Bennett, H. Gallardo-Rincon, M. T. Muñoz, L. A. Sanchez and J. I. Santos, “Evaluation of Immunogenicity and Side Effects of Triple Viral Vaccine (MMR) in Adults, Given by Two Routes: Subcutaneous and Respiratory (aerosol),” Vaccine, vol. 23, No. 8, 2005, pp. 1079-1084. doi:10.1016/j.vaccine.2004.08.018
- J. L. Diaz-Ortega, J. V. Bennett, D. Castañeda, J. R. Vieyra, J. L. Valdespino-Gomez and J. Fernández de Castro, “Successful Seroresponses to Measles and Rubella Following Aerosolized Triviraten Vaccine, but Poor Response to Aerosolized Mumps (Rubini) Component: Comparisons with Injected MMR,” Vaccine, vol. 28, No. 3, 2010, pp. 692-698. doi:10.1016/j.vaccine.2009.10.083
- J. L. Diaz-Ortega, J. V. Bennett, D. Castañeda, D. Martinez and J. Fernández de Castro, “Antibody Persistence in Young Adults 1 year after MMR Immunization by Aerosol or by Subcutaneous Route,” Vaccine, vol. 28, No. 44, 2010, pp. 7228-7232. doi:10.1016/j.vaccine.2010.08.055
- B. Bankamp, M. Takeda, Y. Zhang, W. Yu and P. Rota, “Genetic Characterization of Measles Vaccine Strains,” Journal of Infectious Diseases, vol. 204, No. S1, 2011, pp. S533-S548. doi:10.1093/infdis/jir097
- A. Dilraj, F. Cutts, J. Fernandez de Castro, J. Wheeler, D. Brown, H. Coovadia and J. V. Bennett, “Response to Different Measles Vaccine Strains Given by Aerosol and Subcutaneous Routes to Schoolchildren: A Randomized Trial,” Lancet, vol. 355, No. 9206, 2000, pp. 798-803. doi:10.1016/S0140-6736(99)95140-1
- A. M. Galazka, S. Robertson and A. Kraigher, “Mumps and Mumps Vaccine: A Global Review,” Bulletin of the World Health Organization, vol. 77, No. 1, 1999, pp. 3- 14.
- P. M. Strebel, M. J. Papania, G. H. Dayan and N. A. Halsey, “Measles vaccine,” In: S. A. Plotkin, W. Orenstein and P. Offit, Eds., Vaccines, WB Saunders Elsevier, Philadelphia, 2008, pp. 353-398.
- S. A. Plotkin and S. E. Reef, “Rubella vaccine,” In: S. A. Plotkin, W. Orenstein and P. Offit, Eds., Vaccines, WB Saunders Elsevier, Philadelphia, 2008, pp. 737-772.
- Biológicos y Reactivos de México, “MMR vaccines registered in Mexico,” 2008. http://www.birmex.gob.mx/birmexnuevo/images/vacuna_tripleviral.pdf
- Aerogen, “Aeroneb Go,” 2009. http://www.aerogen.com/aeroneb-go-micropump-nebulizer-system.htmL
- Vacumed, “1029 Disposable Cardboard Mouthpieces with One-Way Valve,” 2009. http://www.vacumed.com/zcom/product/Product.do?compid=27&prodid=202
- B. J. Cohen, S. Audet, N. Andrews and J. Beeler, “Plaque Reduction Neutralization Test for Measles Antibodies: Description of A Standardized Laboratory Method for Use in Immunogenicity Studies of Aerosol Vaccination,” Vaccine, vol. 26, No. 1, 2007, pp. 59-66. doi:10.1016/j.vaccine.2007.10.046
- R. T. Chen, L. Markowitz, P. Albrecht, J. A. Stewart, L. M. Mofenson, S. R. Preblud and W. A. Orenstein, “Measles Antibody: Reevaluation of Protective Titers,” Journal of Infectious Diseases, vol. 162, No. 5, 1990, pp. 1036-1042. doi:10.1093/infdis/162.5.1036
- Behring, Enzygnost® Anti-Rubella-Virus/IgG, Manual de Instrucciones, 2004.
- Behring, Enzygnost® Anti Parotitis-Virus/IgG, Manual de Instrucciones, 2003.
- J. V. Bennett, J. Fernández de Castro, J. L. ValdespinoGomez, L. Garcia-Garcia, R. Islas-Romero, G. EchanizAviles, A. Jimenez-Corona and J. Sepulveda-Amor, “Aerosolized Measles and Measles-Rubella Vaccines Induce Better Measles Antibody Booster Responses than Injected Vaccines: Randomized Trials in Mexican Schoolchildren,” Bulletin of the World Health Organization, vol. 80, No. 10, 2002, pp. 806-812.
- J. Bellanti, B. Zeligs, J. Mendez-Inocencio, L. GarciaGarcia, R. Islas-Romero, B. Omidvar, J. Omidvar, G. Kim, J. Fernandez de Castro, J. Sepulveda-Amor, L. Walls, W. Bellini and J. L. Valdespino-Gomez, “Immunologic Studies of Specific Mucosal and Systemic Immune Responses in Mexican Schoolchildren After Booster Aerosol or Subcutaneous Immunization with Measles Vaccine,” Vaccine, vol. 22, No. 9-10, 2004, pp. 1214-1220. doi:10.1016/j.vaccine.2003.09.032
- J. Sepulveda-Amor, J. L. Valdespino-Gomez, L. GarciaGarcia, J. V. Bennett, R. Islas-Romero, G. Echaniz-Aviles and J. Fernández de Castro, “A Randomized Trial Demonstrating Successful Boosting Responses Following Simultaneous Aerosols of Measles and Rubella (MR) Vaccines in Schoolchildren,” Vaccine, vol. 20, No. 21-22, 2002, pp. 2790-2795. doi:10.1016/S0264-410X(02)00179-2

