World Journal of Nano Science and Engineering
Vol.3 No.4(2013), Article ID:40500,8 pages DOI:10.4236/wjnse.2013.34016
Improvement of Open-Circuit Voltage in Organic Photovoltaic Cells with Chemically Modified Indium-Tin Oxide
1Department of Electronics and Computer, School of Power Engineering, Mongolian University of Science and Technology, Ulaanbaatar, Mongolia
2Center for Nanoscience and Nanotechnology, Department of Chemical Technology, School of Chemistry and
Chemical Engineering, National University of Mongolia, Ulaanbaatar, Mongolia
3Department of Chemistry, Chemical Engineering and Life Science, Yokohama National University, Yokohama, Japan
4Faculty of Engineering, New Mongol Institute of Technology, Ulaanbaatar, Mongolia
Email: ch_ganzorig@num.edu.mn
Copyright © 2013 Khayankhyarvaa Sarangerel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 12, 2013; revised September 13, 2013; accepted September 20, 2013
Keywords: Open-Circuit Voltage; Chemical Modification; Indium-Tin Oxide
ABSTRACT
The possibility of the increase in open-circuit voltage of organic photovoltaic cells based primarily indium-tin oxide (ITO)/rubrene/fullerene/Al structure by changing the work function of ITO anodes and Al cathodes was described in this work. To change built-in potential preferably in order to increase the open-circuit voltage, the work function of ITO should be increased and work function of Al should be decreased. The correlation between the change in work functions of electrodes and performance of the organic photovoltaic cells before and after surface modifications was examined in detail. The enhancement of open-circuit voltage depends on a function of work function change of both ITO and Al electrode. We could show that the built-in potential in the cells played an important role in open-circuit voltage.
1. Introduction
Organic photovoltaic (PV) cells have been attracted much attention in recent decades due to their potentials as fabrication, low-cost production, and technological advantages of semiconductor materials [1-5]. Since the first report of donor-acceptor heterojunction with a power conversion efficiency (hp) of about 1% by Tang [6], new materials and device structures have been developed in PV cells [7-15]. After the first report of organic PV cells, the performances of this type of cells have been significantly improved to reach hp in a range of 3% - 8% [8,9,16,17]. However, such efficiency is not sufficient for practical use, and further improvement is required.
To obtain large open-circuit voltage (Voc), Taima et al. introduced a p-type semiconductor 5, 6, 11, 12-tetraphenylnaphthacene (rubrene), which has the HOMO level of 5.4 eV. They obtained the Voc of 0.91 V [18]. Forrest et al. introduced an excellent p-type semiconductor boron subphthalocyanine chloride (SubPc) with a low HOMO level of 5.6 eV [19].
Indium-tin-oxide (ITO) is the most widely used as a transparent anode in organic PV cells due to its high conductivity, work function, and transparency in the visible spectral range [6]. Thus, various surface treatments of ITO have been attempted to change the work function of ITO in order to improve the properties of ITO substrates and control the charge injection barrier height reviewed in previous reports [20,21]. Although a number of groups have shown that chemical modification of ITO can be used to optimize the performance of organic lightemitting diodes (OLEDs) [20,21], there have been limited attempts to use chemical modification or chemically selfassembled monolayers (SAMs) in organic PV cells [22,23].
To investigate the possibility of increase in Voc by controlling the work functions of the electrodes, we report here the use of chemically modified ITO with different terminal groups (Hand Cl-) of p-benzenesulfonyl chlorides and p-chlorophenyldichlorophospate (-P) forming effective monolayers. We examine the correlation between the change in the work function of ITO and the performance of the PV cells by the chemical modification and find that the large increase in Voc. In this work, we selected tris(8-hydroxyquinoline)aluminum (Alq3) as an electron transport layer (ETL) to substitute for bathocuproine (BCP) in cells based on rubrene (Rub)/buckminsterfullerene (C60) heterojunction. Moreover, to examine the further improvement of Voc, we used a lithium carboxylate (C6H5COOLi) [24] as a cathode interface material with low-work function which was inserted between ETL and Al.
2. Experimental
ITO coated glass substrates with a sheet resistance of ca. 15 W/square (Sanyo Vacuum Industries) were cleaned by sonication successively in two detergents (Extran MA 03, pH 6.8, MERCK and Kontaminon O, pH 10, WAKO), rinsed with deionized water, and stored in isopropanol until being required. After cleaning with acetone and isopropanol (this cleaned ITO will be called hereafter “as-cleaned ITO” with notation of “ac”) the ITO substrates were immersed for 5 min in dichloromethane solutions containing 1 mM of (Hand Cl-) of p-benzenesulfonyl chlorides (Tokyo Chemical Industry) and p-chlorophenyldichlorophospate (Tokyo chemical industry). The modified ITO anodes were rinsed in pure dichloromethane and then vacuum dried for ~1 h.
C60 (purity > 99%) (Tokyo Chemical Industry), the sublimed grade rubrene (Aldrich Co.) and Alq3 (Dojindo Labs), the reagent grade BCP (Kanto Chemical), and lithium benzoate (purity~99%) (Aldrich Co.) were used without further purification. All the materials were deposited using vacuum evaporation under a pressure of 5 - 7 ´ 10−6 Torr at deposition rates of 1 - 1.5 Ǻ/s for organic layers and 3 - 4 Ǻ/s for Al cathode. The active area for all the cells was defined to be 5 ´ 5 mm2 by using a shadow mask. The current density-voltage (J-V) curves were measured under illumination of a simulated solar light with 100 mW ´ cm−2 (AM1.5G) by a solar simulator (Yamashita Denso, YSS-50). Electric data were taken using an Advantest R6145 DC voltage current source unit at room temperature in ambient atmosphere.
The absorption spectral data for all the thin film were taken using an UV-visible spectrophotometer (UV-265 FW, Shimadzu) at room temperature in ambient atmosphere.
3. Results and Discussions
3.1. Expected Energy Diagrams of PV Cells
For a cell based on exciton dissociation by charge transfer at a donor-acceptor (D/A) interface, hp is the product of the efficiencies [1] of four sequential steps 1) photon absorption leading to the generation of an exciton, 2) diffusion of the exciton to the D/A interface, 3) exciton dissociation (or charge separation) by charge-transfer (CT) at the D/A interface, and 4) collection of the free charge carriers at electrodes, i.e., charge transport to the anode (holes) and cathode (electrons), to supply a direct current.
Figure 1 shows the interfacial energy diagrams with shifts of vacuum level (D) at the interfaces due to dipole layer formation in four types of cells studied in the present work. In general, the work function of metal is changed by covering the metal surface with different materials [25]. First, we will discuss the shift at a C60/Al cathode interface. The photoemission study of the C60/Al interface revealed an abrupt vacuum-level shift of D = ~ +0.9 eV [26]. Namely, the work function of the Al electrode (4.2 eV) was increased to 5.1 eV by depositing a C60 film on an Al surface. This shift is schematically illustrated in Figures 1(a) and (b). The same energy level shift at the C60/Al interface was also reported previously [27]. Another group reported the shift of +0.7 eV for the C60/Al interface and that of +0.9 eV for the C60/LiF(0.5 nm)/Al interface [28]. In the latter case, the work function was increased from 3.6 eV (LiF/Al) to 4.5 eV (C60/LiF/Al). The increase in the work function for all cases described above is possibly interpreted by partial electron transfer from Al to C60 [26-28]. The HOMO and LUMO levels of C60 are reported to be 6.2 eV and 3.7 eV, respectively [10]. The increase in the work function of the Al electrode, however, is not preferable to create the built-in potential (Vbi) to separate the charge effectively in the PV cells.
In order to decrease the work function of the Al electrode, we have to put another layer of less electron affinity than C60. As such materials, we examined Alq3 and BCP [10,16,20,29-31] LUMO levels of which are higher (i.e., less electron affinity) than that of C60. In fact, the organic side for these interfaces is charged positively, making this side more comfortable (low energy) for an electron, and making the sign of D negative. Taking into account the D at Alq3/Al interface of ~−1.0 eV [25], the resulting work function of Alq3/Al is decreased from the value of metallic Al (4.2 eV) [16] down to 3.2 eV as shown in Figures 1(c)-(d). The work function of the LiF/Al substrate was also gradually decreased upon Alq3 deposition, from 3.6 eV to 3.1 eV for Alq3 film deposition [28,32]. Toyoshima et al. reported the electronic structure at the interface between BCP and Al by UV photoemission spectroscopy [33]. Their results for BCP /Al interface were similar to the shift in the work function as observed at Alq3/Al interface [25,32]. In this way, we constructed the energy diagrams of the Al cathode side as shown in Figure 1.
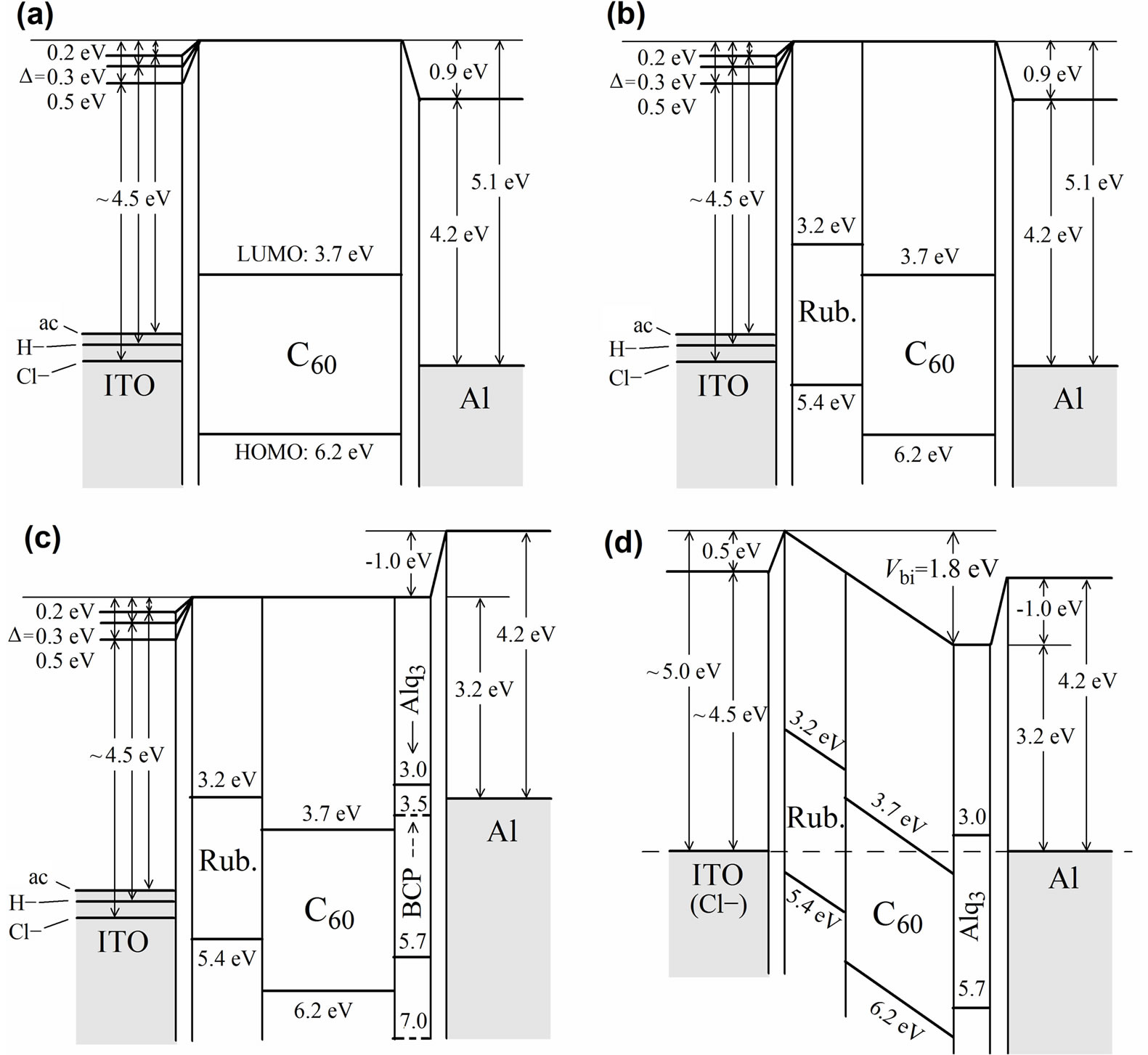
Figure 1. Interfacial energy diagrams with the shifts of vacuum level (D) at the interfaces due to dipole layer formation in the PV cells. These lead to buildup of built-in potential (Vbi) as shown in (d).
Next, we discuss the work function control of the anode side. The molecular approach allows for fine-tuning the work function using organic molecules on ITO depending upon magnitude and direction of the dipole moment [34]. The effective work functions formed by chemical modification of ITO shown in Figure 1 were estimated from the contact potential difference (CPD) values [34,35].
An interface dipole with its negative end pointing toward the organic layer and its positive end toward the electrode surface increases the ITO work function (i.e., the Fermi energy is down) and HOMO energy level in the organic layer is relatively up by adding an electrostatic energy [8] as shown in Figure 1. When the cells studied have the same cathode material, the changes in Vbi obtained for cells with variously modified ITO electrodes are equal to the changes in the ITO work function. This is illustrated on the left side of Figure 1, where we consider that the ITO work function is in the range 4.5 - 5.0 eV. The HOMO and LUMO values for rubrene are reported to be 5.4 eV and 3.2 eV, respectively [18]. The work function control at the anode as well the cathode leads to buildup of a large Vbi as shown in Figure 1(d). The dipole layers at interfaces may have a deep impact on the Vbi and consequently on the Voc of organic PV cells.
3.2. Characteristics of PV Cells
Figure 2 shows the effect of ITO work function on the current density-voltage (J-Vbias) characteristics under 100 mW ´ cm−2 illumination and in dark of four kinds of the PV cells with various surface treatments of ITO. Figure 2(a) shows the room temperature J-Vbias characteristics of ITO(variously treated)/C60(60 nm)/Al single-layer cells with a focus on the dark conduction properties. A linear fitting of the log-log plot (not shown) for these cells shows that the current for forward bias (electrons injection from the top contact) increases much slower (a slope is ~1) than the space-charge limited conduction (SCLC) [36]. Conducting charge transfer complex formed on C60/metal interface was studied in previous report [37]. The gap state, pinning the Fermi level close to the LUMO of C60 molecules, is originating from the C60-metal complex formation at the interface [37]. The unoccupied

Figure 2. The effect of ITO work function on the J-Vbias characteristics under 100 mW ´ cm−2 illumination and in dark of the cells with various surface treatments of ITO: (a) single-layer C60, (b) two-layer Rub/C60, and three-layers (c) with Alq3, and (d) with BCP.
states of the C60-metal complex appeared between the Fermi level and LUMO of C60 molecules lower the injection barrier which directly explains the improved device characteristics [37]. These states lead to the formation of Ohmic contact at the C60/Al. A thin LiF interlayer inserted between C60 film and Al cathode gave an effective passivation for the contacts by preventing Al oxidation [38]. It appears that a thin interlayer can help to perverse the SCLC in C60 film and cells under exposure to air, by considerably suppressing the oxygen diffusion into the C60 film and reaction at the C60/Al interface [38].
In Figures 2(b) and (c), the Voc was most effectively increased when Cl-terminated benzenesulfonyl chloride was used. With ITO modified with H-terminated benzenesulfonyl chloride without any appreciable dipole moment in the para-position, the characteristics of PV cells were much better than those on as-cleaned ITO. From the results, the PV characteristics, in particular the Voc, which were well correlated with the work function change, were dramatically improved by the chemical modification of ITO.
The changes in Voc resulting from changes in the self-assembling dipole molecules used to coat the ITO surfaces follow the work function changes for the resulting ITO electrodes. Higher work function anodes result in larger values of the Voc in the cells studied in the present work. In Figure 2(c), it can be seen, for example, that the Voc can be increased from 0.35 V (as-cleaned ITO anode) to 0.48 V by grafting benzenesulfonyl chloride onto the ITO electrode. By grafting a monolayer of p-chlorobenzenesulfonyl chloride molecules results in a further increase in the Voc to 0.84 V.
A dramatic effect of Alq3 (10 nm) or BCP (10 nm) as a buffer layer deposited between C60 and Al on the measured J-Vbias characteristics is clearly observed in Figures 2(b)-(d). The PV cells with Alq3 or BCP exhibited the best J-Vbias characteristics and gave the typical diode characteristics. BCP is the material selected as an exciton blocking layer [16]. Alq3 is a good ETL material widely used in OLEDs [34]. It can be seen in Figures 2(c) and (d) that the cells exhibited similar PV characteristics, indicating that band gaps and LUMO levels of the materials of Alq3 and BCP have minor effects on the PV performances. As shown in Figure 1(c), while bandgaps and LUMO levels of Alq3 and BCP are different, the performance of the cells is not significantly changed by different ETL materials (Table 1). This indicates that electron transport in these cells should not be via LUMO levels of these ETL materials. The results suggest that the most important role of Alq3 or BCP is to establish an Ohmic contact between Al and C60 i.e., a protective film on C60 [39].
While alkali metal-doped organic materials such as Alq3 have been used as an ETL in OLEDs [40,41] this approach has been not been explored in organic PV cells. Recently, we observed the alkali metal formation by thermal decomposition during vapor deposition of alkali metal carboxylates without post-deposition of Al cathode [42].
The improvement was attributed to the reaction of hot Al atoms with C6H5COOLi to form metallic Li during Al vapor deposition [41]. The resulting metallic Li was believed to dope the Alq3 layer [40] and to alloy with the Al cathode [24]. To examine the influence of induced Vbi on the performance of the cells, a 2 nm C6H5COOLi as a cathode interface material deposited between C60 or C60/Alq3 (or C60/BCP) and Al cathode was used. The J-Vbias characteristics of the ITO (modified with Cl-)/ Rub(20 nm)/C60(40 nm)/Al with variously configured cathodes under 100 mW ´ cm−2 illumination and in dark are shown in Figure 3. The PV performances of the cells are summarized in Table 1. Among all the cells studied, we found that the large increase in Voc for the ITO (modified with Cl-)/Rub (20 nm)/C60 (40 nm)/Alq3 (10 nm)/Li/Al cell. We obtained the Voc of 0.92 ± 0.1 V.
In Figure 3 and Table 1, the Voc of cell with the Alq3/Li/Al is the highest, and followed by the BCP/Li/Al, Alq3/Al or BCP/Al, Al, and C6H5COOLi/Al cells. The results suggest the importance of lower work function of Li (2.9 eV) for the larger Voc [41]. The Vbi is increased to larger value, giving an interfacial dipole, which may be attributed to both the alloy formation at the Al cathode [24,41] and doping of Alq3 with Li [40-43]. The ITO (modified with Cl-)/Rub (20 nm)/C60(40 nm)/Li/Al cell exhibited the lowest Voc. It may be considered, however, from our recent study of quenching by the presence of excess and colored species [44] that the doping of C60

Table 1. The performances of organic PV cells with a structure of ITO(Cl-)/Rub/C60/Al with different configured cathodes under 100 mW ´ cm−2 illumination.
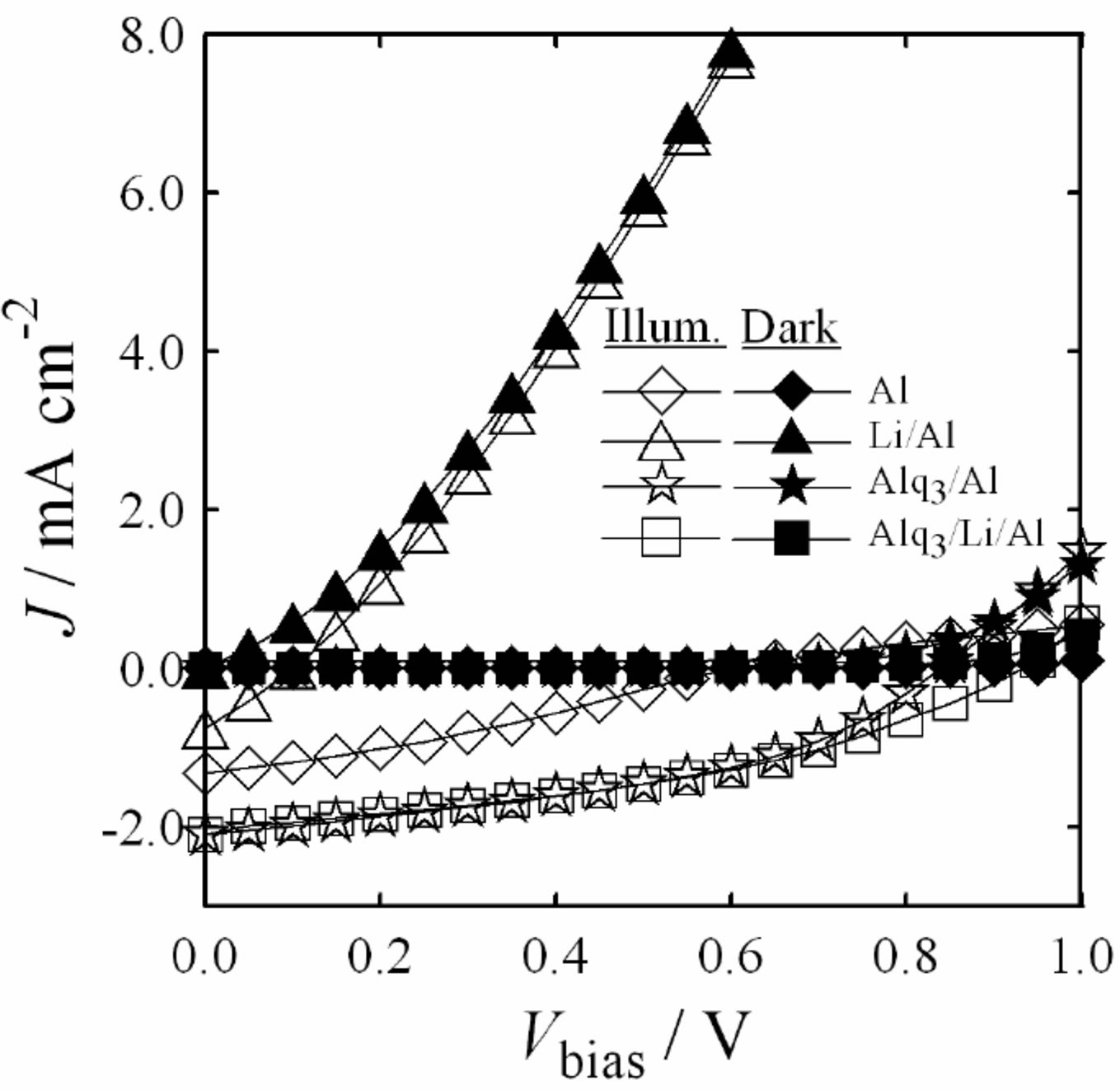
Figure 3. J-Vbias characteristics of the ITO(Cl-)/Rub/C60/ Al with variously configured cathodes under 100 mW ´ cm−2 illumination and in dark.
with Li increases exciton quenching and so that more excitons cannot contribute to the generation of electricity. Srdanov et al. reported results of an in-situ optical absorption study on alkali metal-doped thin films of C60 [45].
Gregg and Hanna proposed that the Voc is controlled by a chemical potential energy gradient of the organic PV cells [46]. The chemical potential gradient is equivalent to the carrier concentration gradient and would depend on the carrier mobility. Based on this idea, Voc is dependent on the hole mobility [47]. The Vbi still plays a role in most organic PV cells. Both the chemical potential and the electrical potential must be taken into account [46].
In bilayer cells, the Voc scales linearly with the work function difference, however, with an additional contribution depending on the light intensity. This contribution is due to the accumulation of charge carriers at the D/A interface, giving rise to a diffusion current which must be compensated by a drift current at open circuit [48]. Under illumination, charges are separated across the D/A interface. Due to the concentration gradient, carriers will diffuse away from the interface, leading to a net diffusion current. The effect of diffusion on Voc in single-layer PV cells has been studied by Malliaras et al. [49]. The accumulated charges at the interface will create a bandbending, which leads to a reduction of the electric field in the bulk of the cell.
In addition to attempts to optimize the components and composition of the active layer, modification of the electrodes has also lead to an improvement in device performance [50]. It is evident that the work function of the negatively charged electrode is relevant for the Voc of the cells. In the classical metal-insulator-metal (MIM) concept, the Voc is in first order approximation governed by the work function difference of the anode and the cathode, respectively. It should be noted that this only holds for the case where the Fermi levels of the contacts are within the bandgap of the insulator and are sufficiently far way from the HOMO and the LUMO levels, respectively [51]. In the case of ohmic contacts, meaning that the negative and positive electrodes match the LUMO level of the acceptor and the HOMO level of the donor, respectively, the situation is different; charge transfer of electrons or holes from the metal into the semiconductor occurs in order to align the Fermi level at the negative and positive electrode, respectively [51]. As a result, the electrode work functions become pinned close to the LUMO/HOMO level of semiconductor [50]. Because of this pinning, the Voc will be governed by the energetics of the LUMO of the acceptor and the HOMO of the donor. Indeed, in bilayer cells, a linear correlation of the Voc with the reduction potential of the acceptor has been reported [52].
For cells with non-ohmic contacts, the observed Voc is in agreement the expected values [50]. In this case, the Voc is determined by the work function differences of the electrodes. However, for the Ohmic contact the measured value is lower that the predicted value, possibly due to the energetic disorder of the charge transport levels [50]. Furthermore, generation of free charges is enhanced by an electric field in the appropriate direction. Possible mechanism by which balance between electron and hole escape currents can be maintained in the steady state is by the build-up of a net charge density within the cell. This acts to reduce the electric field (and hence suppress the generation of free charges) at one electrode and increase it at the other [53]. Snaith et al. found that a charge injection barrier from electrodes into the polymer film helps to retain a high Voc in the blend cell [53].
4. Conclusion
We have studied the use of chemically modified ITO with 4-chlorophenyldichlorophospate and different terminal groups (Hand Cl-) of p-benzenesulfonyl chlorides forming effective monolayers to control work function of ITO for enhancing the Voc in organic PV cells. We have examined the correlation between the change in the work functions of electrodes and the performance of the PV cells before and after the surface modification and found that there is an large increase in Voc for the ITO (modified with Cl-)/Rub(20 nm)/C60(40 nm)/Alq3(10 nm)/ C6H5COOLi (2 nm)/Al. We obtained the Voc of 0.92 ± 0.1 V. Controlling the work functions of electrodes by surface modification at the interfaces is a key parameter, which is useful for interpreting the origin of the opencircuit voltage and leads to improvements in the J-Vbias characteristics of the cells.
REFERENCES
- C. Lee, P. Linneman, P. Peumans, A. Yakimow and S. R. Forrest, “Small Molecular Weight Organic Thin-Film Photodetectors and Solar Cells,” Journal of Applied Physics, Vol. 93, No. 7, 2003, pp. 3693-3723. http://dx.doi.org/10.1063/1.1534621
- S. R. Forrest, “The Path to Ubiquitous and Low-Cost Organic Electronic Applications on Plastic,” Nature, Vol. 428, No. 6994, 2004, pp. 911-918. http://dx.doi.org/10.1038/nature02498
- T. Kietzke, “Recent Advances in Organic Solar Cells,” Advances in OptoElectronics, Vol. 2007, No. 40285, 2007, pp. 1-15. http://dx.doi.org/10.1155/2007/40285
- J. Xue, “Perspectives on Organic Photovoltaics,” Polymer Reviews, Vol. 50, No. 4, 2010, pp. 411-419. http://dx.doi.org/10.1080/15583724.2010.515766
- F. C. Krebs, “Polymeric Solar Cells: Materials, Design, Manufacture,” DEStech Publications, Inc., Lancaster, 2010.
- C. W. Tang, “Two-Layer Organic Photovoltaic Cell,” Applied Physics Letters, Vol. 48, No. 2, 1986, pp. 183-185. http://dx.doi.org/10.1063/1.96937
- Z. R. Dai, Z. W. Pan and Z. L. Wang, “Novel Nanostructures of Functional Oxides Synthesized by Thermal Evaporation,” Advanced Functional Materials, Vol. 13, No. 1, 2003, pp. 9-24. http://dx.doi.org/10.1002/adfm.200390013
- J. Xue, S. Uchida, B. P. Rand and S. R. Forrest, “4.2% Efficient Organic Photovoltaic Cells With Low Series Resistances,” Applied Physics Letters, Vol. 84, No. 16, 2004, pp. 3013-3016. http://dx.doi.org/10.1063/1.1713036
- J. Xue, S. Uchida, B. P. Rand and S. R. Forrest, “Asymmetric Tandem Organic Photovoltaic Cells with Hybrid Planar-Mixed Molecular Heterojunctions,” Applied Physics Letters, Vol. 85, No. 23, 2004, pp. 5757-5759. http://dx.doi.org/10.1063/1.1829776
- C. F. Lin, M. Zhang, S. W. Liu, T. L. Chiu and J. H. Lee, “High Photoelectric Conversion Efficiency of Metal Phthalocyanine/Fullerene Heterojunction Photovoltaic Device,” International Journal of Molecular Sciences, Vol. 12, No. 1, 2011, pp. 476-505. http://dx.doi.org/10.3390/ijms12010476
- G. Dennler, M. C. Scharber and C. J. Brabec, “PolymerFullerene Bulk-Heterojunction Solar Cells,” Advanced Materials, Vol. 21, No. 13, 2009, 1323-1338. http://dx.doi.org/10.1002/adma.200801283
- H. Y. Chen, et al., “Polymer Solar Cells with Enhanced Open-Circuit Voltage and Efficiency,” Nature Photonics, Vol. 3, No. 11, 2009, pp. 649-653. http://dx.doi.org/10.1038/nphoton.2009.192
- G. E. Morse and T. P. Bender, “Boron Subphthalocyanines as Organic Electronic Materials,” ACS Applied Materials & Interfaces, Vol. 4, No. 10, 2012, pp. 5055-5068. http://dx.doi.org/10.1021/am3015197
- G. Li, R. Zhu and Y. Yang, “Polymer Solar Cells,” Nature Photonics, Vol. 6, No. 3, 2012, pp. 153-161. http://dx.doi.org/10.1038/nphoton.2012.11
- J. Yang, L. Qian, R. Zhou, Y. Zheng, A. Tang and P. H. Holloway, “Hybrid Polymer: Colloidal Nanoparticle Photovoltaic Cells Incorporating a Solution-Processed, MultiFunctioned ZnO Nanoscrystal Layer,” Journal of Applied Physics, Vol. 111, No. 4, 2012, pp. 044323-044330. http://dx.doi.org/10.1063/1.3689154
- P. Peumans and S. R. Forrest “Very-High-Efficiency Double-Heterostructure Copper Phthalocyanine/C60 Photovoltaic Cells,” Applied Physics Letters, Vol. 79, No. 1, 2001, pp. 126-128. http://dx.doi.org/10.1063/1.1384001
- M. A. Green, K. Emery, Y. Hishikawa and W. Warta, “Solar Cell Efficiency Tables (Version 37),” Progress in Photovoltaics, Vol. 19, No. 1, 2011, pp. 84-92. http://dx.doi.org/10.1002/pip.1088
- T. Taima, J. Sakai, T. Yamanari and K. Saito, “Realization of Large Open-Circuit Photovoltage in Organic ThinFilm Solar Cells by Controlling Measurement Environment,” Japanese Journal of Applied Physics, Vol. 45, No. 37, 2006, pp. L995-L997. http://dx.doi.org/10.1143/JJAP.45.L995
- K. L. Mutolo, E. I. Mayo, B. P. Rand, S. R. Forrest and M. E. Thompson, “Enhanced Open-Circuit Voltage in Subphthlocyanine/C60 Organic Photovoltaic Cells,” Journal of the American Chemical Society, Vol. 128, No. 25, 2006, pp. 8108-8109. http://dx.doi.org/10.1021/ja061655o
- M. Fujihira and C. Ganzorig, “Conjugated Polymer and Molecular Interfaces,” In: A. Kahn, J. J. Pireaux, W. R. Salaneck and K. Seki, Eds., Marcel Dekker, New York, 2002, pp. 817-858.
- C. Ganzorig and M. Fujihira, “Chemically Modified Oxide Electrodes,” In: A. J. Bard and M. Stratmann, Eds., Modified Electrodes, WILEY-VCH Verlag GmbH, Weinheim, 2007, pp. 261-334.
- N. R. Armstrong, et al., “Interface Modification of ITO Thin Films: Organic Photovoltaic Cells,” Thin Solid Films, Vol. 445, No. 2, 2003, pp. 342-352. http://dx.doi.org/10.1016/j.tsf.2003.08.067
- S. Khodabakhsh, B. M. Sanderson, J. Nelson and T. S. Jones, “Using Self-Assembling Dipole Molecules to Improve Charge Collection in Molecular Solar Cells,” Advanced Functional Materials, Vol. 16, No. 1, 2006, pp. 95-100. http://dx.doi.org/10.1002/adfm.200500207
- C. Ganzorig and M. Fujihira, “A Lithium Carboxylate Ultrathin Film on an Aluminum Cathode for Enhanced Electron Injection in Organic Electroluminescent Devices,” Japanese Journal of Applied Physics, Vol. 38, No. 11B, 1999, pp. L1348-L1350. http://dx.doi.org/10.1143/JJAP.38.L1348
- H. Ishii, K. Sugiyama, E. Ito and K. Seki, “Energy Level Alignment and Interfacial Electronic Structures at Organic/Metal and Organic/Organic Interfaces,” Advanced Materials, Vol. 11, No. 8, 1999, pp. 605-625. http://dx.doi.org/10.1002/(SICI)1521-4095(199906)11:8<605::AID-ADMA605>3.0.CO;2-Q
- A. J. Maxwell, P. A. Bronwiler, D. Arvanitis, J. Hasselstrom, M. K. J. Johansson and N. Martensson, “Electronic and Geometric Structure of C60 on Al(111) and Al(110),” Physical Review B, Vol. 57, No. 12, 1998, pp. 7312-7326. http://dx.doi.org/10.1103/PhysRevB.57.7312
- J. Y. Lee, “Efficient Hole Injection in Organic Light Emitting Diodes Using C60 as a Buffer Layer for Al Reflective Anodes,” Applied Physics Letters, Vol. 88, No. 7, 2006, pp. 073512-073514. http://dx.doi.org/10.1063/1.2174838
- S. K. M. Jonsson, W. R. Salaneck and M. Fahlman, “Photoemission of Alq3 and C60 film on Al and LiF Substrates,” Journal of Applied Physics, Vol. 98, No.1, 2005, pp. 014901-014907. http://dx.doi.org/10.1063/1.1929884
- R. Mitsumoto, et al., “Electronic Structures and Chemical Bonding of Fluorinated Fullerenes Studied by NEXAFS, UPS and Vacuum-Absorption Spectroscopies,” Journal of Physical Chemistry A, Vol. 102, No. 3, 1998, pp. 552- 560. http://dx.doi.org/10.1021/jp972863t
- M. Hayashi, H. Ishii, Y. Ouchi and K. Seki, “Examination of Band Bending at Buckminsterfullerene (C60) Metal Interfaces by the Kelvin Probe Method,” Journal of Applied Physics, Vol. 92, No. 7, 2002, pp. 3784-3793. http://dx.doi.org/10.1063/1.1504495
- Y. Tanaka, K. Kanai, Y. Ouchi and K. Seki, “Oxygen Effect in the Interfacial Electronic Structure of C60 Film Studied by Photoelectron Spectroscopy,” Chemical Physics Letters, Vol. 441, No. 1-3, 2007, pp. 63-70. http://dx.doi.org/10.1016/j.cplett.2007.04.080
- T. Yokoyama, D. Yoshimura, E. Ito, H. Ishii, Y. Ouchi and K. Seki, “Energy Level Alignment at Alq3/LiF/Al Interfaces Studied by Electron Spectroscopies: Island Growth of LiF and Size-Dependence of the Electronic Structures,” Japanese Journal of Applied Physics, Vol. 42, No. 6A, 2003, pp. 3666-3675. http://dx.doi.org/10.1143/JJAP.42.3666
- S. Toyoshima, K. Kuwabara, T. Sakurai, T. Taima, K. Saito, H. Kato and K. Akimoto, “Electronic Structure of Bathocuproine on Metal Studied by Ultraviolet Photoemission Spectroscopy,” Japanese Journal of Applied Physics, Vol. 46, No. 4B, 2007, pp. 2692-2695. http://dx.doi.org/10.1143/JJAP.46.2692
- C. Ganzorig, K. J. Kwak, K. Yagi and M. Fujihira, “Fine Tuning Work Function of Indium Tin Oxide by Surface Molecular Design: Enhanced Hole Injection in Organic Electroluminescent Devices,” Applied Physics Letters, Vol. 79, No. 2, 2001, pp. 272-274. http://dx.doi.org/10.1063/1.1384896
- K. Sarangerel, C. Ganzorig, M. Fujihira, M. Sakomura and K. Ueda, “Influence of the Work Function of Chemically Modified Indium-Tin-Oxide Electrodes on the OpenCircuit Voltage of Heterojunction Photovoltaic Cells,” Chemistry Letters, Vol. 37, No. 7, 2007, pp. 778-779. http://dx.doi.org/10.1246/cl.2008.778
- C. Ganzorig, M. Sakomura, K. Ueda and M. Fujihira, “Current-Voltage Behavior in Hole-Only Single-Carrier Devices with Self-Assembling Dipole Molecules on Indium Tin Oxide Anodes,” Applied Physics Letters, Vol. 89, No. 26, 2006, pp. 263501-253603. http://dx.doi.org/10.1063/1.2420792
- S. W. Cho, et al., “Origin of Charge Transfer Complex Resulting in Ohmic Contact at the C60/Cu Interface,” Synthetic Metals, Vol. 157, No. 2-3, 2007, pp. 160-164. http://dx.doi.org/10.1016/j.synthmet.2007.01.006
- C. J. Huang, D. Glozea, A. Turakn and Z. H. Lu, “Passivation Effect of Al/LiF Electrode on C60 Diodes,” Applied Physics Letters, Vol. 86, No. 3, 2005, pp. 033107-033109. http://dx.doi.org/10.1063/1.1854193
- M. Vogel, S. Doka, Ch. Breyer, M. Ch. Lux-Steiner and K. Fostiropoulos, “On the Function of a Bathocuproine Buffer Layer in Organic Photovoltaic Cells,” Applied Physics Letters, Vol. 89, No. 16, 2006, pp. 163501- 163503. http://dx.doi.org/10.1063/1.2362624
- J. Kido and T. Matsumoto, “Bright Organic Electroluminescent Devices Having a Metal-Doped Electron-Injecting Layer,” Applied Physics Letters, Vol. 73, No. 20, 1998, pp. 2866-2868. http://dx.doi.org/10.1063/1.122612
- C. Ganzorig, K. Suga and M. Fujihira, “Alkali Metal Acetates as Effective Electron Injection Layers for Organic Electroluminescent Devices,” Materials Science and Engineering: B, Vol. 85, No. 2-3, 2001, pp. 140-143. http://dx.doi.org/10.1016/S0921-5107(01)00547-5
- C. Ganzorig and M. Fujihira, “Evidence for Alkali Metal Formation at a Cathode Interface of Organic Electroluminescent Devices by Thermal Decomposition of Alkali Metal Carboxylates during Their Vapor Deposition,” Applied Physics Letters, Vol. 85, No. 20, 2004, pp. 4774- 4776. http://dx.doi.org/10.1063/1.1819984
- N. Johansson, T. Osada, S. Stafstrom, W. R. Salaneck, V. Parente, D. A. dos Santos, X. Crispin and J. L. Bredas, “Electronic Structure of Tris(8-Hydroxyquinoline) Aluminum Thin Films in the Pristine and Reduced States,” Journal of Chemical Physics, Vol. 111, No. 5, 1999, pp. 2157-2163. http://dx.doi.org/10.1063/1.479486
- C. Ganzorig and M. Fujihira, “A Possible Mechanism for Enhanced Electrofluorescence Emission through TripletTriplet Annihilation in Organic Electroluminescent Devices,” Applied Physics Letters, Vol. 81, No. 17, 2002, pp. 3137-3139. http://dx.doi.org/10.1063/1.1515129
- V. I. Srdanov, C. H. Lee and N. S. Sariciftci, “Spectral and Photocarrier Dynamics in Thin Films of Pristine and Alkali-Doped C60,” Thin Solid Films, Vol. 257, No. 2, 1995, pp. 233-243. http://dx.doi.org/10.1016/0040-6090(94)05707-9
- B. A. Gregg and M. C. Hanna, “Comparing Organic to Inorganic Photovoltaic Cells: Theory, Experiment, and Simulation,” Journal of Applied Physics, Vol. 93, No. 6, 2003, pp. 3605-3614. http://dx.doi.org/10.1063/1.1544413
- Y. Terao, H. Sasabe and C. Adachi, “Correlation of the Hole Mobility Exciton Diffusion Length, and Solar Cell Characteristics in Phthalocyanine/Fullerene Organic Solar Cells,” Applied Physics Letters, Vol. 90, No. 10, 2007, 103515-103517. http://dx.doi.org/10.1063/1.2711525
- C. M. Ramsdale, et al., “The Origin of the Open-Circuit Voltage in Polyfluorene-Based Photovoltaic Devices,” Journal of Applied Physics, Vol. 92, No. 8, 2002, pp. 4266-4270. http://dx.doi.org/10.1063/1.1506385
- G. G, Malliaras, J. R. Salem, P. J. Brock and J. C. Scott, “Photovoltaic Measurement of the Built-In Potential in Organic Light Emitting Diodes and Photodiodes,” Journal of Applied Physics, Vol. 84, No. 3, 1998, pp. 1583- 1587. http://dx.doi.org/10.1063/1.368227
- V. D. Mihailetchi, P. W. M. Blom, J. C. Hummelen and M. T. Rispens, “Cathode Dependence of The Open-Circuit Voltage of Polymer: Fullerene Bulk Heterojunction Solar Cells,” Journal of Applied Physics, Vol. 94, No. 10, 2003, pp. 6849-6864. http://dx.doi.org/10.1063/1.1620683
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, “Device Physics of Polymer: Fullerene Bilk Heterojunction Solar Cell,” Advanced Materials, Vol. 19, No. 12, 2007, pp. 1551-1566. http://dx.doi.org/10.1002/adma.200601093
- C. J. Brabec, et al., “Origin of the Open Circuit Voltage of Plastic Solar Cells,” Advanced Functional Materials, Vol. 11, No. 5, 2001, pp. 374-380. http://dx.doi.org/10.1002/1616-3028(200110)11:5<374::AID-ADFM374>3.0.CO;2-W
- H. J. Snaith, N. C. Greenham and R. H. Friend, “The Origin of Collected Charge and Open-Circuit Voltage in Blended Polyfluorene Photovoltaic Devices,” Advanced Materials, Vol. 16, No. 18, 2004, pp. 1640-1645. http://dx.doi.org/10.1002/adma.200305766

